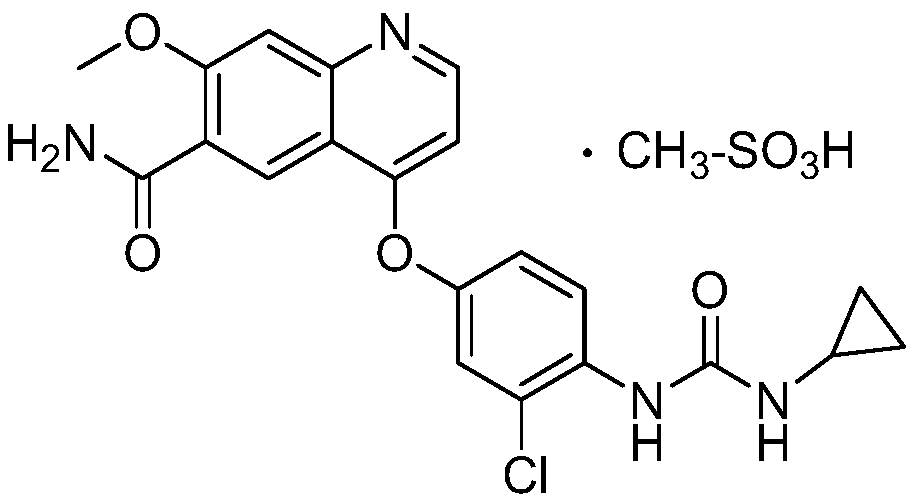
Preparation method of lenvatinib and its salts
A technology of lenvatinib and compounds, which is applied in the field of drug synthesis, can solve the problems of lenvatinib finished product residue, low lenvatinib yield, and difficult monitoring of intermediates, so as to avoid potential safety hazards, reduce production costs, React Safe Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
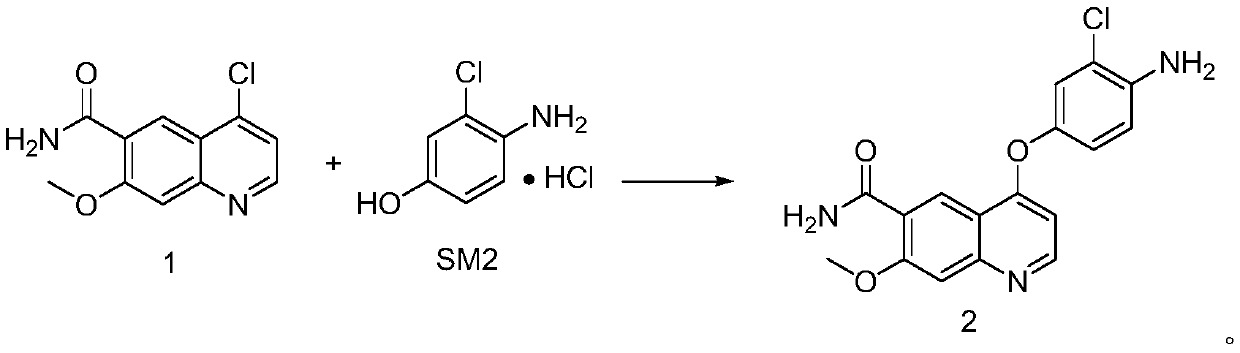
[0051] The preparation of formula 2 compound
[0052]Potassium hydroxide (41.50 g, 3.5 eq) was added into a three-neck reaction flask containing 500 ml of dimethyl sulfoxide containing 10% water. At room temperature, SM2 (57.00 g, 1.5 eq) was added in one portion. After the addition, stir at room temperature for about 3 minutes. Add 50.00 g of the compound of formula 1 at one time, raise the temperature to 110°C, keep it warm for 2 hours, add acetone:water=1:10 (1.5L) while it is hot, cool down to room temperature for crystallization, collect the filter cake by filtration, and dry it under vacuum at 45°C overnight . 68g of the reddish-brown solid compound of Formula 2 was collected, the purity of the reaction solution was 97.81%, the purity of the crude product was 98.51%, and the yield was 94.44%.
Embodiment 2
[0054] The preparation of formula 3 compound
[0055] 28.7g of the compound of formula 2 was added to a reaction flask containing 215ml of N-methylpyrrolidone, and pyridine (14.5g, 2.2eq) was added at the same time, the temperature was lowered to 1°C and 1eq of water was added at the same time, and phenyl chloroformate ( 28.9 g, 2.2 eq). After about 50 minutes of dripping, keep at 2°C for 2 hours, take a drop of the reaction solution and dilute it with 1ml of methanol for HPLC detection. HPLC showed that the purity of the reaction liquid was 96.205%, and the remaining reaction liquid was used to prepare the compound of formula 4.
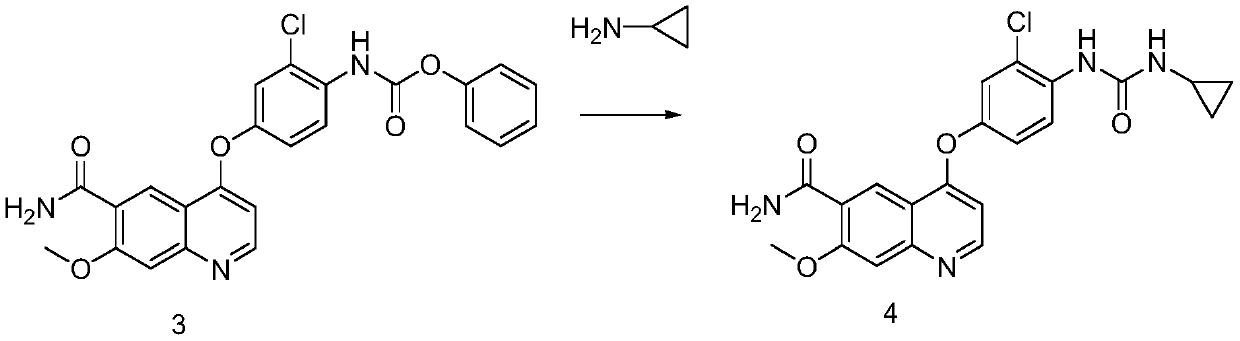
Embodiment 3
[0057] The preparation of formula 4 compound
[0058] At room temperature, add 21.45 g of cyclopropylamine dropwise to the reaction solution of the compound of formula 3 prepared above, drop it in about 20 minutes, raise it to room temperature, keep stirring and react for 2 hours. The liquid purity is 96.58%. Add 1.29L of acetone-water mixed solution (acetone:water=1:5), crystallize overnight at room temperature, collect the filter cake by filtration, and vacuum-dry the filter cake at 50°C to obtain 30 g, the purity of which is 99.6% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com