Lenvatinib mesilatee polymorph and preparation method thereof
A polymorphic form, lenvatinib technology, applied in the field of medicine, can solve problems such as not being able to be used as a pharmaceutical crystal form, thermodynamic instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: Preparation of crystal form AZT-15
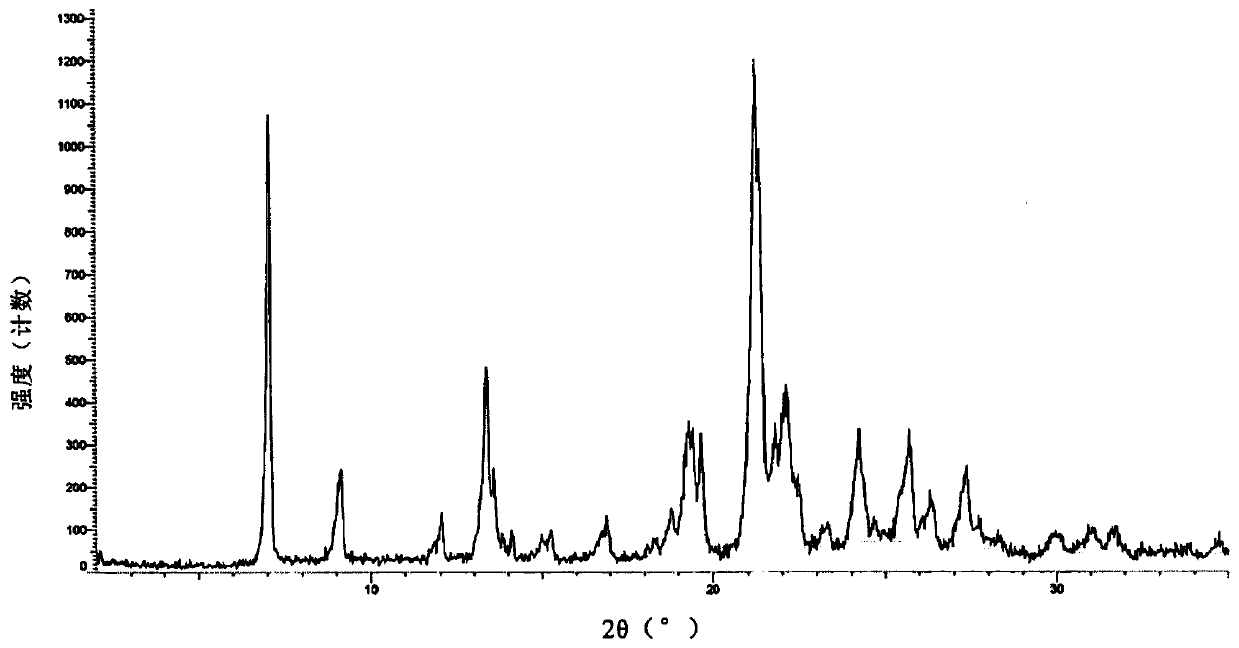
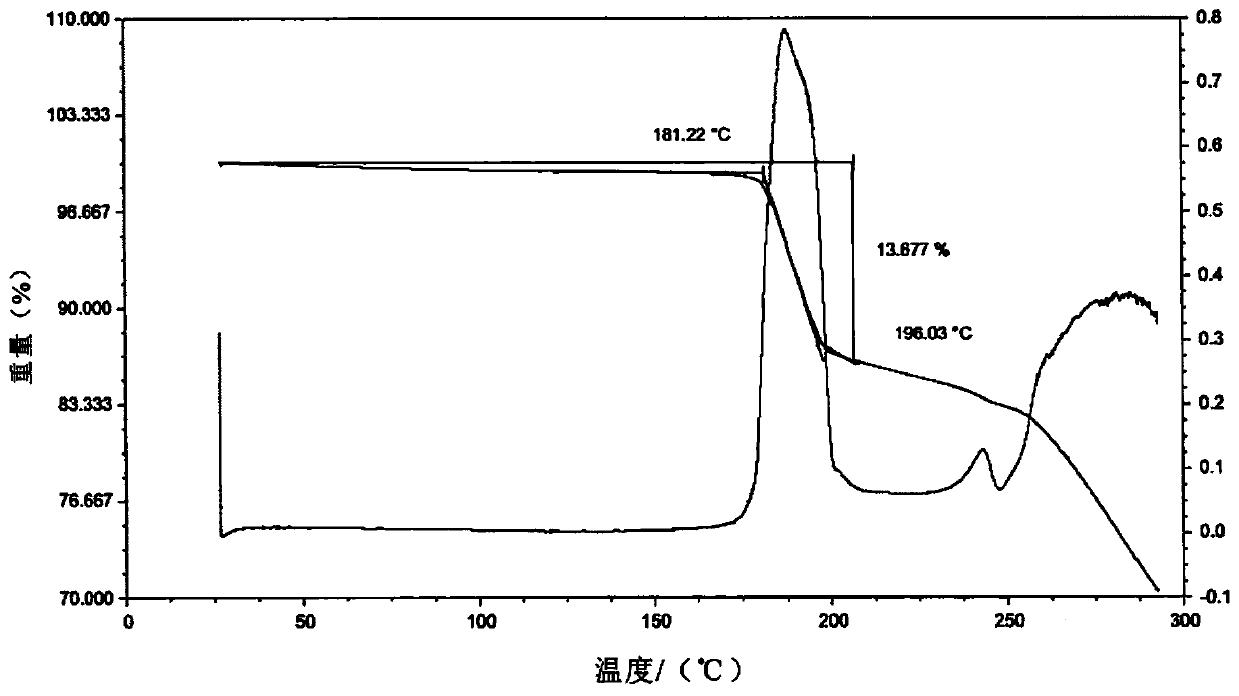
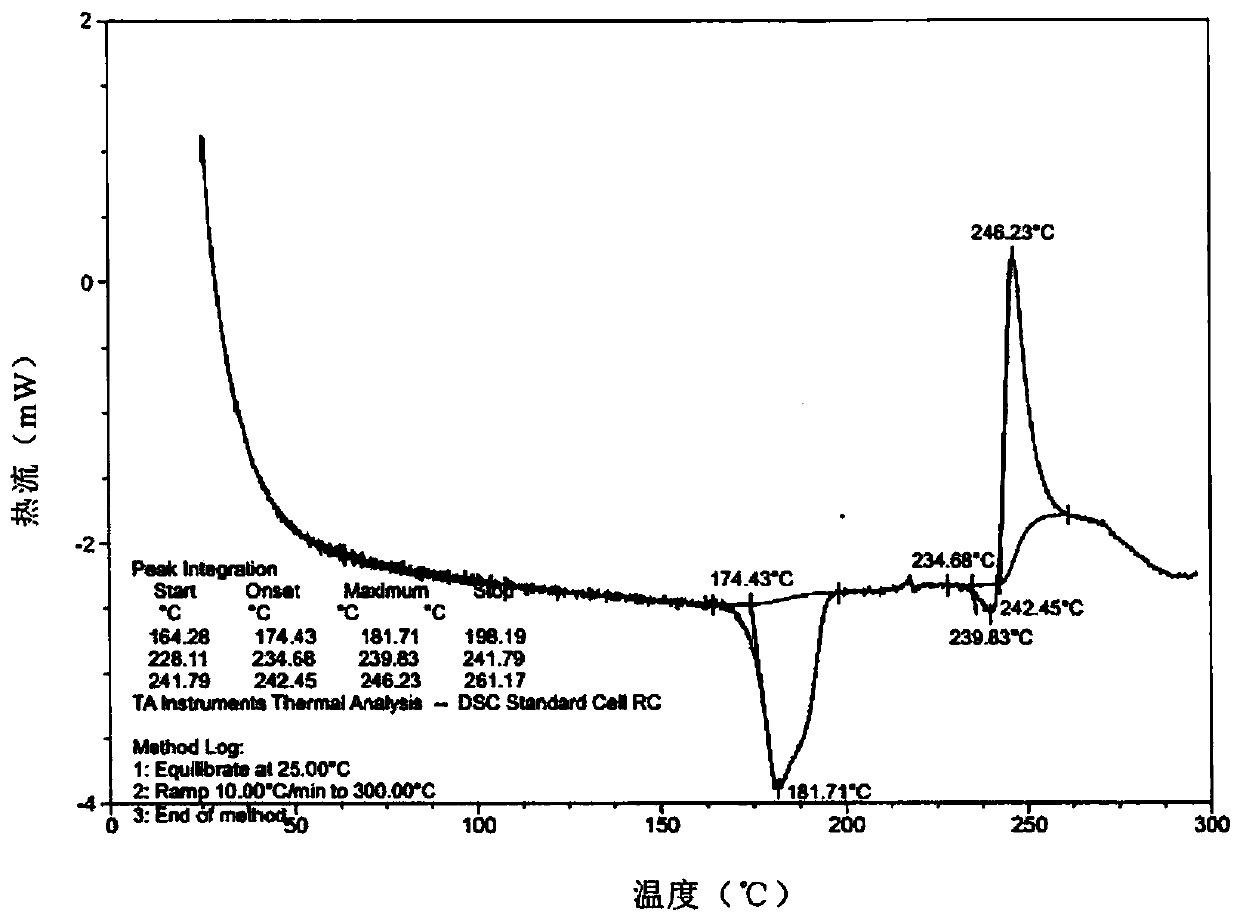
[0105]Weigh 2.0 g of lenvatinib free base and add it into 20 mL of DMSO:acetone=3:1 mixed solvent, heat and stir at 50°C. After the solution was clear, 0.5 g of methanesulfonic acid was added and stirred for 10 minutes. Add 20 mL of MTBE dropwise for 30 minutes. After the dropwise addition, the temperature was lowered to 10-15° C., and the stirring was continued for 24 hours. Filter and dry under vacuum at 25°C to obtain flaky light yellow crystals. Gained crystal is tested by XRPD, and its X-ray powder diffraction pattern is as follows figure 1 As shown; by TGA test, the TGA figure of crystal form AZT-15 appears a weight loss peak at 181 ° C, and its spectrum is as follows figure 2 As shown; after the DSC test, the DSC diagram of the crystal form AZT-15 has a desolvation endothermic peak at 174 ° C, and the Onset temperature is 174 ± 2 ° C. The spectrum is as follows image 3 shown by 1 H-NMR test, the spectrum ...
Embodiment 2
[0110] Embodiment 2: Preparation of crystal form AZT-15
[0111] Weigh 2.0 g of lenvatinib free base and add it into 30 mL of DMSO:ethanol=4:1 mixed solvent, heat and stir at 50°C. After the solution was clear, 0.5 g of methanesulfonic acid was added and stirred for 10 minutes. Slowly add 30 mL of MTBE dropwise for 30 minutes. After the dropwise addition, the temperature was lowered to 10-15° C., and the stirring was continued for 24 hours. Filter and dry under vacuum at 25°C to obtain flaky light yellow crystals. The obtained crystals were subjected to XRPD, TGA, DSC, 1 H-NMR test, the obtained spectrograms are all substantially consistent with that of Example 1.
Embodiment 3
[0112] Embodiment 3: Preparation of crystal form AZT-15
[0113] Weigh 2.0 g of lenvatinib free base and add it into 20 mL of DMSO, heat and stir at 60°C. After the solution was clear, 0.5 g of methanesulfonic acid was added and stirred for 10 minutes. Cool down to 20° C., add 0.02 g of the crystal form AZT-15 obtained in Example 1, and stir for 10 minutes. Slowly add 20 mL of MTBE dropwise for 30 minutes. After the dropwise addition, the temperature was lowered to 10-15° C., and the stirring was continued for 24 hours. Filter and dry in a vacuum oven at 25°C to obtain flaky light yellow crystals. The obtained crystals were subjected to XRPD, TGA, DSC, 1 H-NMR test, the obtained spectrograms are all substantially consistent with that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com