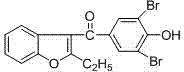
Benzbromarone crystal form A and preparation method thereof
A technology of benzbromarone crystal form and benzbromarone, which is applied in the field of chemical pharmaceuticals, can solve the problems of poor industrial applicability of crystallization purification methods, poor drug form stability, and inestimable market potential, achieving low cost and good crystal form stability , the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of benzbromarone crystal form A:
[0019] Take 27.5g of benzbromarone raw material and completely dissolve it in 90ml of acetone solvent under the condition of reflux at 56°C. After dissolving, the temperature is rapidly lowered, and solids are gradually precipitated, left to stand at 5°C for crystallization for 10 hours, filtered, and rinsed with a small amount of cold acetone , the benzbromarone crystal form A can be obtained.
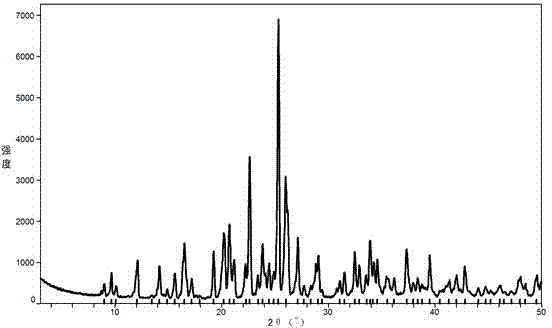
[0020] The powder X-ray diffraction pattern of gained benzbromarone crystal form A is as attached figure 2 shown.
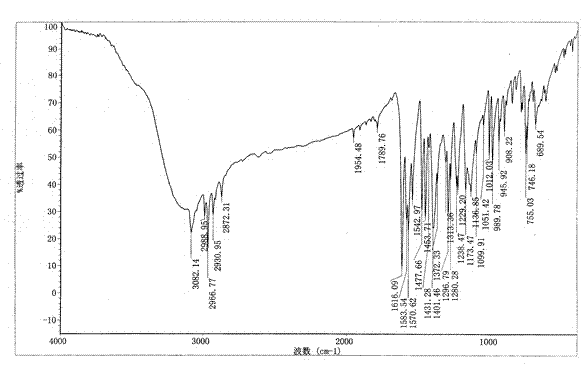
[0021] The infrared absorption spectrum figure of gained benzbromarone crystal form A is as attached image 3 shown.
[0022] The differential scanning calorimetry differential thermal analysis spectrum of gained benzbromarone crystal form A is as attached Figure 4 shown.
[0023] The detection method and result of differential scanning calorimetry differential thermal analysis spectrum are as follows:
[0024]...
Embodiment 2
[0032] Take 27.5g of benzbromarone raw material and completely dissolve it in 82ml of acetone solvent at 50°C. After dissolving, the temperature is rapidly lowered, and solids are gradually precipitated. Stand at 0°C for crystallization for 2 hours, filter, and rinse with a small amount of cold acetone. The crystal form A of benzbromarone can be obtained.
Embodiment 3
[0034] Take 27.5g of benzbromarone raw material and completely dissolve it in 138ml of acetone solvent at 40°C. After dissolving, the temperature is rapidly lowered, and solids are gradually precipitated. Stand at 20°C for crystallization for 15 hours, filter, and rinse with a small amount of cold acetone. The crystal form A of benzbromarone can be obtained.
[0035] The content of related substances of the benzbromarone crystal form A obtained by the preparation method of the present invention is far less than the standard of European Pharmacopoeia Version 7.0 (page 1465-1466), and the contents of related substances are shown in Table 1.
[0036] Table 1 Comparison of related substances of benzbromarone raw materials and crystal form A samples with European Pharmacopoeia standards
[0037]
[0038] The solid state of benzbromarone crystal form A obtained by this preparation method is respectively subjected to 10 days, 20 days, and 30 days under the conditions of 92.5% humi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com