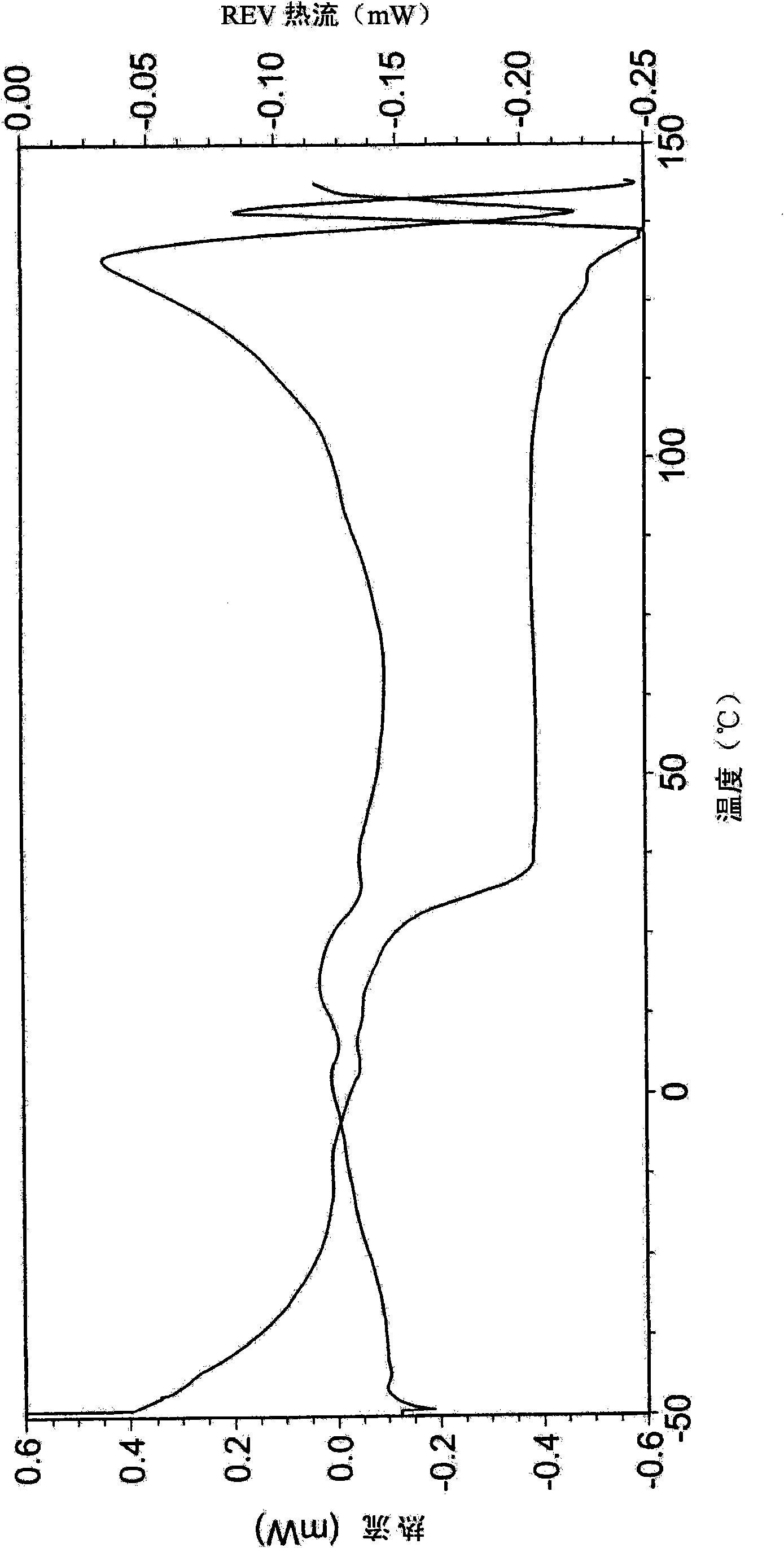
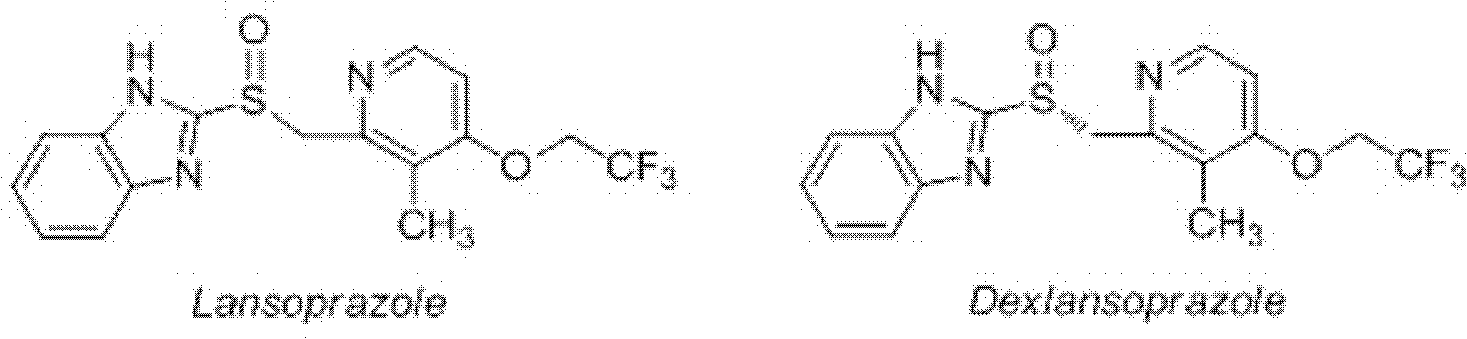
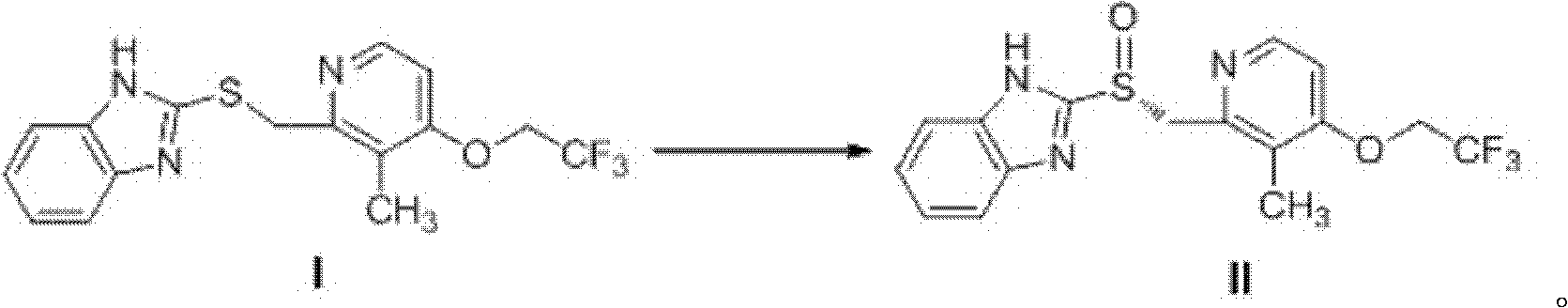
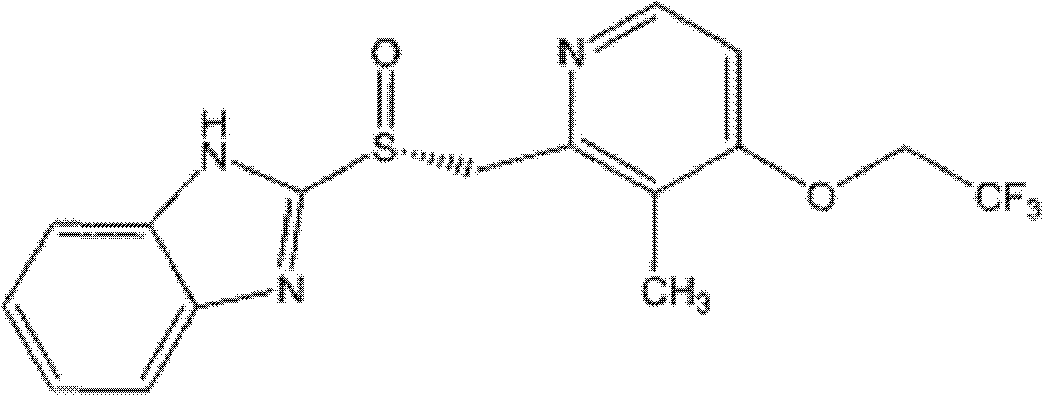
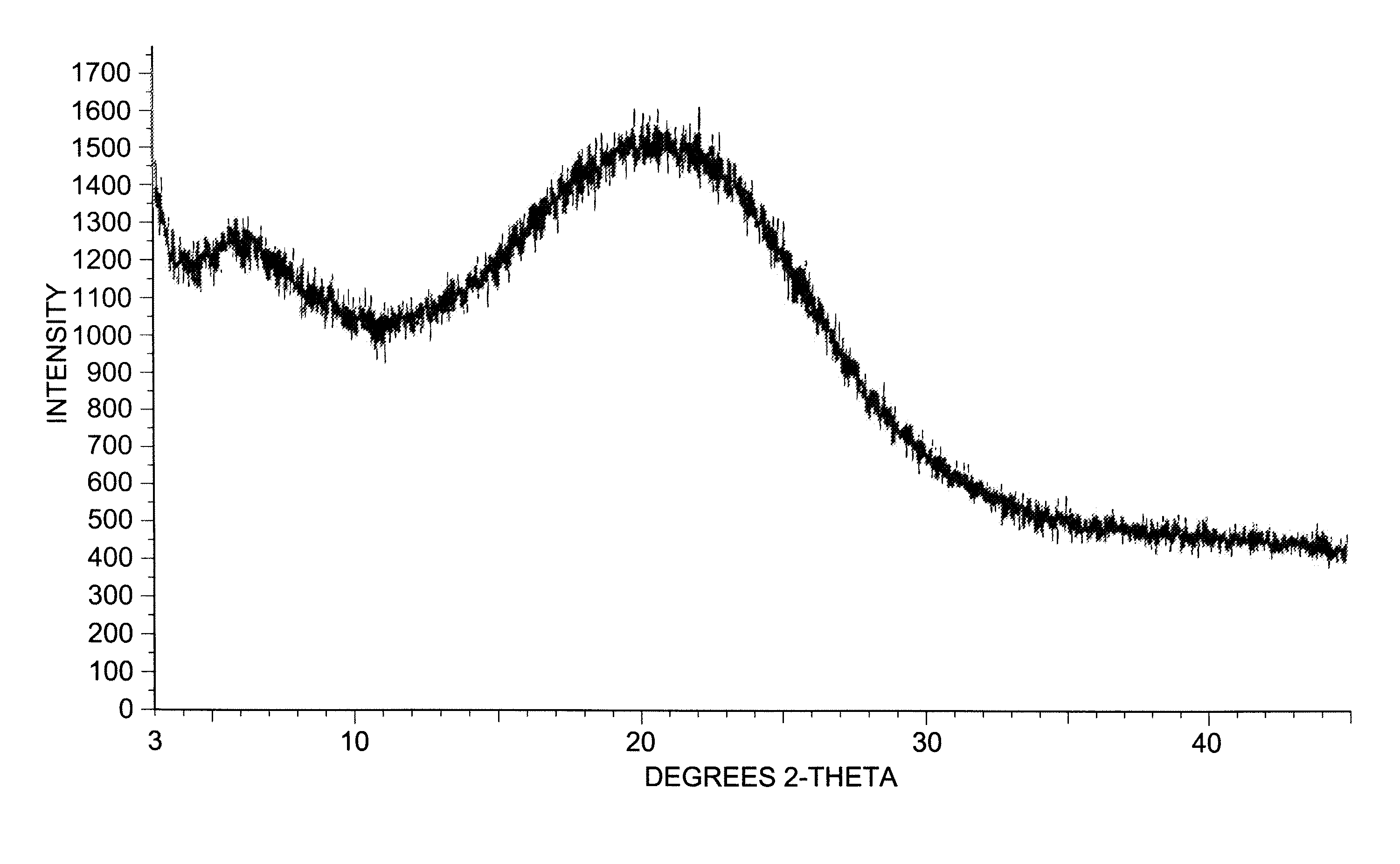
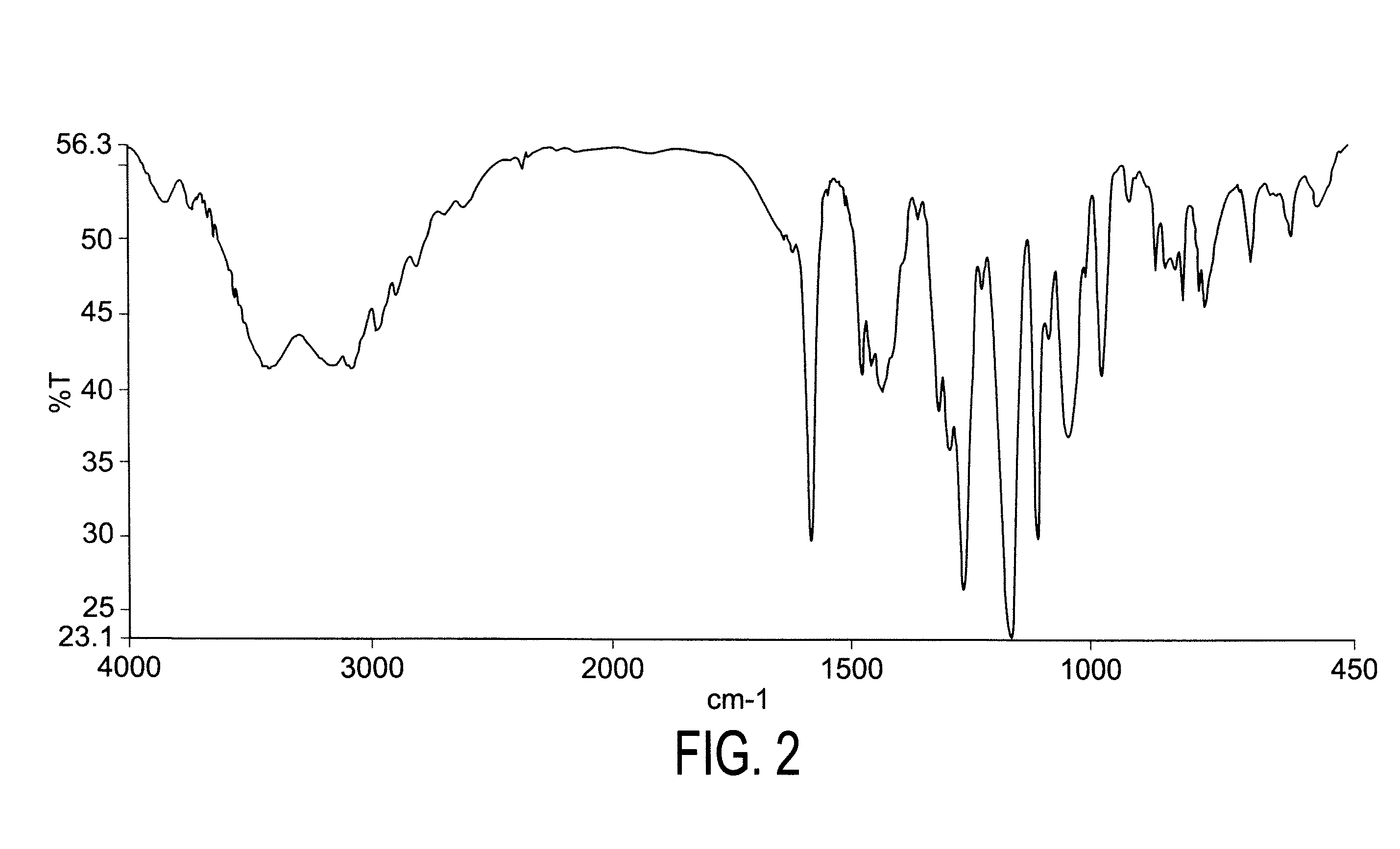
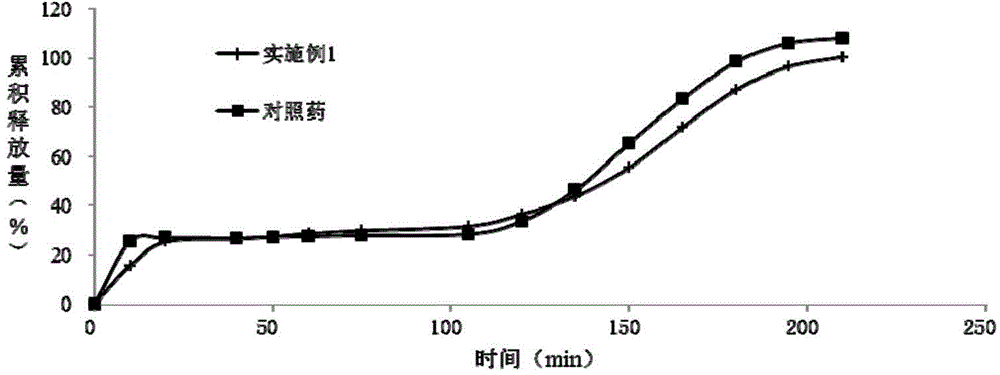
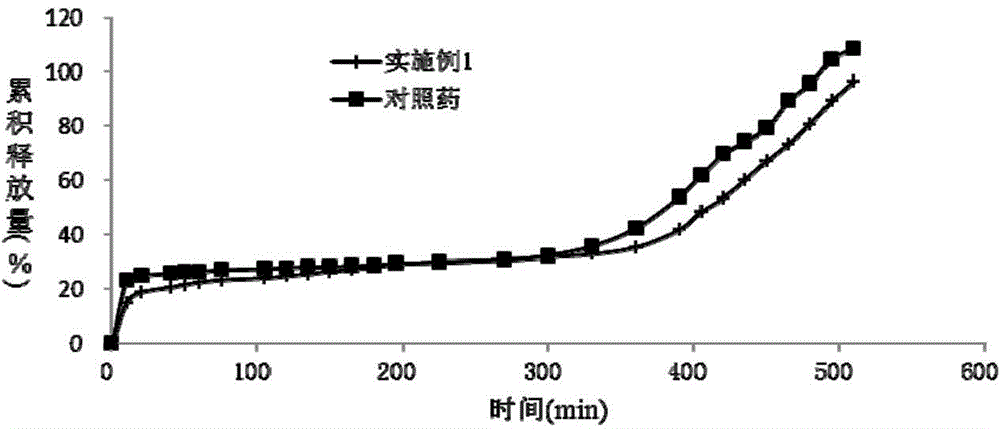
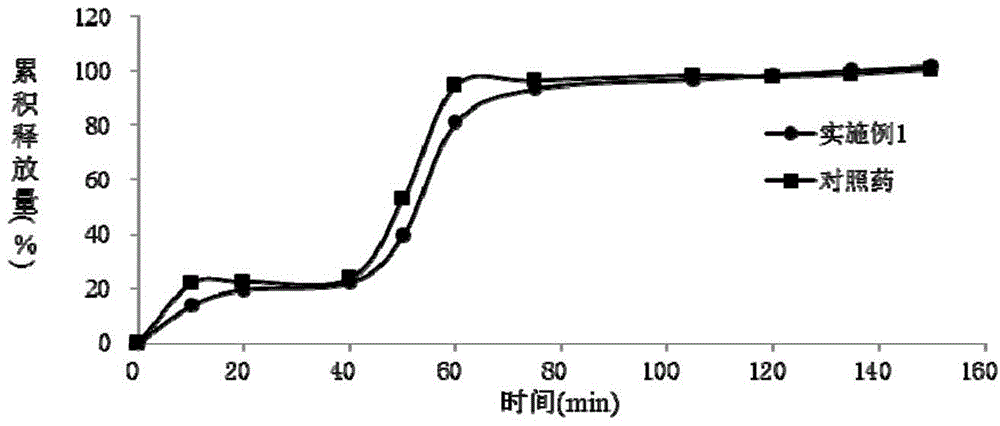
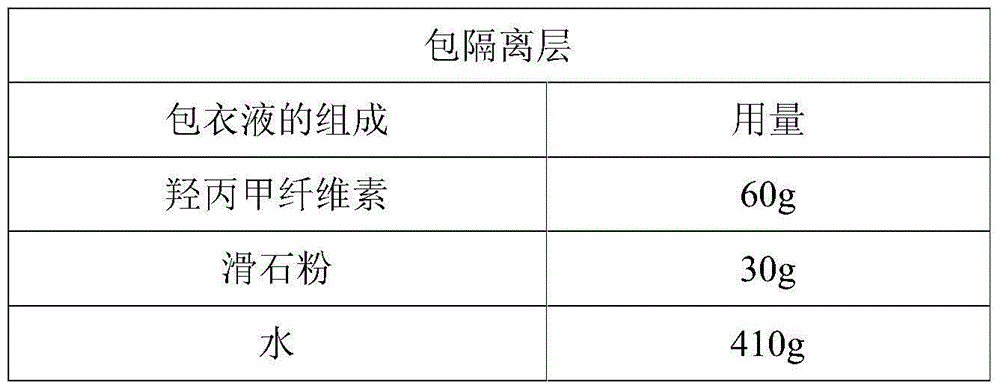
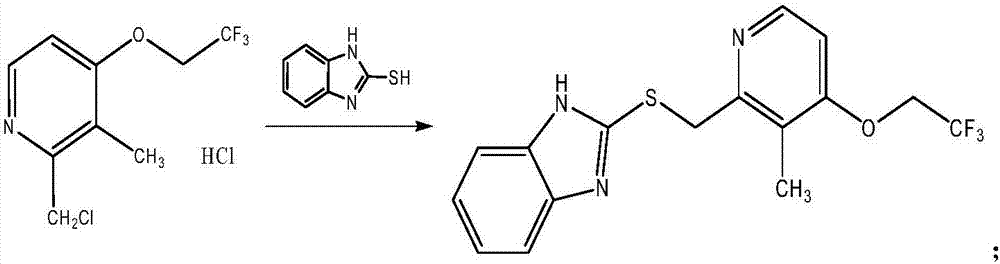
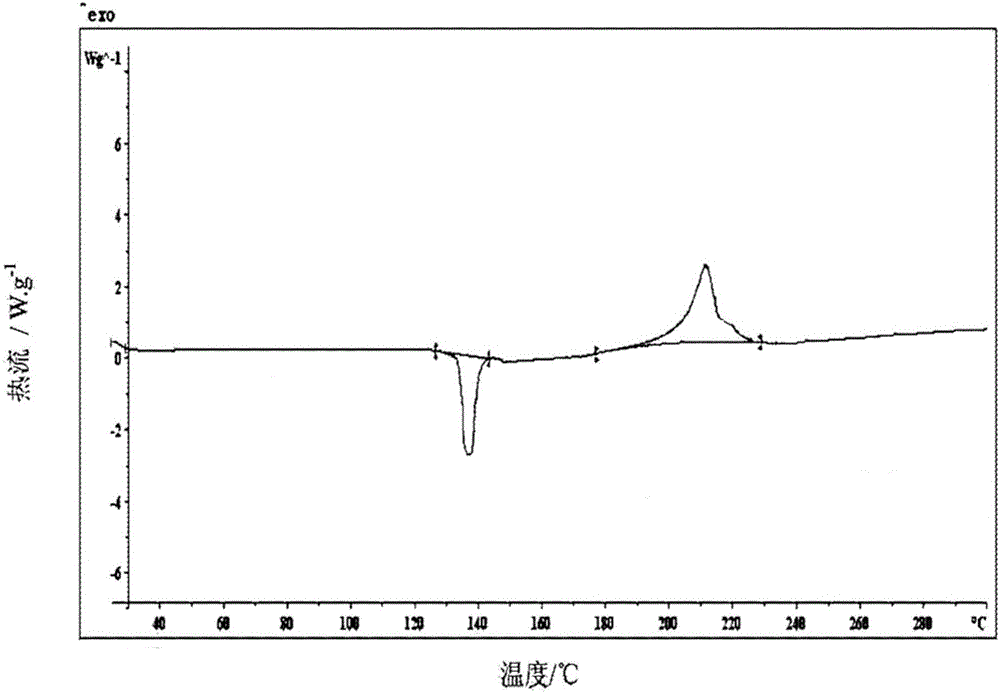
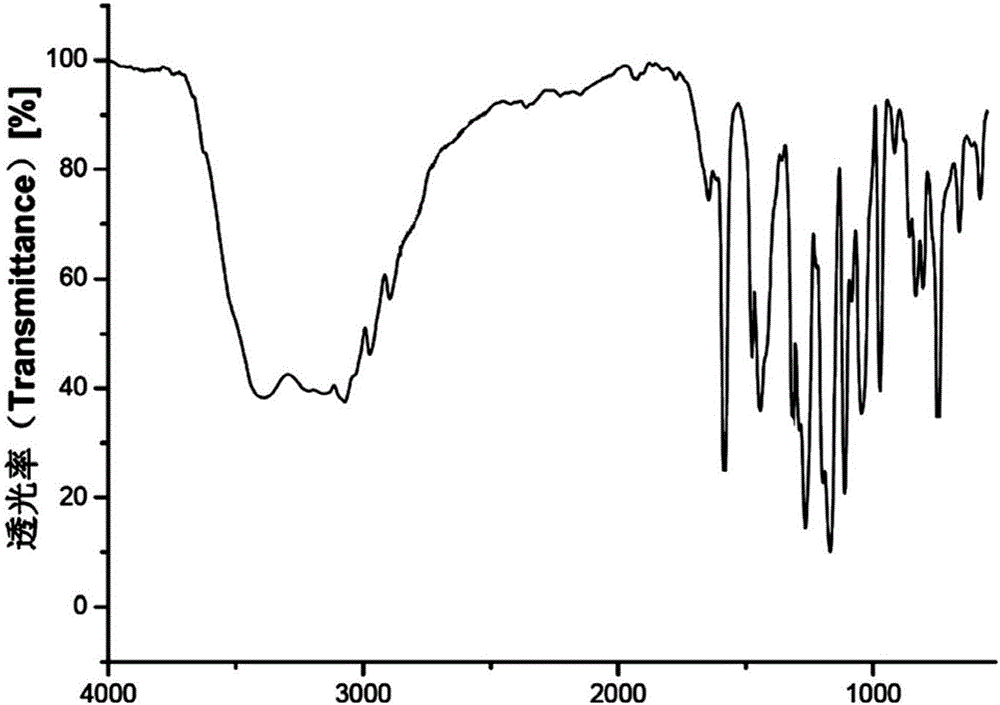
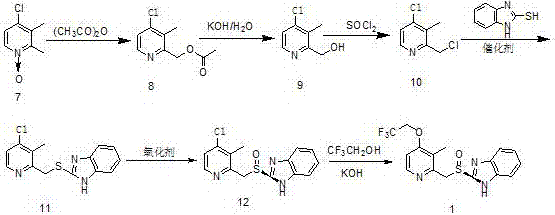
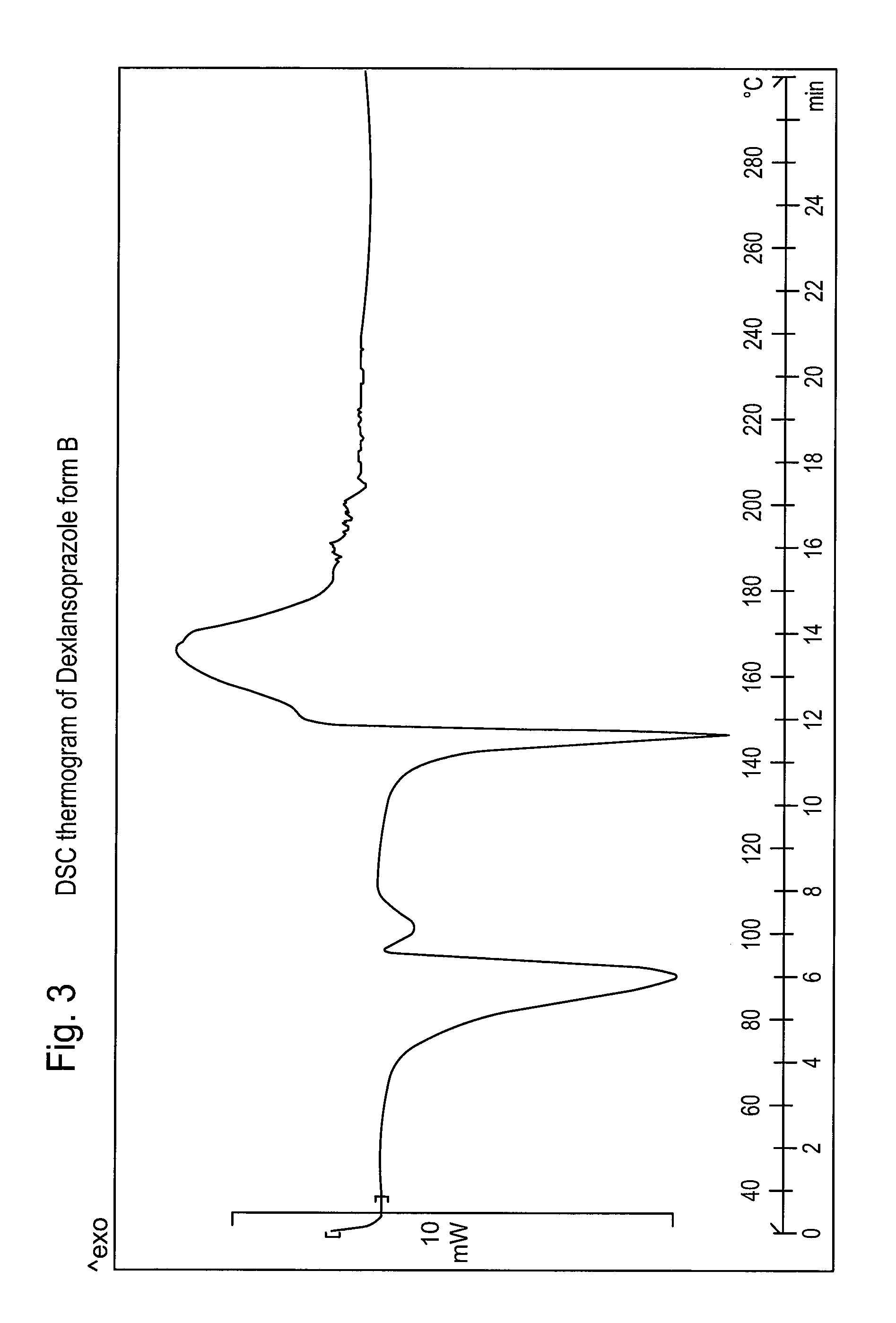
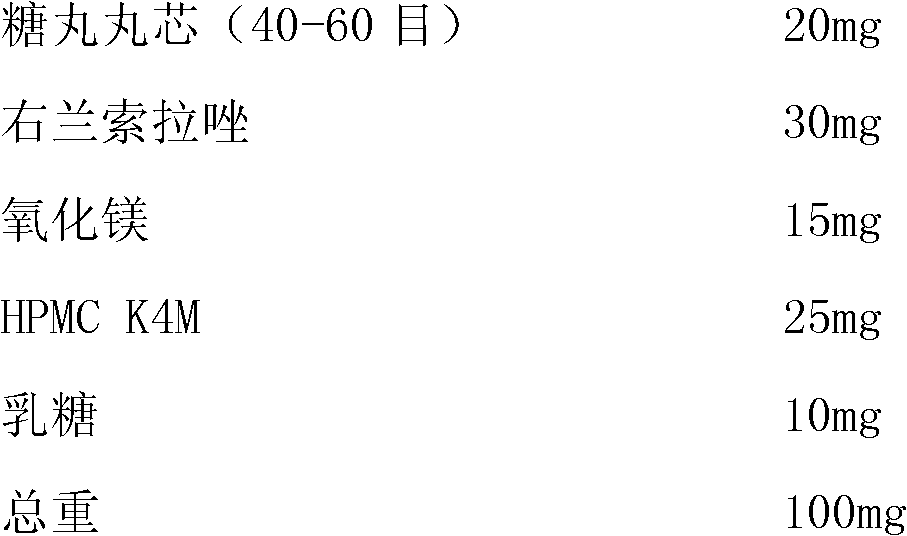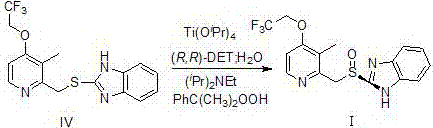Patents
Literature
121 results about "Dexlansoprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
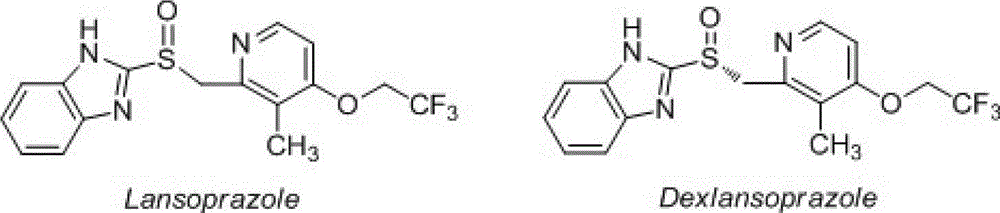
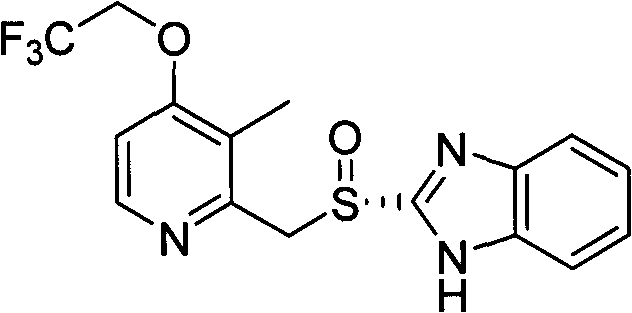
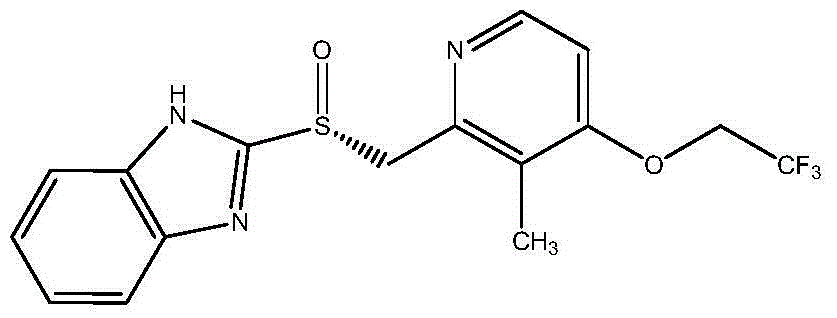
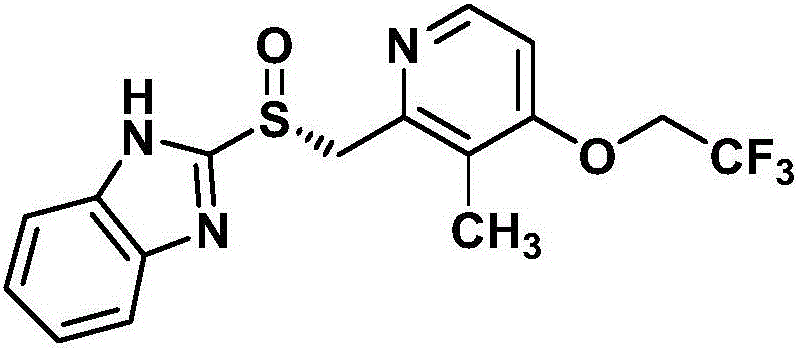
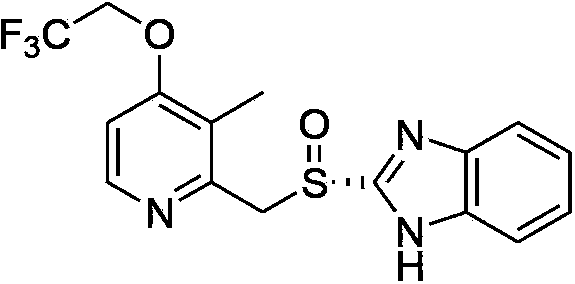
Dexlansoprazole is used to treat certain stomach and esophagus problems (such as acid reflux).
Pharmaceutical formulations of acid-labile drugs
InactiveUS20110189271A1Chemical stabilityGood storage stabilityBiocidePowder deliveryDexlansoprazolePharmaceutical formulation
Pharmaceutical formulations comprising inert cores that are coated with a layer comprising an amorphous dexlansoprazole sodium salt formed in-situ, further sequentially coated with an intermediate layer and an enteric coating layer. Coated cores may be further formulated to produce tablets or capsules.
Owner:DR REDDYS LAB LTD +1
Dexlansoprazole compositions
Premixes of dexlansoprazole with pharmaceutical excipients, processes for preparing premixes, pharmaceutical formulations containing the premixes, and their use in treatment of erosive esophagitis and heartburn associated with non-erosive gastroesophageal reflux disease.
Owner:DR REDDYS LAB LTD +1
Dexlansoprazole process and polymorphs
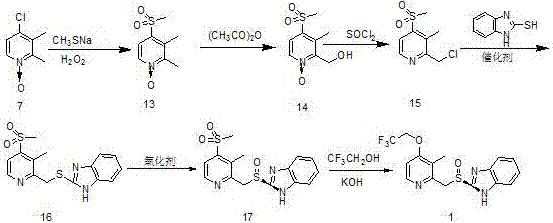
Processes for the preparation of dexlansoprazole, an amorphous form of dexlansoprazole, a solid dispersion of amorphous dexlansoprazole and a pharmaceutically acceptable carrier, and processes for their preparation are provided. Also provided are crystalline compounds 2-[(R)-[(4-chloro-3-methyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole and 2-[(R)-[(4-nitro-3-methyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, and methods for their preparation.
Owner:DR REDDYS LAB LTD
Method for synthesizing and purifying dexlansoprazole
ActiveCN102659763AHigh purityAvoid heating operationsOrganic chemistryDexlansoprazoleImproved method
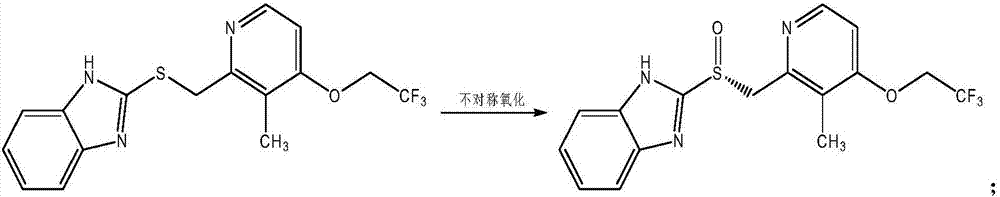
The invention mainly relates to an improved method for synthesizing dexlansoprazole by utilizing a Sharpless asymmetric oxidation method and an optimized method for purifying dexlansoprazole. According to the method, the total yield is above 40% and the purity of the product and the enantiomeric excess (e.e.) are both above 99.5%. The preparation method is simple, is low in cost and is suitable for industrial production.
Owner:南京博德生物制药有限公司
Dexlansoprazole enteric-coated slow controlled-release pellet tablets
InactiveCN103565770AEasy product industrializationTrouble swallowingAntibacterial agentsOrganic active ingredientsDexlansoprazoleBULK ACTIVE INGREDIENT
The invention discloses dexlansoprazole enteric-coated slow controlled-release pellet tablets belonging to the field of slow controlled-release preparations, and in particular relates to a preparation method of acid-sensitive proton pump inhibitor (PPI) slow controlled-release pellet tablets. The pellet tablets are prepared by tabletting and coating two types of pellets with different release speeds as well as a filler, a disintegrating agent and a lubricant, and release active ingredients at different release speeds, wherein one type of the pellets are enteric-coated quick-release pellets, and the other type of the pellets are enteric-coated slow-release pellets.
Owner:FUKANGREN BIO PHARMA
Dexlansoprazole process and polymorphs
InactiveUS20110028518A1Extended shelf lifeImprove stabilityBiocideOrganic chemistryDexlansoprazoleMethyl group
Processes for the preparation of dexlansoprazole, an amorphous form of dexlansoprazole, a solid dispersion of amorphous dexlansoprazole and a pharmaceutically acceptable carrier, and processes for their preparation. Also provided are crystalline compounds 2-[(R)-[(4-chloro-3-methyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole and 2-[(R)-[(4-nitro-3-methyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, and methods for their preparation.
Owner:DR REDDYS LAB LTD +1
Dexlansoprazole sustained-release capsule and preparation method thereof
The invention relates to a dexlansoprazole sustained-release capsule and a preparation method thereof. The capsule comprises two dexlansoprazole micropills, wherein the two micropills consist of hollow pill cores, active medicine layers, isolating coating layers and sustained-release coating layers. The dexlansoprazole sustained-release capsule provided by the invention can be released in the intestinal tract in position so as to persistently inhibit acid release.
Owner:BEIJING RED SUN PHARMA
Dextral lansoprazole freeze-drying preparation and preparation method thereof
InactiveCN102908322AImprove convenienceImprove securityOrganic active ingredientsPowder deliveryFreeze-dryingDexlansoprazole
The invention relates to a dextral lansoprazole freeze-drying preparation, which comprises the following components: dextral lansoprazole, Hydroxypropyl-Beta-Cyclodextrin and PH modifier. The invention also provides a preparation method for the freeze-drying preparation. The freeze-drying preparation obtained according to the technical scheme of the invention can maintain better redissolving performance and solution stability, and enhance the convenience and security of clinical application; and furthermore, the Hydroxypropyl-Beta-Cyclodextrin used in the preparation method plays the function of stabilizer and excipient, that is, on one hand, the racemization of the dextral lansoprazole is effectively reduced, and on the other hand, the freeze-drying preparation obtained through the preparation method is loose, full and integral in appearance and is suitable for industrialized production.
Owner:NANJING YOKO PHARMA GRP CO LTD
Dexlansoprazole sustained release capsule and preparation method thereof
ActiveCN102600109ASolve the speed problemSolve the problem of bioavailabilityOrganic active ingredientsDigestive systemSustained Release CapsuleDexlansoprazole
The invention belongs to the technical field of pharmaceutical preparation, and particularly relates to a dexlansoprazole sustained release capsule and a preparation method thereof. Two kinds of pellets are arranged in the capsule, and the weight ratio of dexlansoprazole contained in the first kind of pellets and the second kind of pellets is 1:3-4. The dexlansoprazole sustained release capsule disclosed by the invention can avoid the destruction of the dexlansoprazole in gastric acid, and is released in the intestinal tract in a fixed position so as to achieve the purposes of taking effect rapidly and improving bioavailability.
Owner:乐普药业科技有限公司
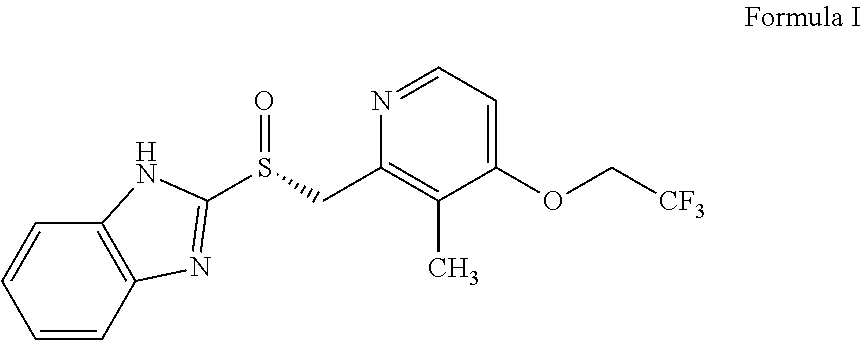
Method for preparing dexlansoprazole
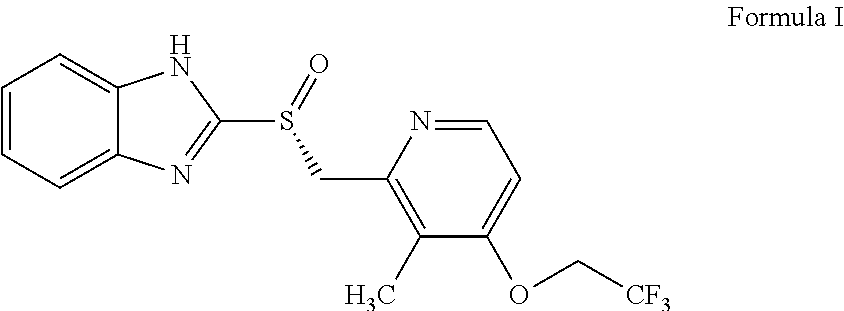
InactiveCN102108077AImprove stabilityHigh purityOrganic chemistryDigestive systemDexlansoprazoleMedicinal chemistry
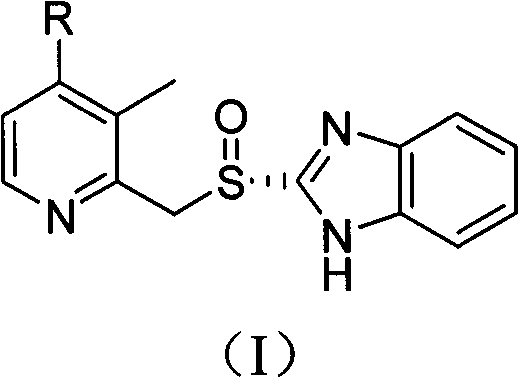
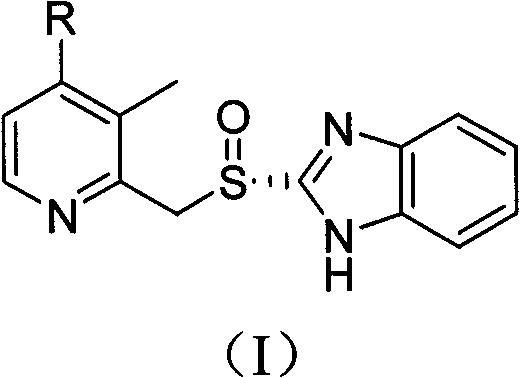
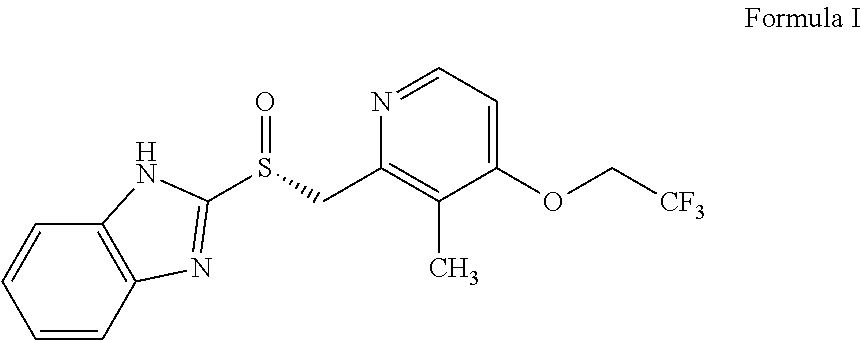
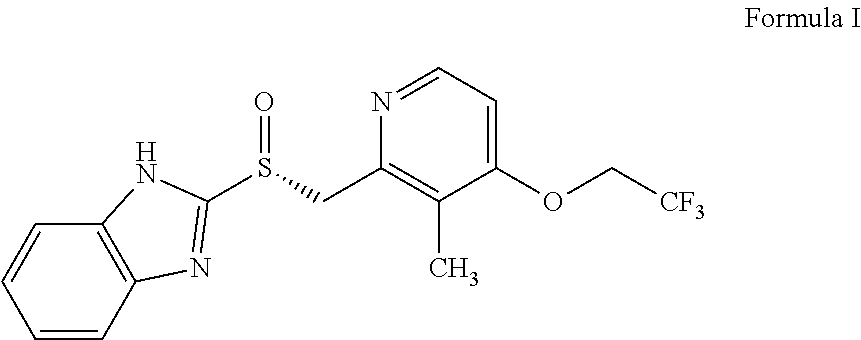
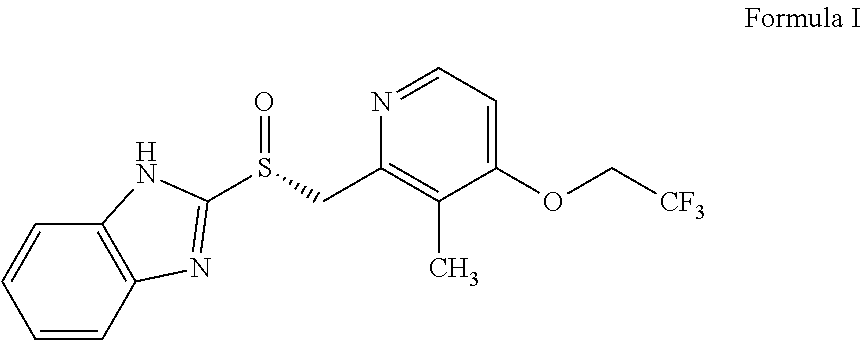
The invention relates to a method for preparing dexlansoprazole. The method is as follows: the compound in the formula (I) is substituted by trifluoroethanol under an alkaline condition to prepare dexlansoprazole.
Owner:JIANGSU HANSOH PHARMA CO LTD
Process for the preparation of crystalline dexlansoprazole
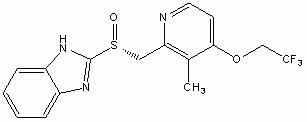
Process for the preparation of crystalline anhydrous (R)-2-[[[3-methyl-4-(2,2,2-trifluoroetoxy)-2-piridyl]methyl]sulphinyl]benzimidazole (dexlansoprazole).
Owner:DIPHARMA FRANCIS
Dexlansoprazole sustained release capsule and preparation method thereof
ActiveCN104940169AAvoid destructionAvoid residueOrganic active ingredientsDigestive systemSustained Release CapsuleDexlansoprazole
The invention belongs to the technical field of pharmaceutical preparation and aims at improving bioavailability of dexlansoprazole in vivo. The dexlansoprazole sustained release capsule provided by the invention is hardly released in gastric acid, and can be disintegrated in intestines, and active ingredients are dissolved out, so that destruction of dexlansoprazole in the gastric acid is avoided; the dexlansoprazole sustained release capsule provided by the invention contains two different types of enteric micropelets, so that two-time dual drug release (DDR) is realized; in a process of preparing eudragit S100 aqueous dispersion, different amounts of alkaline substances are added, different mol numbers of carboxyls in polymers are neutralized, and an enteric-coating material is controlled to be dissolved at different pH values, so that two-time release is realized; besides, an aqueous dispersion coating is adopted, so that ethanol residue is effectively avoided.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Dual release oral tablet compositions of dexlansoprazole
Dual release oral tablet compositions of dexlansoprazole or pharmaceutically acceptable salts or hydrated forms thereof and processes for the manufacture of the tablet composition and its use in the treatment of gastrointestinal disorders.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Dexlansoprazole and its preparation method and use
The invention provides a dexlansoprazole and its preparation method and use. The preparation method of dexlansoprazole comprises synthesizing a dexlansoprazole crude product through a simple process and carrying out further optical purity refinement and purification to obtain a dexlansoprazole product having optical purity close to 100%. The dexlansoprazole product has optical purity close to 100%, has high purity, can be used as a medicinal component and has a better effect.
Owner:乐普药业科技有限公司
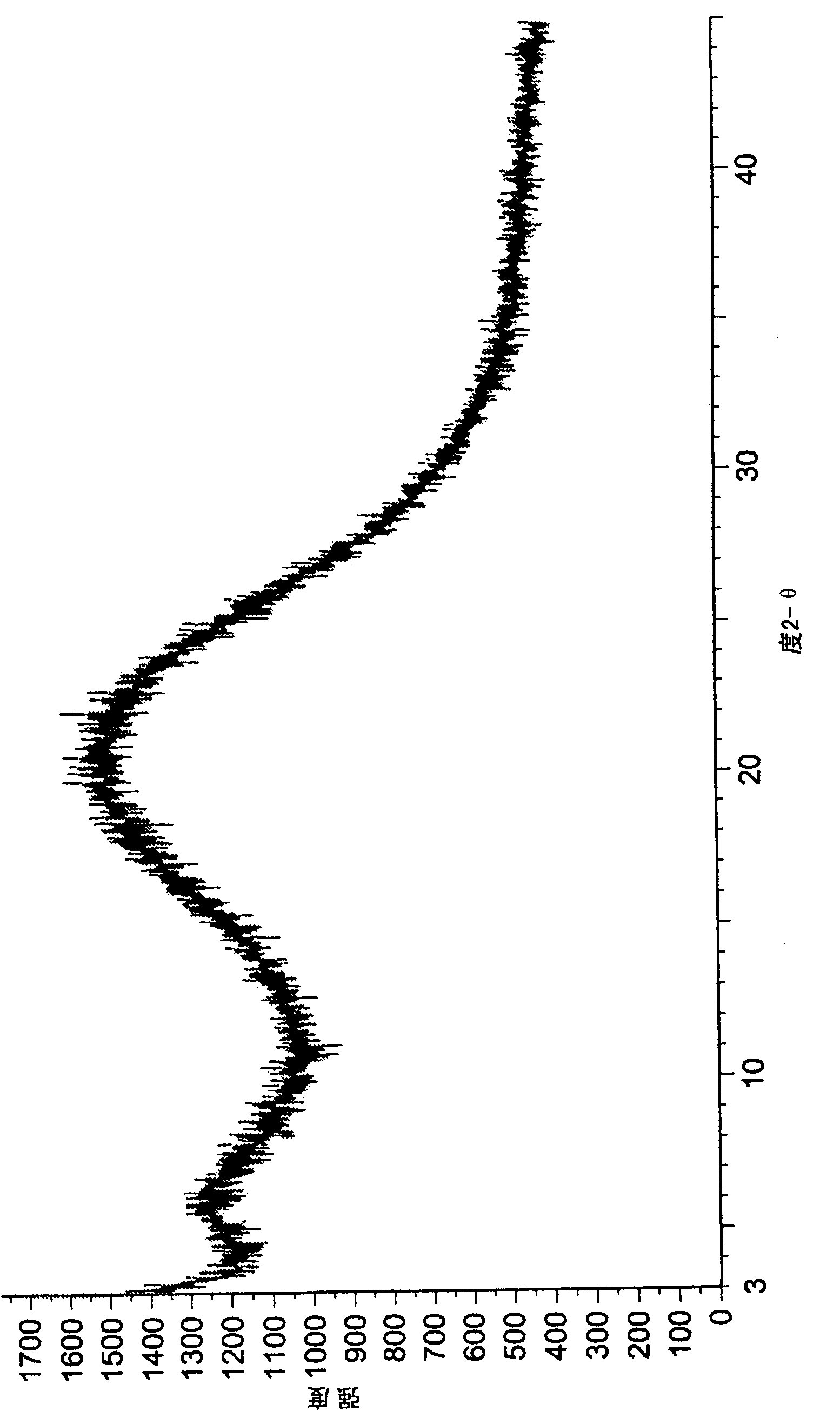
Method for preparing amorphous dexlansoprazole
InactiveCN102108076AGood repeatabilityHigh optical purityOrganic chemistryPolymer scienceDexlansoprazole
The invention relates to a method for preparing amorphous dexlansoprazole, which comprises the steps of dissolving dexlansoprazole or the crystal thereof in a single or mixed solvent, precipitating solids under alkaline conditions at a temperature of 5 DEG C below zero to 25 DEG C below zero, further filtering, washing and drying, and finally getting the amorphous dexlansoprazole.
Owner:JIANGSU HANSOH PHARMA CO LTD
Preparation method for dexlansoprazole
InactiveCN106543146AImprove product qualityShort reaction timeOrganic chemistryOrganic synthesisDexlansoprazole
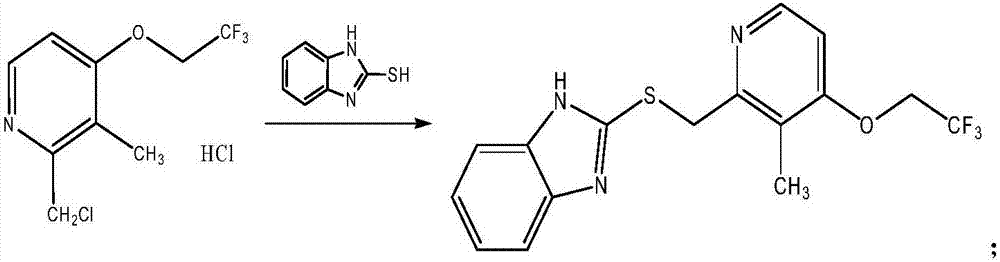
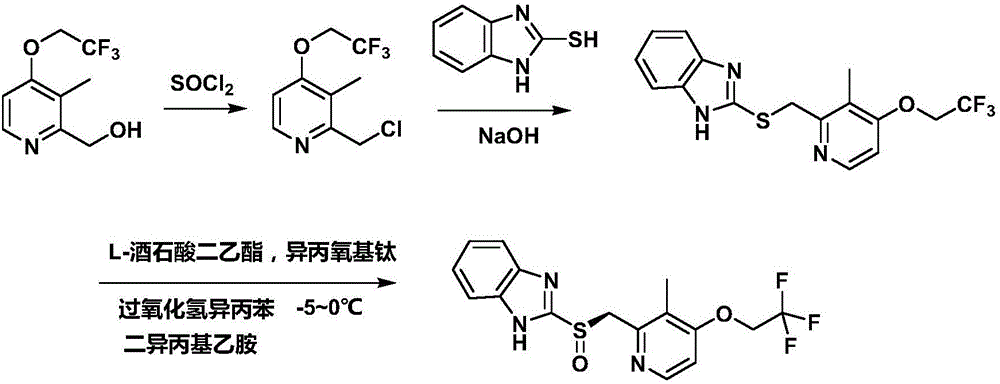
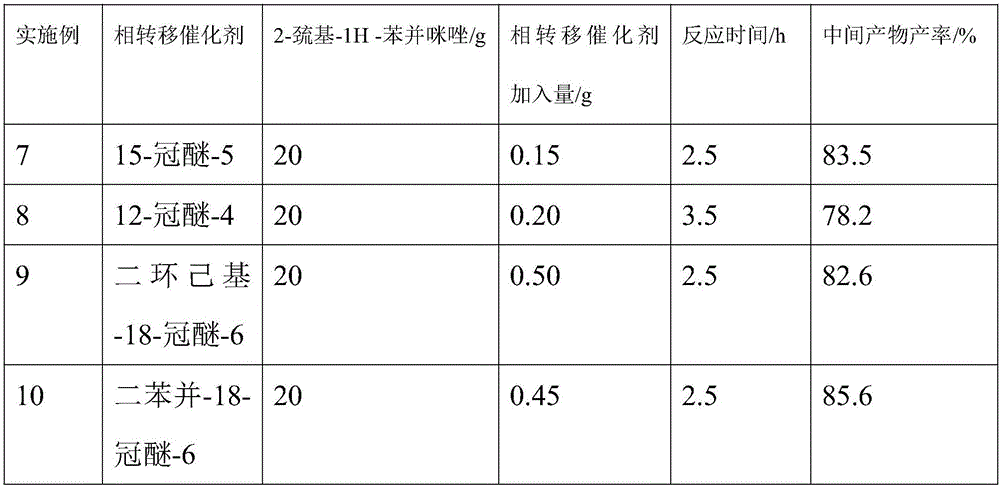
The invention discloses a preparation method for dexlansoprazole, belonging to the field of organic synthesis. The method comprises the following steps: (1) carrying out reaction on 2-hydroxymethyl-3-methyl-4-(2,2,2-trifluoro ethyoxyl) pyridine and thionyl chloride, and compounding 2-chloromethyl-3-methyl-4-(2,2,2-trifluoro ethyoxyl) pyridine; (2) putting 2-chloromethyl-3-methyl-4-(2,2,2-trifluoro ethyoxyl) pyridine acquired in the step (1) and 2-sulfydryl-1H-benzimidazole into an aqueous liquid, and then adding a phase transfer catalyst and sodium hydroxide, thereby acquiring 2-[[3-methyl-4-(2,2,2-trifluoro ethyoxyl) pyridine-2-group] methylmercapto]-1H-benzimidazole; (3) taking L-ethyl tartrate as a chiral assistant agent, titanium isopropoxide and diisopropylethylamine as a catalyst and cumyl hydroperoxide as an oxidizing agent and reacting with 2-[[3-methyl-4-(2,2,2-trifluoro ethyoxyl) pyridine-2-group] methylmercapto]-1H-benzimidazole acquired in the step (2) at low temperature, thereby acquiring dexlansoprazole. The preparation method for dexlansoprazole disclosed by the invention is simple and efficient, is capable of obviously shortening the reaction time under the condition of guaranteeing the product yield and is capable of improving the product quality.
Owner:石家庄市度智医药科技有限公司
Dexlansoprazole compositions
Premixes of dexlansoprazole with pharmaceutical excipients, processes for preparing premixes, pharmaceutical formulations containing the premixes, and their use in treatment of erosive esophagitis and heartburn associated with non-erosive gastroesophageal reflux disease.
Owner:DR REDDYS LAB INC +1
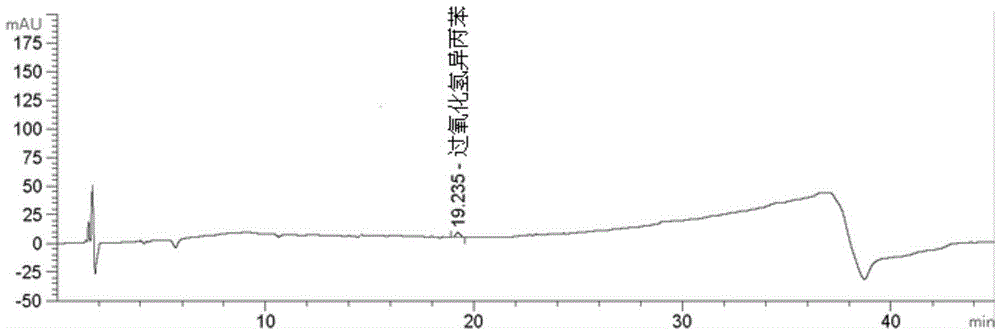
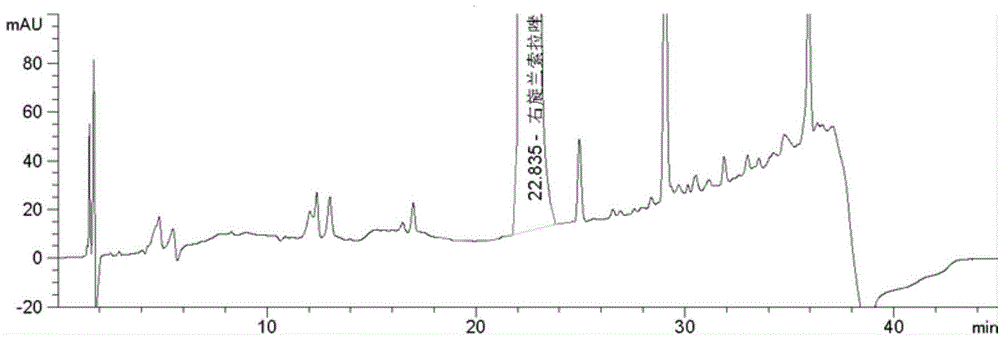
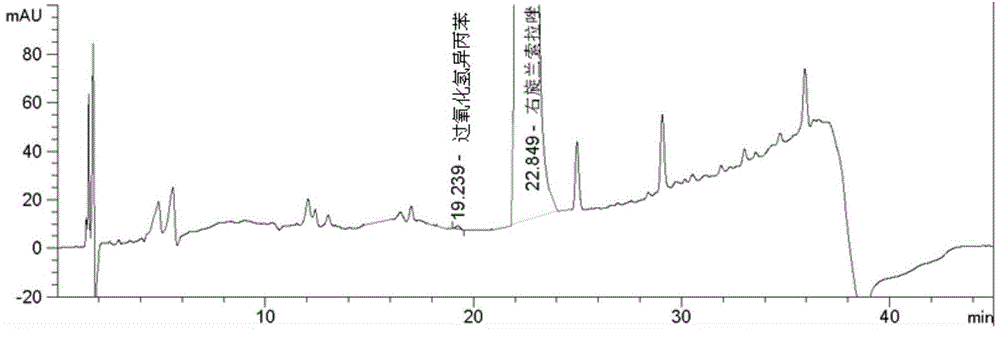
Method for determining cumene hydroperoxide impurity
ActiveCN104535675AFast and efficient separationFast and efficient content determinationComponent separationDexlansoprazoleGradient elution
The invention discloses a method for determining a cumene hydroperoxide impurity. The method comprises the step of carrying out gradient elution by selecting a siloxane bonded silica gel as a stationary phase and a first mixed solvent of an organic solution and an alkaline aqueous solution as a mobile phase. The detection method is strong in specificity, high in precision, strong in accuracy and simple, convenient and quick to operate, and the quality of a drug can be effectively controlled. The method is wide in applicability and can be used for detecting a cumene hydroperoxide compound in medicinal raw materials such as right-handed lansoprazole and the like.
Owner:WATERSTONE PHARMA WUHAN
Modified release pharmaceutical compositions of dexlansoprazole
InactiveUS20110274752A1Improve stabilityMaximize mechanical resistanceBiocideDigestive systemGastrointestinal disorderDexlansoprazole
Modified release oral pharmaceutical compositions of dexlansoprazole or pharmaceutically acceptable salts or hydrated forms thereof in the form of a bilayer tablet and processes for the manufacture of the tablet composition and its use in the treatment of gastrointestinal disorders.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
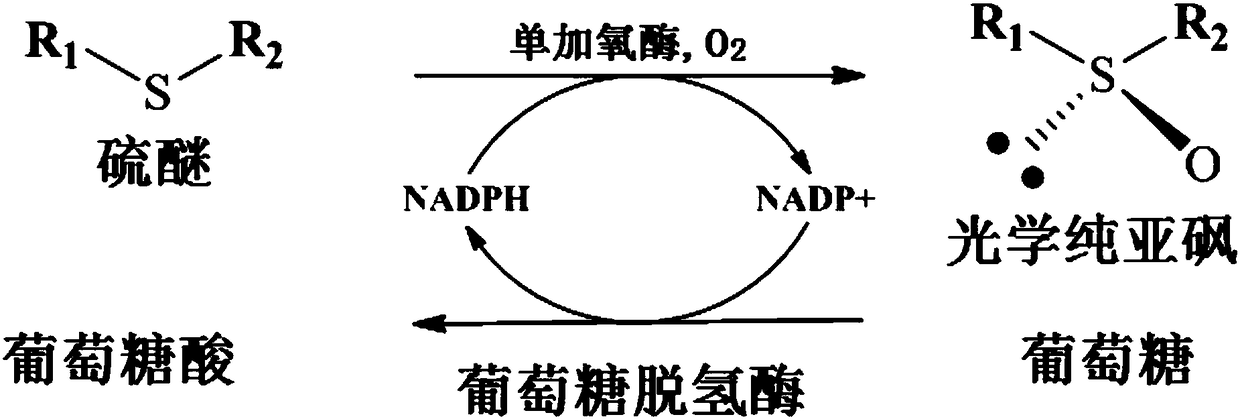
Bradyrhizobium monooxygenase and application thereof to preparation of chiral sulfoxide
ActiveCN108570425AIncrease concentrationHigh optical purityBacteriaMicroorganism based processesSulfur EthersDexlansoprazole
The invention discloses bradyrhizobium monooxygenase, a gene for encoding the monooxygenase, a recombinant expression vector comprising the gene, a recombinant expression transformant, a method for preparing the monooxygenase by the recombinant expression transformant and an application of the monooxygenase to preparation of optical pure chiral sulfoxide, and particularly relates to a method for preparing a razole medicine by asymmetric oxidation of catalytic razole precursor sulfur ether. Compared with other methods for preparing optical pure sulfoxide, a product prepared by the monooxygenaseserving as a catalyst is high in optical purity, avoids generation of sulphone serving as a by-product and has the advantages of mild reaction conditions, simplicity and convenience in operation, easiness in amplification and the like. Therefore, the monooxygenase has an excellent industrial application prospect in synthesis of a series of medicine intermediates and razole medicines, particularlyright-handed rotation lansoprazole.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Oral tablet compositions of dexlansoprazole
InactiveUS20110274754A1Overcome problemsFacilitated releaseBiocideDigestive systemGastrointestinal disorderDexlansoprazole
Oral tablet compositions of dexlansoprazole or pharmaceutically acceptable salts or hydrated forms thereof having a gradual release and processes for the manufacture of the tablet composition and its use in the treatment of gastrointestinal disorders.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Dextrorotation lansoprazole diphasic spansule preparation and preparing method thereof
InactiveCN104940168AAvoid complexitySmall particle sizeOrganic active ingredientsDigestive systemDexlansoprazoleStereochemistry
The invention belongs to the pharmacy field, and particularly relates to a dextrorotation lansoprazole diphasic spansule preparation and a preparing method thereof. According to the method, drug applying is carried out through the ball grinding technology and a centrifugal granulator, and the dextrorotation lansoprazole diphasic spansule preparation has good stability and a high releasing rate.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Dry suspension of sodium dexlansoprazole and preparation method of dry suspension
ActiveCN106619520AImprove medication safetyGood physical and chemical stabilityOrganic active ingredientsPowder deliverySodium bicarbonateSustained release pellets
The invention relates to a dry suspension of sodium dexlansoprazole and a preparation method of the dry suspension. The dry suspension is prepared from a new crystal form A of sodium dexlansoprazole, sodium bicarbonate, a solubilizer, gastric-soluble quick-release pellets A, enteric sustained-release pellets B, filler, a suspending agent, a flavoring agent and other pharmaceutically acceptable auxiliary materials, wherein the gastric-soluble quick-release pellets A are prepared by coating quick-release pellet cores A prepared from a raw material, namely, the new crystal form A of sodium dexlansoprazole with gastric-soluble coats, and the enteric sustained-release pellets B are prepared by coating slow-release pellet cores B prepared from a raw material, namely, the new crystal form A of sodium dexlansoprazole with enteric coats; the rest new crystal form A of sodium dexlansoprazole is supplemented by sodium bicarbonate and the solubilizer for micronization pretreatment. The dry suspension is moderately released in the stomach and can quickly relieve excessive gastric acid, stomachache and other symptoms, residual medicines are slowly dissolved and released in intestinal tracts, and the efficacy is prolonged. Meanwhile, the dry suspension has the advantages that the dry suspension tastes good, dissolves out quickly, is convenient to take, can improve patient compliance and the like, the preparation process is advanced, simple to operate, safe, pollution-free and suitable for industrial production.
Owner:NANJING HERON PHARM CO LTD +1
Preparation method of (R)-lansoprazole
InactiveCN107141280AObvious superiorityReduce dosageOrganic chemistry methodsReaction temperatureDexlansoprazole
The invention provides a preparation method of (R)-lansoprazole. Through a condensation reaction and an asymmetric oxidation reaction of thioether, the (R)-lansoprazole is prepared. The preparation method comprises refining a (R)-lansoprazole finished product. In the first reaction step, cheap sodium hydroxide replaces sodium methylate, a reaction temperature is reduced to the room temperature from a return temperature, ethanol is used as a solvent and a high yield of 99.5% is realized. In the second asymmetric oxidation step, a yield is 80% or more. The preparation method has simple processes, is free of multiple complex extraction and separation processes and is suitable for industrial production. In the third step, through reaction condition optimization, a reaction conversion rate is greater than 85% and enantioselectivity is greater than 97%. Through purification, the product quality satisfies the FDA same-type product standards, optical purity and chemical purity are greater than 99.5%, the content of thioether is less than 0.1% and sulphone content is less than 0.1%. The preparation method has stable processes and an industrialization prospect.
Owner:长沙康普大药房有限责任公司
Freeze-dried dexlansoprazole composition for injection and preparation method thereof
ActiveCN103961322AOvercoming the disadvantage of being easily destroyedQuick effectPowder deliveryOrganic active ingredientsDiseaseArginine
The invention provides a freeze-dried dexlansoprazole composition powder injection for injection and a preparation method thereof. The powder injection comprises the active components consisting of dexlansoprazole and arginine, and meglumine and / or excipients like mannitol. The preparation method comprises the following steps: dissolving raw and adjuvant materials with water; adding a proper amount of a sodium hydroxide solution to allow a solution obtained in the previous step to be clear; then adding active carbon for decoloring; carrying out active carbon removal and then adjusting a pH value; and successively carrying out filtering with a filter membrane, split charging and freeze-drying. The powder injection is clinically applied to treatment of diseases like gastric ulcer, duodenal ulcer, erosive gastro-oesophageal reflux disease, helicobacter pylori infection and Zollinger-Ellison syndrome. The freeze-dried dexlansoprazole composition powder injection for injection provided by the invention overcomes the problem of poor redissolvability of dexlansoprazole, has stable quality and good redissolvability and improves medication security.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
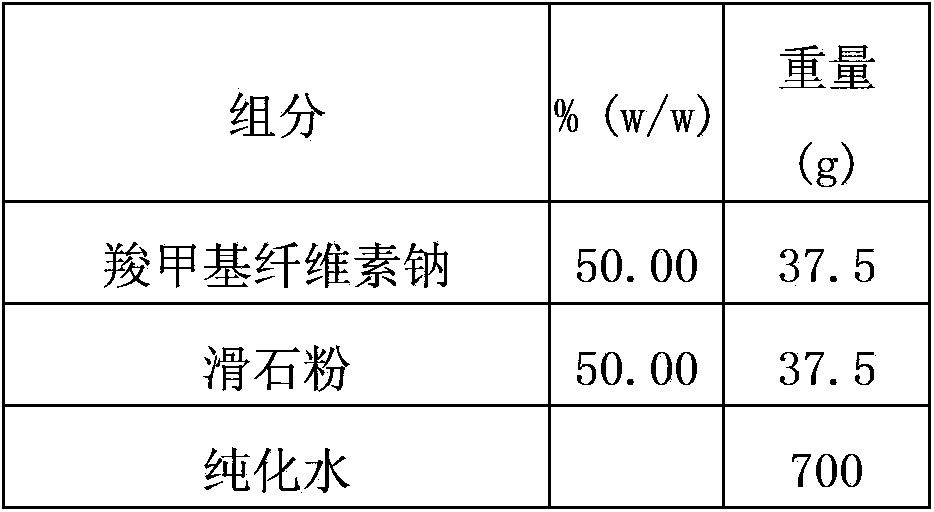
Multilayer coating system enteric preparation for dexlansoprazole
ActiveCN103961329ATo achieve the effect of multi-level releaseIncrease the effect of acid suppressionOrganic active ingredientsDigestive systemIsolation effectCoating system
The invention provides a novel multilayer coating system enteric preparation for dexlansoprazole. The preparation comprises a drug layer I, a drug layer II, enteric coating layers IV and isolating layers III, wherein the isolating layers III are arranged among the drug layer I or II and the enteric coating layers IV, the drug layer I and the drug layer II are respectively composed of dexlansoprazole, a filler, a binder, a stabilizing agent and a lubricant, and the preparation comprises, from interior to exterior, the drug layer I, the isolating layer III-1, the enteric coating layer IV-1, the isolating layer III-2, the drug layer II, the isolating layer III-3 and the enteric coating layer IV-2. The novel multilayer coating system enteric preparation for dexlansoprazole provided by the invention can improve the acid inhibition effect of dexlansoprazole and prolongs action time of dexlansoprazole, the isolation effect of the isolating layers in the whole preparation system is optimized, and stability of product quality is ensured; and a preparation method provided by the invention is applicable to industrial production and has a great application value.
Owner:SHANGHAI SUNTECH PHARMA +1
Preparation method of right-handed lansoprazole crystal form
InactiveCN106279107AHigh melting pointHigh bulk densityOrganic chemistry methodsMethylene DichlorideDexlansoprazole
The invention provides a preparation method of a right-handed lansoprazole crystal form. The preparation method is carried out in an ethyl acetate-isopropyl ether system. The preparation method comprises the following steps: (1) dissolving a purified lansoprazole hydrate in five times of mass / volume ratio of ethyl acetate, adding an appropriate amount of saturated sodium bicarbonate solution, stirring for 10 min, carrying phase separation, adding an appropriate amount of anhydrous sodium sulfate and 0.2g of active carbon into an organic phase, stirring for 30 min, filtering, and washing with ethyl acetate; (2) merging filtrate and washing liquor, slowly adding isopropyl ether two times of the volume of the ethyl acetate, adding a crystal seed A, and stirring for 10 min; (3) cooling to 0 to 10 DEG C, and precipitating solids; (4) performing suction filtration, and washing a filter cake with isopropyl ether; and (5) drying in vacuum for 2h at 40 DEG C, thus obtaining white crystals with relatively large density. By adopting the preparation method, the defect in the original patent that a great amount of solvent is consumed by using a methylene dichloride-isopropyl ether system can be overcome, an obtained crystal form is high in melt point, large in bulk density and high in stability, and the preparation method is small in environmental pollution during the preparation process, simple in operation, high in production efficiency and suitable for mass production.
Owner:成都尚药科技有限公司
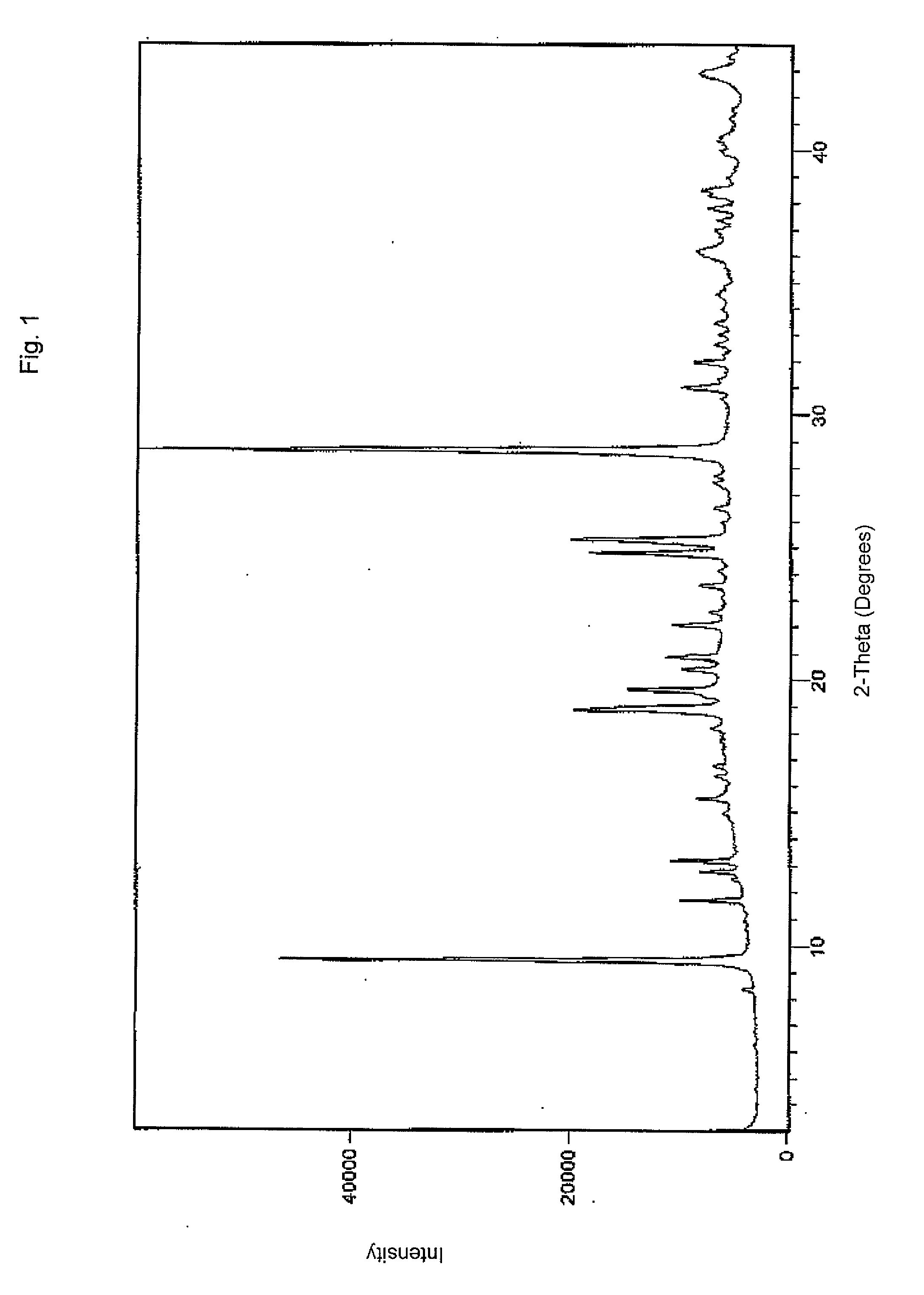
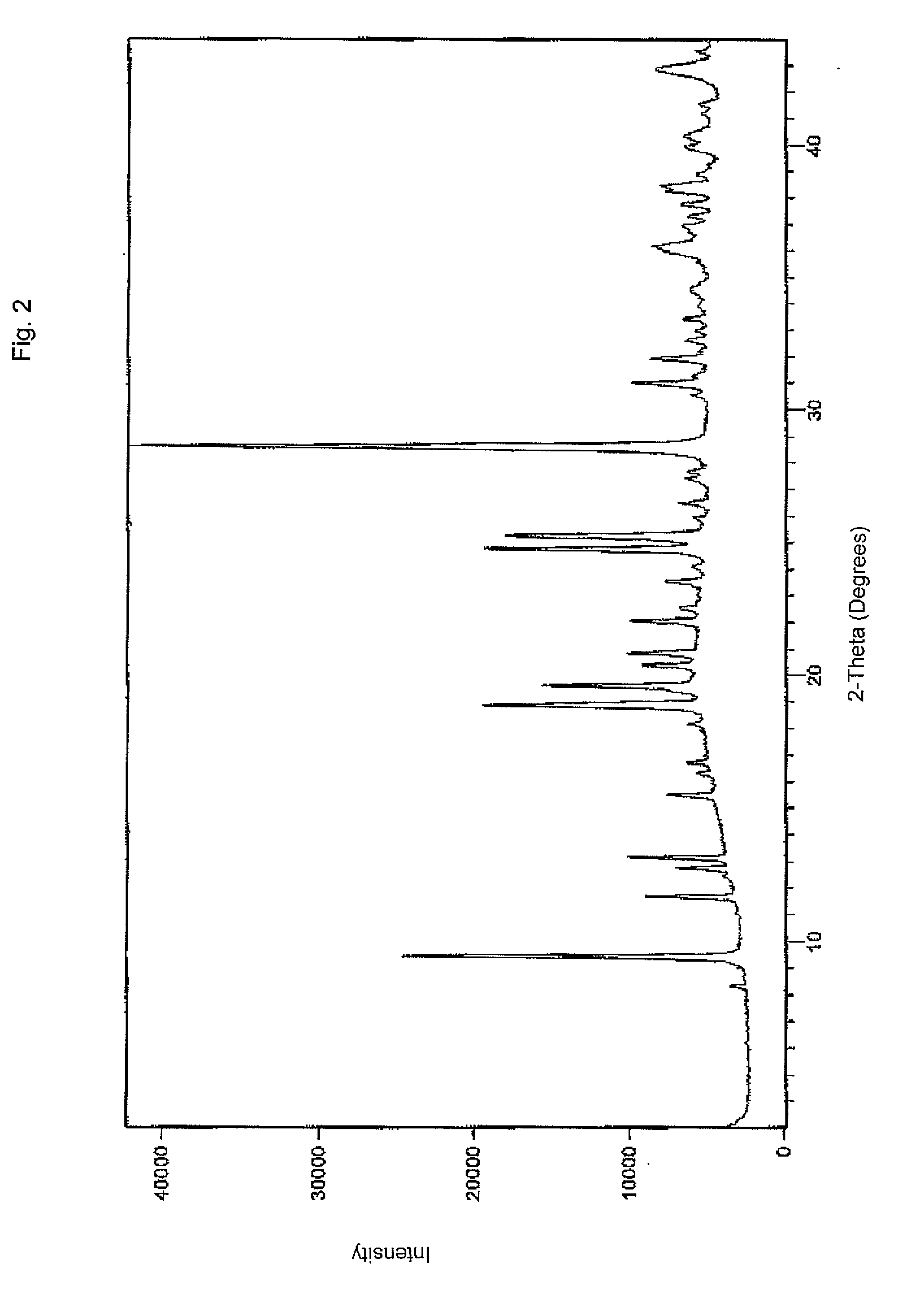
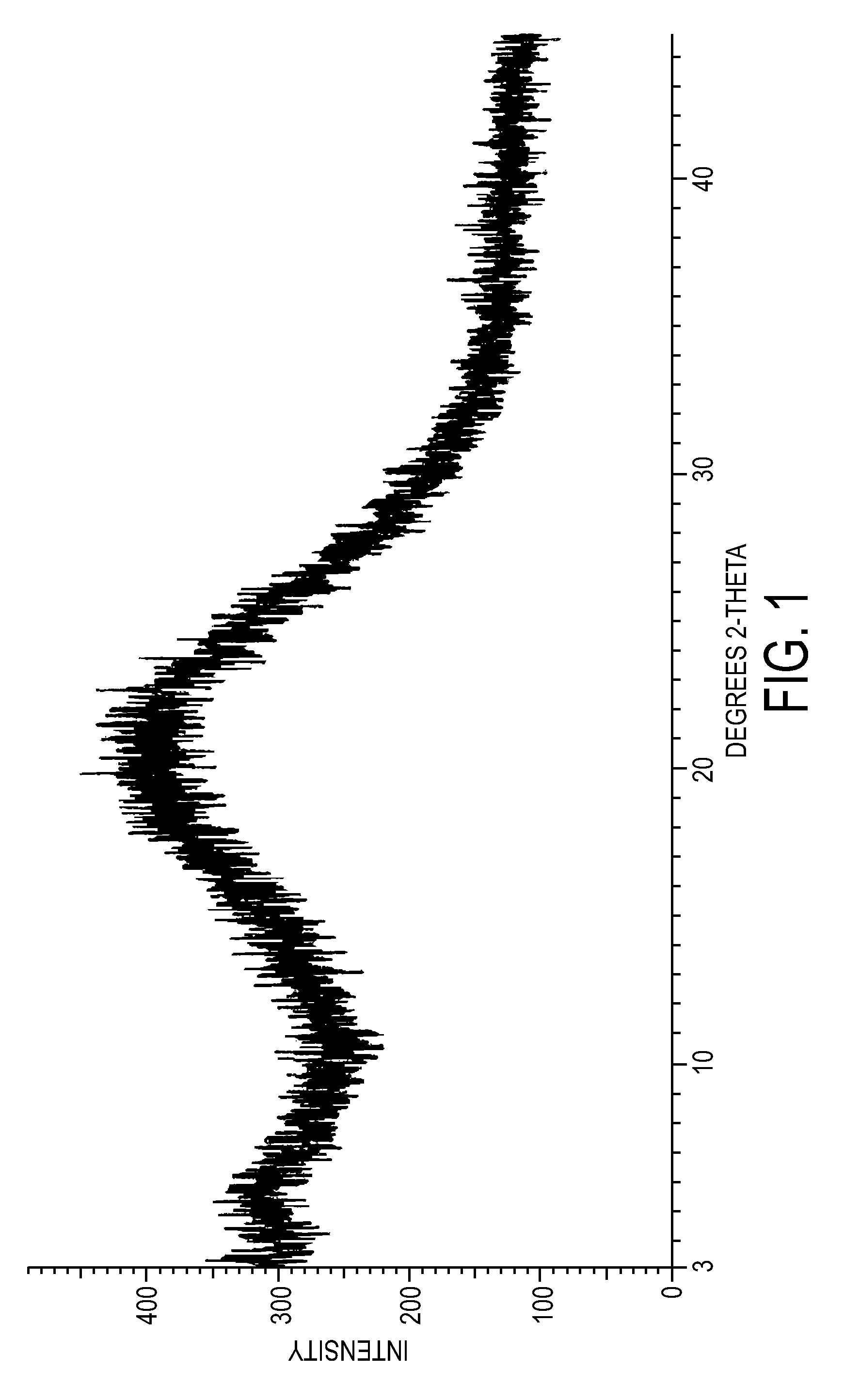
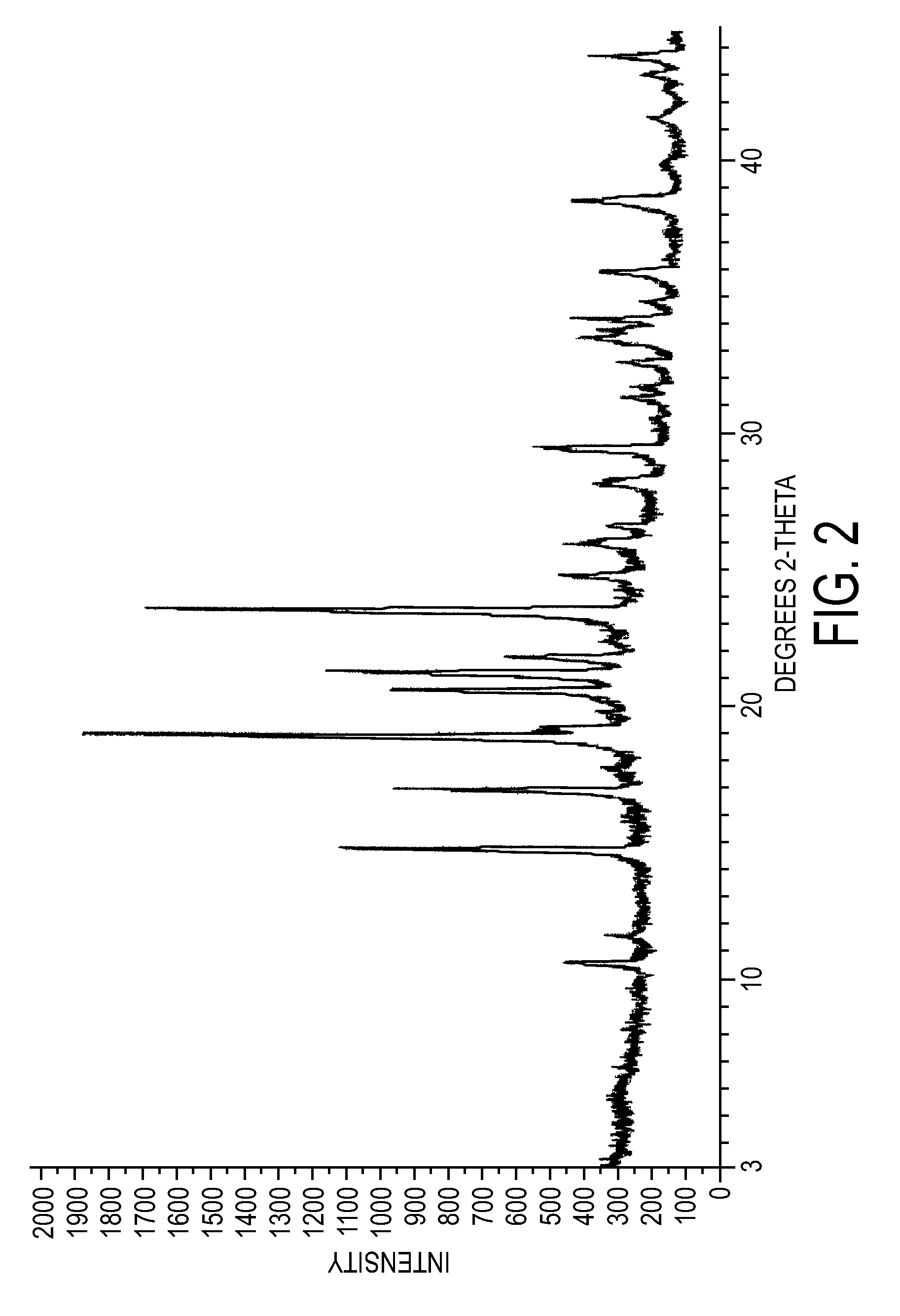
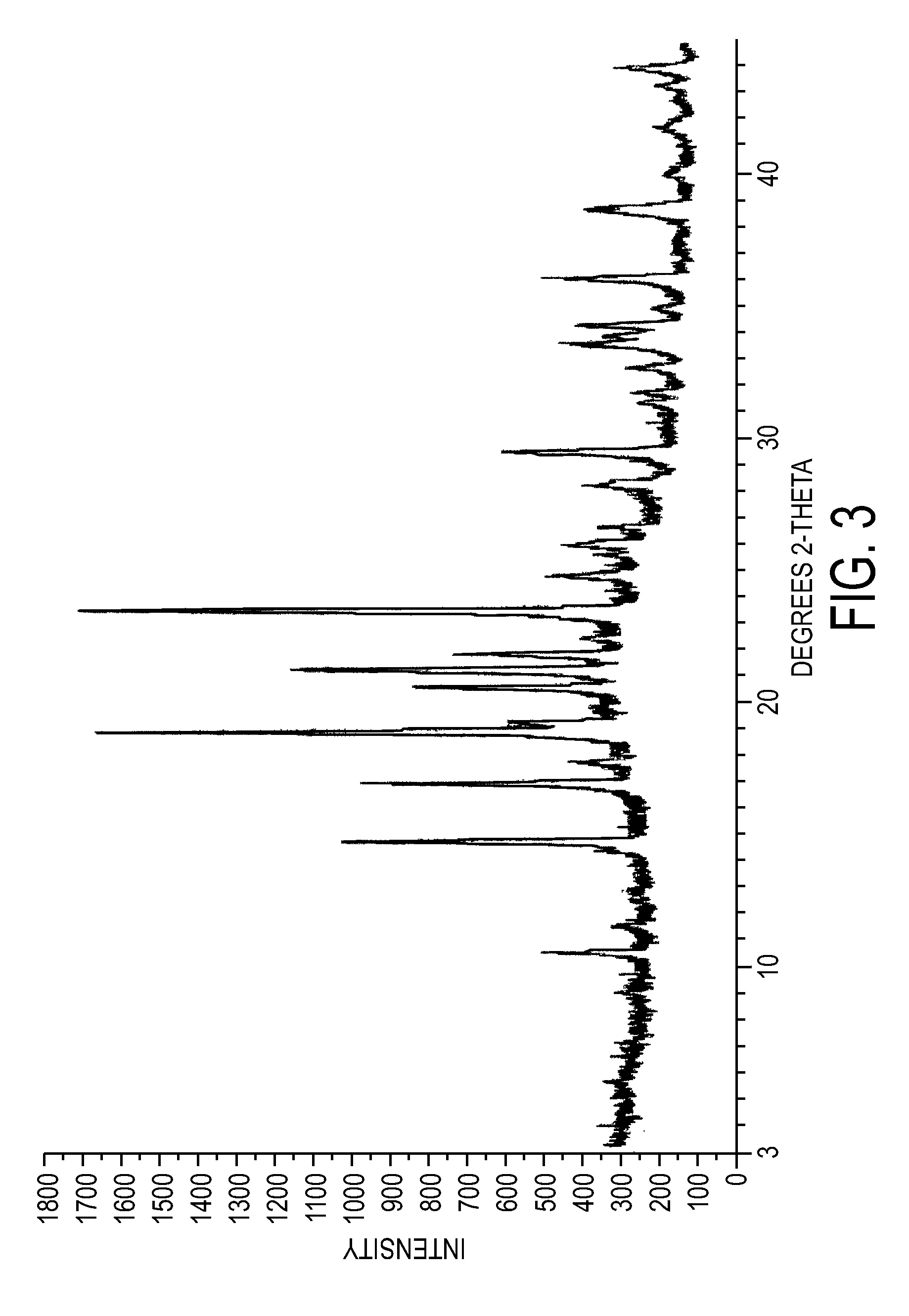
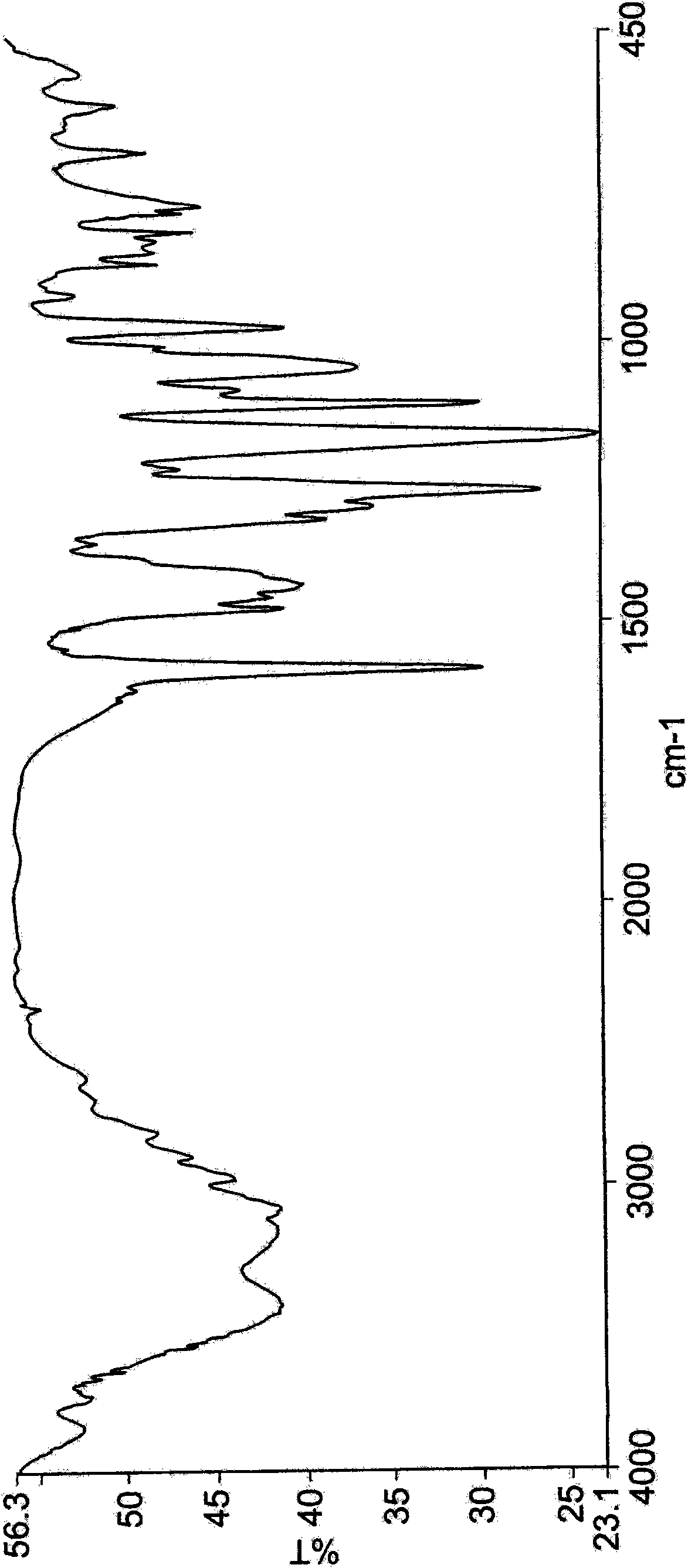
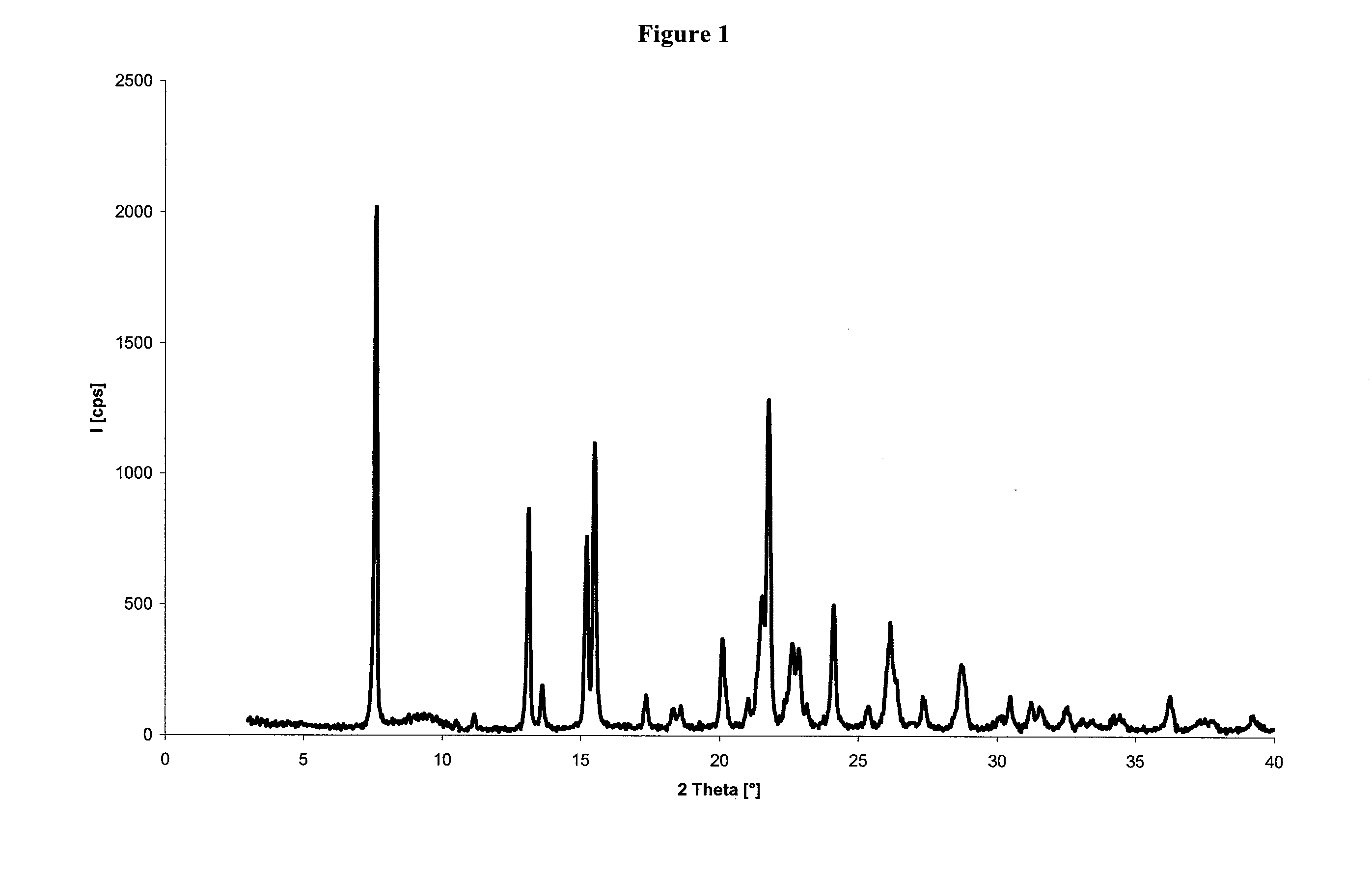
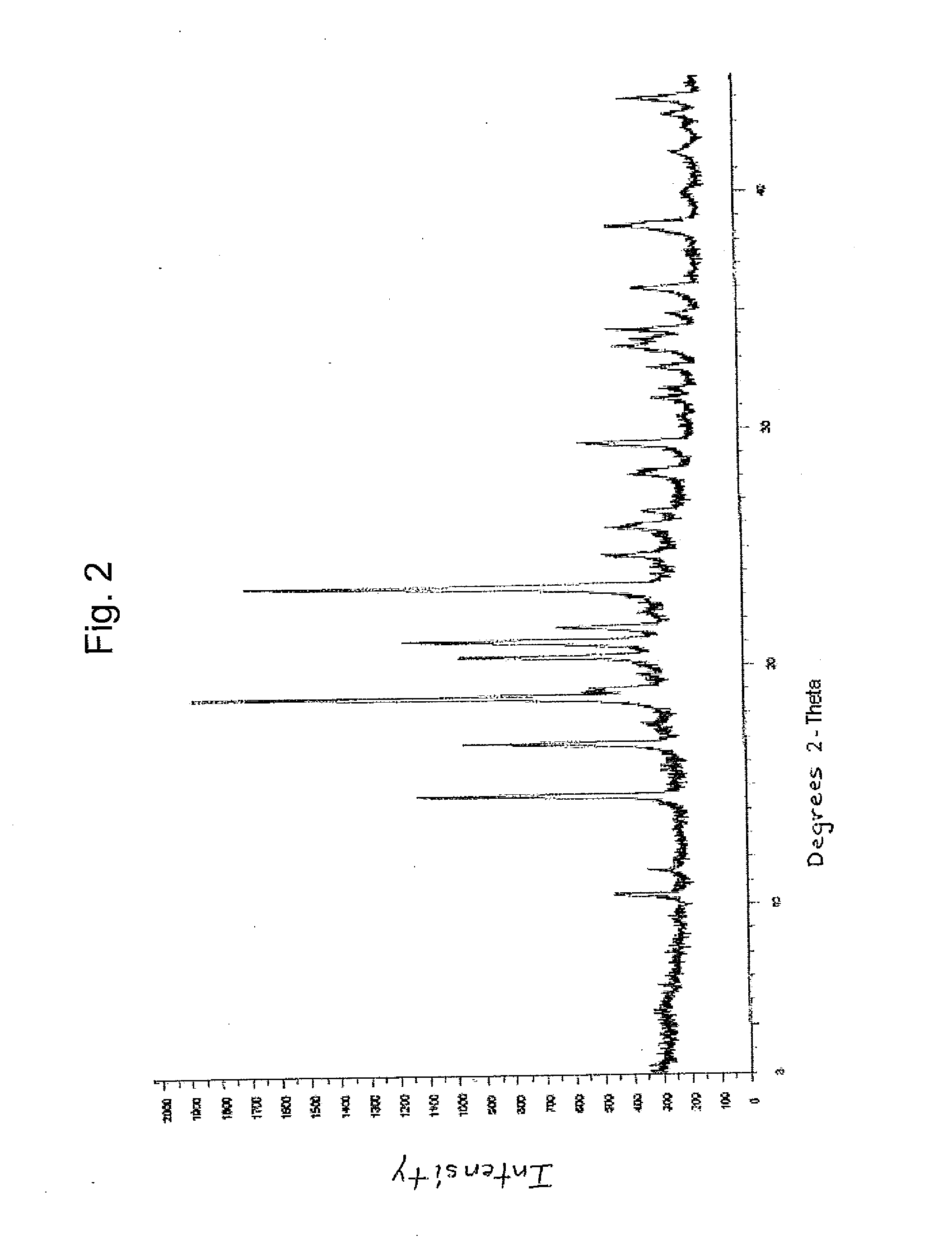
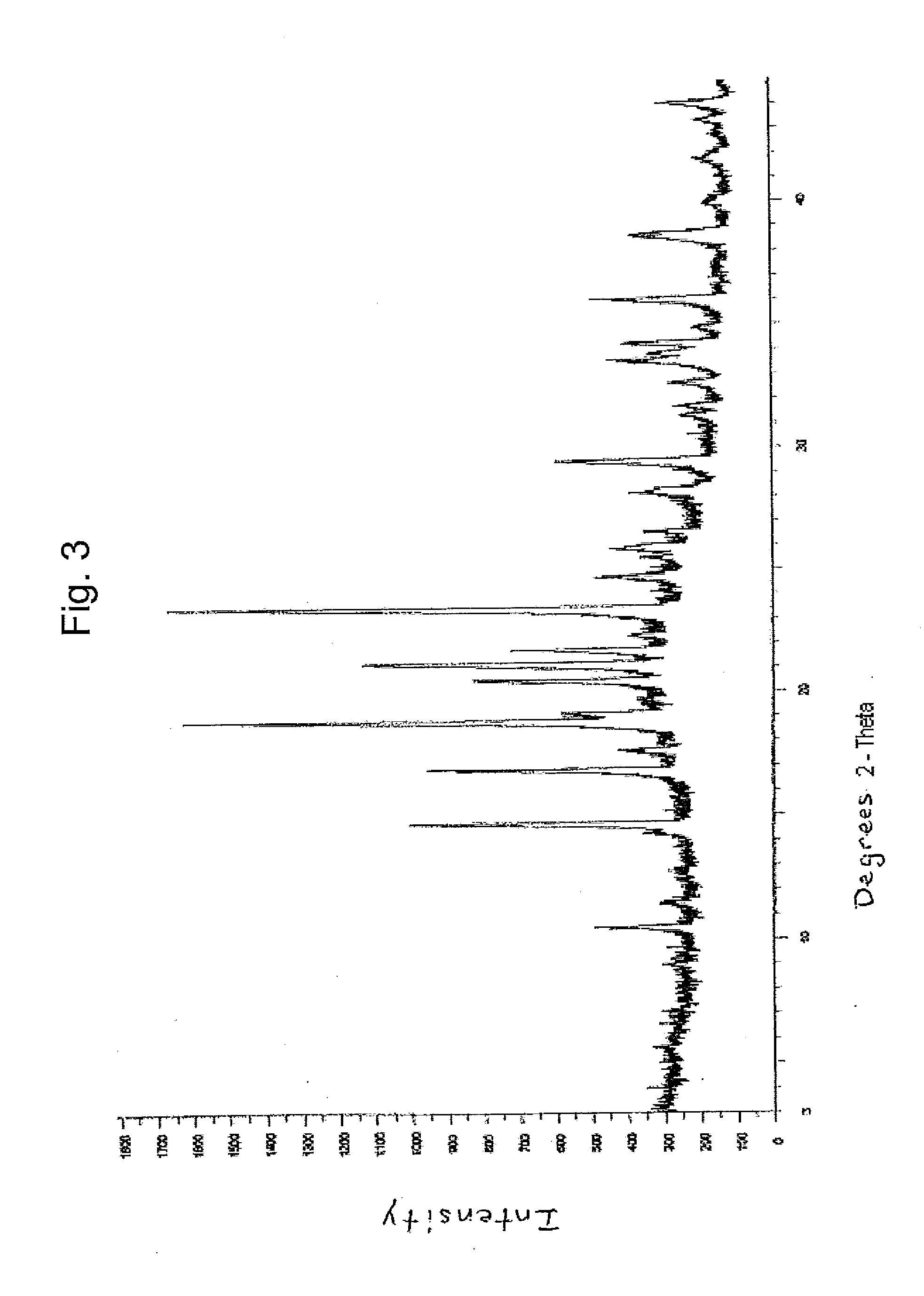
Crystalline forms of dexlansoprazole
The present invention relates to novel crystalline forms of Dexlansoprazole and a process for the preparation of amorphous Dexlansoprazole.
Owner:DIPHARMA FRANCIS
Oral tablet compositions of dexlansoprazole
Oral tablet compositions of dexlansoprazole or pharmaceutically acceptable salts or hydrated forms thereof having a gradual release and processes for the manufacture of the tablet composition and its use in the treatment of gastrointestinal disorders.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Method for synthesizing dexlansoprazol by titanium catalyst ligand
ActiveCN103694225AHigh structural asymmetryHigh ee valueOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystDexlansoprazole
The invention discloses a method for synthesizing dexlansoprazol by a titanium catalyst ligand. The chemical name of the titanium catalyst ligand is (1R,2S)-N-p-methylbenzene sulfonic acid-1,2-diphenyl ethylenediamine. The method comprises the following steps: mixing the ligand with tetraisopropyl titanate, N,N-diisopropylethylamine and purified water according to a certain ratio, and catalyzing 2-[[[3-methyl-4-(2,2,2 trifluoroethyoxyl)-2-pyridine]methyl]thioxo]-1H-benzimidazole by heating to synthesize dexlansoprazol. According to dexlansoprazol, the enantiomer excess is not less than 99.2% and the yield is not less than 80%. The process is good in stability and high in enantioselectivity, and can be better applied to industrialization production.
Owner:福州基石医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com