Bradyrhizobium monooxygenase and application thereof to preparation of chiral sulfoxide
A technology of monooxygenase and thioether monooxygenase, which is applied in the field of bioengineering technology, can solve the problems of low conversion rate, low conversion rate of large sterically hindered sulfoxide compounds, poor selectivity, etc., and achieves simple operation, Good industrial application prospect, easy industrial amplification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] That is, the present invention discloses a method for preparing the above-mentioned monooxygenase, culturing the above-mentioned recombinant expression transformant, and then isolating the monooxygenase therefrom.
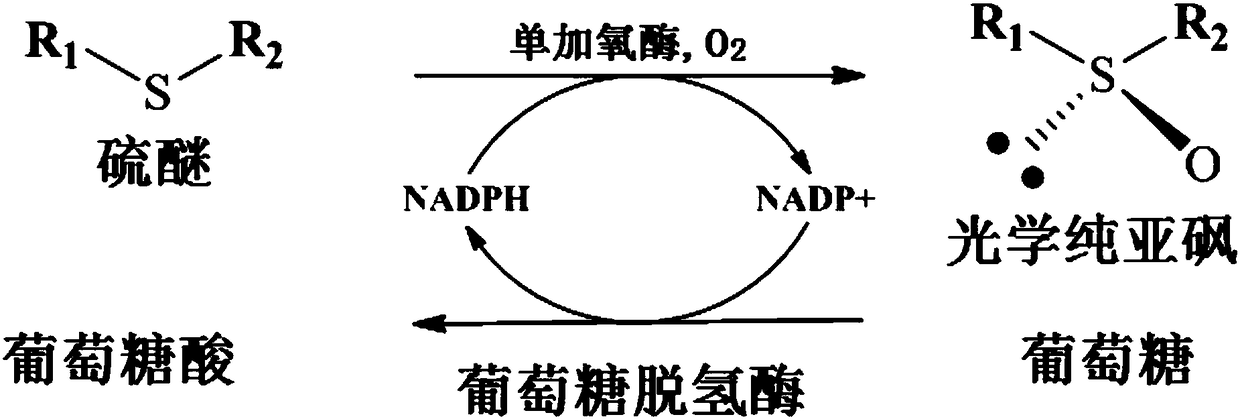
[0070] The present invention also provides an application of the above-mentioned Bradyrhizobium oligotrophicum ECU1212 or monooxygenase in the asymmetric catalytic oxidation of latent chiral sulfide compounds. Alternatively, Bradyrhizobium oligotrophicum ECU1212 can be used in its quiescent whole-cell form for the application of asymmetric catalytic oxidation of latent chiral thioether compounds.
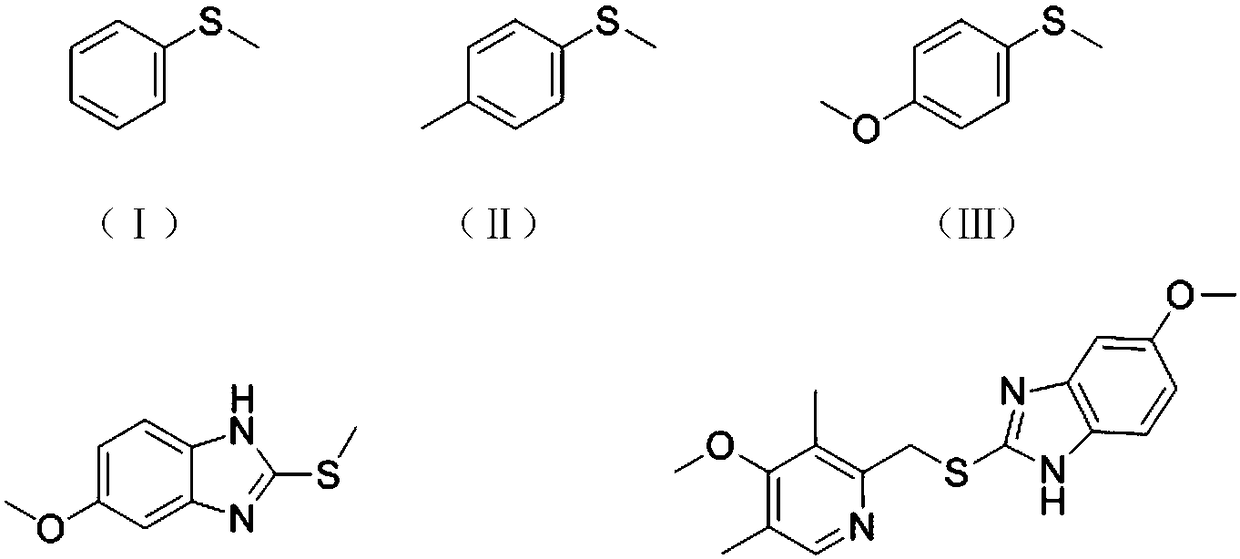
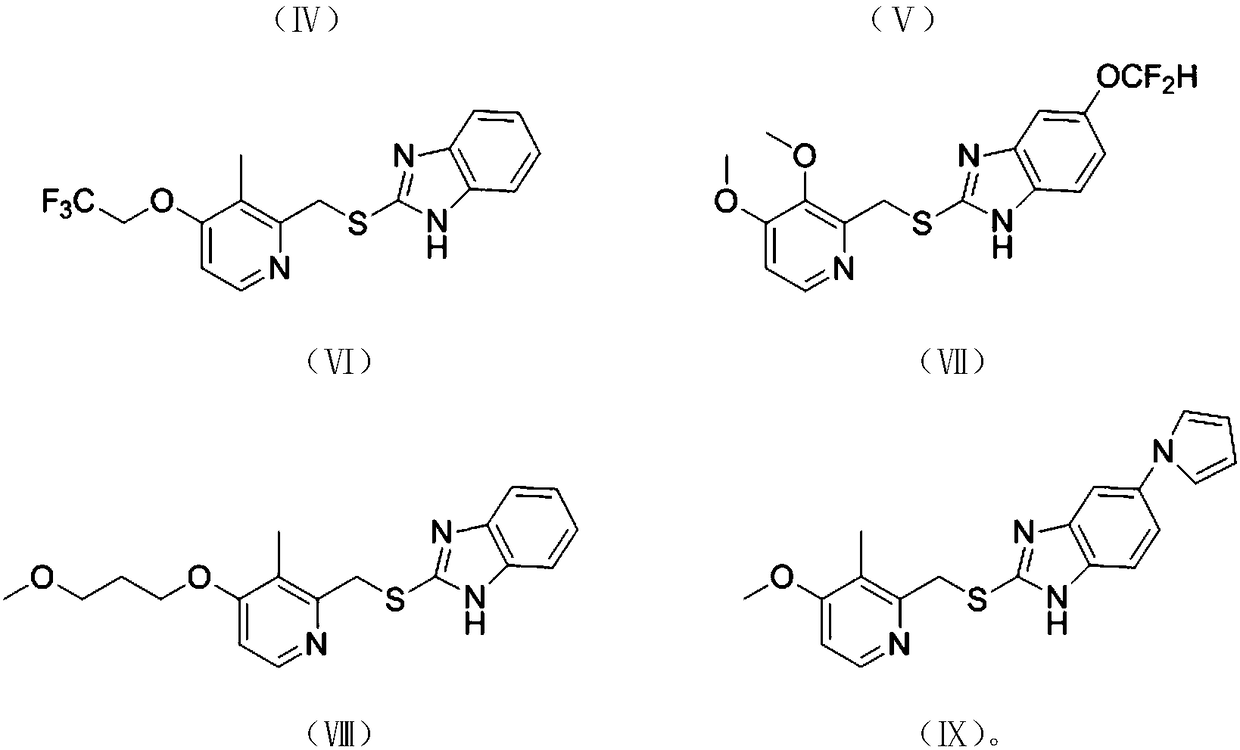
[0071] Further optionally, the latent chiral thioether compound is selected from compounds represented by any of the following chemical formulas:
[0072]
[0073]
[0074] Among them, in the present invention, the Chinese names of the above chemical formulas I to IX are represented by sulfide anisole, p-methylanisole, p-methoxyanisole, 5-methoxy-2-(methylsu...
Embodiment 1
[0086] Screening of Bradyrhizobium oligotrophicum ECU1212
[0087] Soil collection is mainly divided into two parts, the direct collection of soil samples and the collection of soil samples after the pre-embedded substrate, a total of 252 soil samples.
[0088] Direct soil sample collection: collect relatively moist soil, generally mostly water sources, plants, contaminated substrates, etc., dig out the soil 2-3cm from the ground, about 3-5g, put the used soil samples in a low-temperature, Store in a dry place, or put it directly in a 1.5mL Eppendorf tube and store it in a refrigerator at 4°C. The collection locations are as follows: Shanghai Fengxian Chemical Industry Zone, Xinhua Hospital, orchards, vegetable farms, near garbage bins, near rivers, green belts, campuses (Xuhui or Fengxian campus of East China University of Science and Technology), greening of residential areas, botanical gardens, etc.
[0089] Embedded substrate: Lansoprazole sulfide is white powder, insolub...
Embodiment 2
[0093] Preparation of resting cells of Bradyrhizobium oligotrophicum ECU1212
[0094] The Bradyrhizobium oligotrophicum ECU1212 obtained by screening as in Example 1 was inoculated into rich medium (glucose 15g / L, peptone 10g / L, yeast extract 5g / L, NaH 2 PO4 0.5g / L, MgSO 4 0.5 g / L, NaCl 10 g / L, pH 7.0), 28°C, 180 rpm shaker for 24 hours, then centrifuged at 5000×g for 10 min to collect wet cells. The collected wet cells were frozen at -80°C for 12h, and then dried at a low temperature for 20h with a freeze dryer to obtain freeze-dried cells, which were stored in a refrigerator at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com