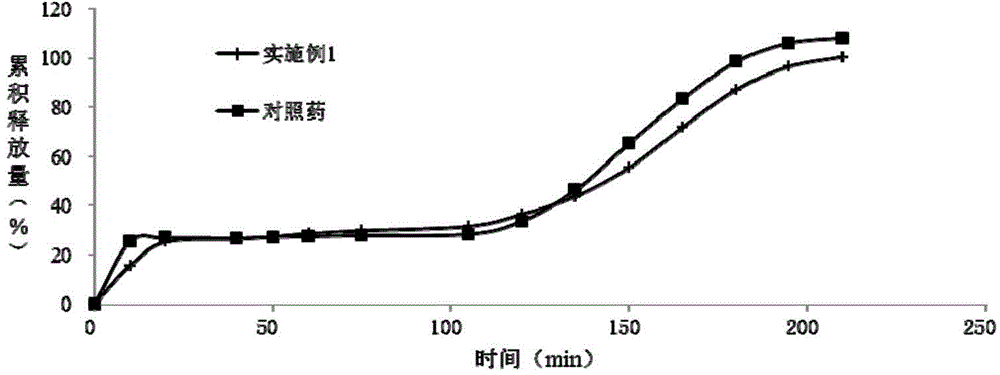
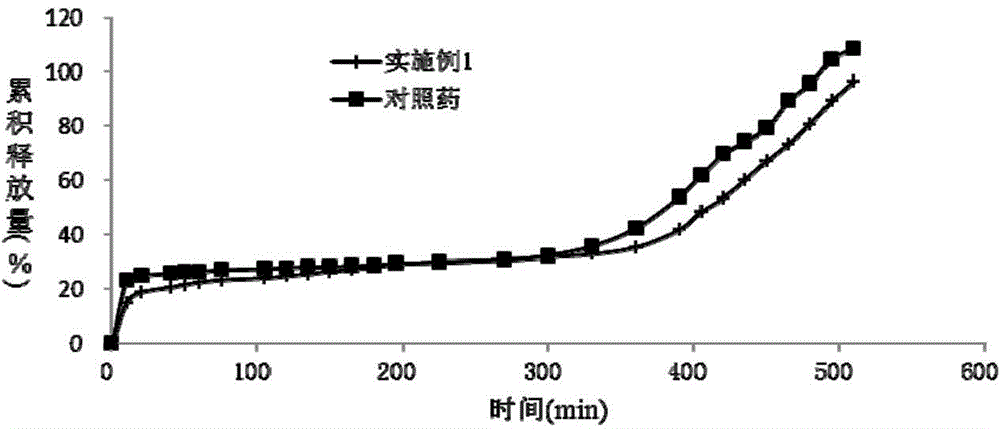
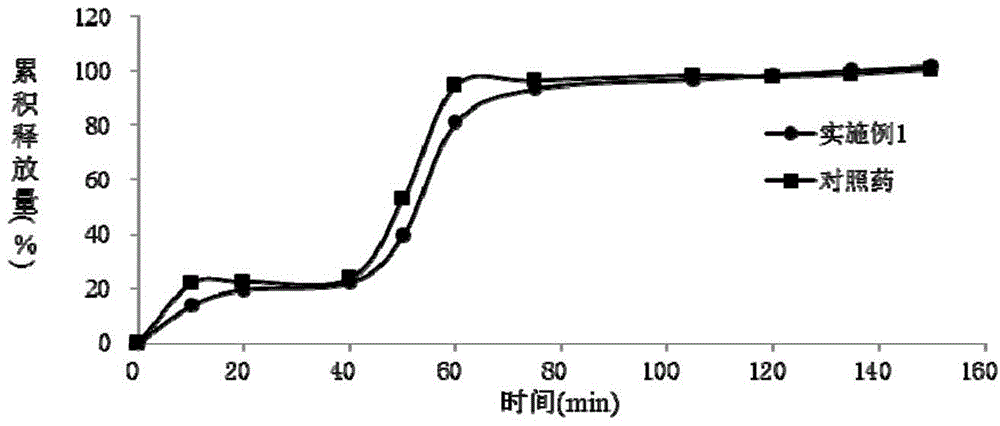
Dexlansoprazole sustained-release capsule and preparation method thereof
A technology of dexlansoprazole and sustained-release capsules, applied in the field of dexlansoprazole sustained-release capsules and its preparation, can solve the problems of dexlansoprazole instability, difficulty in development, unstable properties of aqueous solution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 A kind of dexlansoprazole sustained-release capsule and preparation method thereof
[0030] Dexlansoprazole sustained-release capsules consist of enteric-coated pellets and sustained-release pellets.
[0031] 1. Composition of enteric-coated pellets:
[0032]
[0033] 2. Sustained-release pellets
[0034]
[0035] 3. Preparation process
[0036] Preparation process of enteric-coated pellets: (1) Mix the prescription amount of dexlansoprazole, magnesium carbonate, sucrose, and low-substituted hydroxypropyl cellulose evenly to obtain solid powder, disperse with hydroxypropyl methylcellulose (HPMC E5) A 3% solution of the binder in water was obtained. Fill 200g of blank microcrystalline cellulose pellet cores (28-32 mesh) into a centrifugal coating granulator, and spray with the above-mentioned spray solution under the following conditions: inlet air temperature: 25-30°C, spray the coating solution Rate: 1.0g / min, spray pressure 0.5kg / cm 2 , the obt...
Embodiment 2
[0045] Embodiment 2 A kind of dexlansoprazole sustained-release capsule and preparation method thereof
[0046] Dexlansoprazole sustained-release capsules consist of enteric-coated pellets and sustained-release pellets.
[0047] 1. Composition of enteric-coated pellets:
[0048]
[0049] 2. Sustained-release pellets
[0050]
[0051] 3. Preparation process
[0052] Preparation process of enteric-coated pellets: (1) Mix the prescription amount of dexlansoprazole, magnesium carbonate, sucrose, and low-substituted hydroxypropyl cellulose evenly to obtain solid powder, disperse with hydroxypropyl methylcellulose (HPMC E5) A 3% solution of the binder in water was obtained. Fill 200g of blank microcrystalline cellulose pellet cores (28-32 mesh) into a centrifugal coating granulator, and spray with the above-mentioned spray solution under the following conditions: inlet air temperature: 25-30°C, spray the coating solution Rate: 1.0g / min, spray pressure 0.5kg / cm 2 , the obt...
Embodiment 3
[0061] Embodiment 3 a kind of dexlansoprazole sustained-release capsules and preparation method thereof
[0062] Dexlansoprazole sustained-release capsules consist of enteric-coated pellets and sustained-release pellets.
[0063] 1. Composition of enteric-coated pellets:
[0064]
[0065] 2. Sustained-release pellets
[0066]
[0067] 3. Preparation process
[0068] Preparation process of enteric-coated pellets:
[0069] (1) Mix the prescription amount of dexlansoprazole, magnesium carbonate, sucrose, and low-substituted hydroxypropyl cellulose evenly to obtain solid powder, and disperse hydroxypropyl methylcellulose (HPMC E5) in water to obtain 3% bonding agent solution. Fill 200g of blank microcrystalline cellulose pellet cores (28-32 mesh) into a centrifugal coating granulator, and spray with the above-mentioned spray solution under the following conditions: inlet air temperature: 25-30°C, spray the coating solution Rate: 1.0g / min, spray pressure 0.5kg / cm 2 , th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com