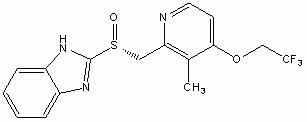
Dexlansoprazole sustained release capsule and preparation method thereof
A technology of lansoprazole late and dexlansoprazole, which is applied in the field of dexlansoprazole delayed-release capsules and its preparation, can solve the problem that the stability is susceptible to light, heavy metal ions, oxidizing and reducing components, etc. Influence of various factors, reduction of drug onset speed and bioavailability, changes in the chemical structure of dexlansoprazole, etc., to achieve the effects of high predictability of drug efficacy, improvement of bioavailability, and enhanced solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Dexlansoprazole delayed-release capsules, two kinds of micropills are installed in the capsule, the mass ratio of the first kind of micropills to the second kind of micropills (based on dexlansoprazole) is 1:3;
[0043] The first micropill is successively a blank core, a drug-containing layer, an isolation layer and an enteric coating layer from the inside to the outside, and the mass parts of the blank core are 800 parts, and the composition and mass parts of the drug-containing layer are dexlansoprazole: 300 copies,
[0044] Sodium hydroxide: 16 parts,
[0045] Tween 80: 5 servings,
[0046] Sodium carboxymethyl starch: 50 parts,
[0047] Colloidal silica: 20 parts,
[0048] Hypromellose: 200 parts,
[0049] The composition and mass parts of the isolation layer are hypromellose: 50 parts,
[0050] Tween 80: 6 servings,
[0051] Titanium dioxide: 6 parts,
[0052] Macrogol 8000: 10 parts,
[0053] The composition and mass parts of the casing layer are methacryl...
Embodiment 2
[0062] Dexlansoprazole delayed-release capsules, two kinds of micropills are installed in the capsule, the mass ratio of the first kind of micropills to the second kind of micropills (based on dexlansoprazole) is 1:3;
[0063] The first micropill is successively a blank core, a drug-containing layer, an isolation layer and an enteric coating layer from the inside to the outside, and the mass parts of the blank core are 800 parts, and the composition and mass parts of the drug-containing layer are dexlansoprazole: 300 copies,
[0064] Magnesium hydroxide: 5 parts,
[0065] Poloxamer: 1 part,
[0066] Microcrystalline cellulose: 200 parts,
[0067] Magnesium stearate: 5 parts,
[0068] Polyvinylpyrrolidone: 50 parts,
[0069] The composition and mass parts of isolation layer are polyvinylpyrrolidone: 30 parts,
[0070] Poloxamer: 3 parts,
[0071] Titanium dioxide: 3 parts,
[0072] Micronized silica gel: 5 parts,
[0073]The composition and mass parts of the casing laye...
Embodiment 3
[0082] Dexlansoprazole delayed-release capsules, two kinds of micropills are installed in the capsule, the mass ratio of the first kind of micropills to the second kind of micropills (based on dexlansoprazole) is 1:4;
[0083] The first micropill is successively a blank core, a drug-containing layer, an isolation layer and an enteric coating layer from the inside to the outside, and the mass parts of the blank core are 800 parts, and the composition and mass parts of the drug-containing layer are dexlansoprazole: 300 copies,
[0084] Sodium carbonate: 20 parts,
[0085] Tween 80: 3 servings,
[0086] Low-substituted hydroxypropyl cellulose: 100 parts,
[0087] Talcum powder: 30 parts,
[0088] Dextrin: 300 parts,
[0089] The composition and mass parts of isolation layer are dextrin: 60 parts,
[0090] Tween 80: 10 servings,
[0091] Titanium dioxide: 8 parts,
[0093] The composition and mass parts of the casing layer are methacrylic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com