Dry suspension of sodium dexlansoprazole and preparation method of dry suspension
A kind of technology of dexlansoprazole sodium, lansoprazole sodium, is applied in the dry suspension of dexlansoprazole sodium and preparation field thereof, can solve the poor stability of dexlansoprazole, high production cost, temperature Sensitivity to humidity etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 Preparation of new crystal form A of dexlansoprazole sodium dimethylacetamide of the present invention
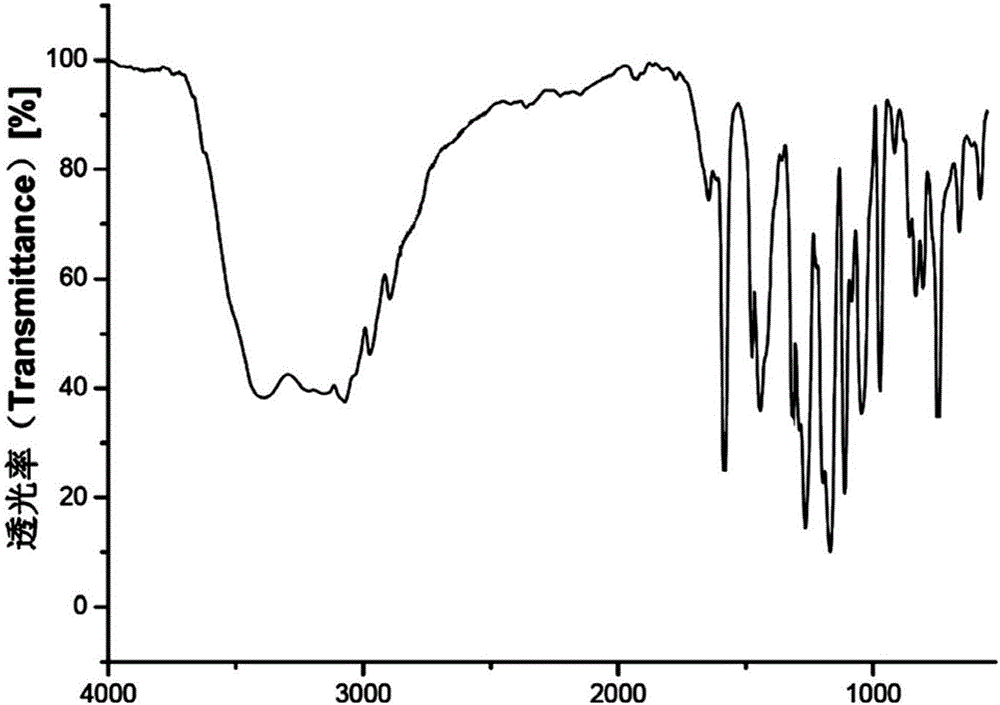
[0085] The new crystal form A of a kind of dexlansoprazole sodium dimethylacetamide solvate provided by the present invention, every mole of this new crystal form A contains 1 mole of dimethylacetamide solvent molecule, structural formula is as follows:
[0086] .
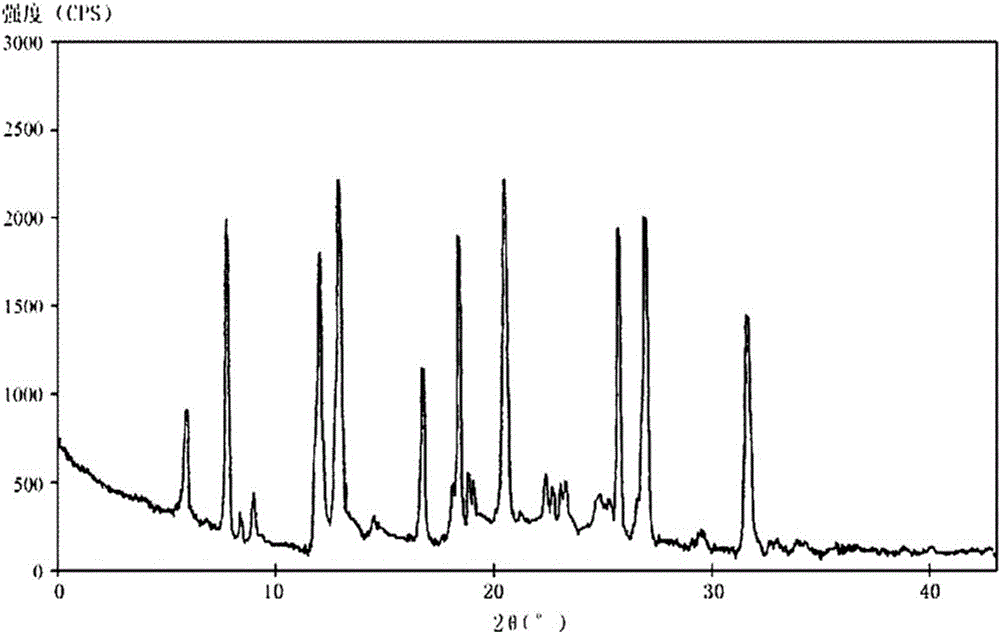
[0087] The new crystal form A of dexlansoprazole sodium according to the present invention has characteristics at diffraction angles 2θ of 5.9, 7.6, 12.2, 12.7, 16.6, 18.4, 20.5, 25.8, 26.8, and 31.4 degrees. Diffraction peaks, where the 2θ value error range is ±0.2, and the spectrum is as attached to the description figure 1 shown.
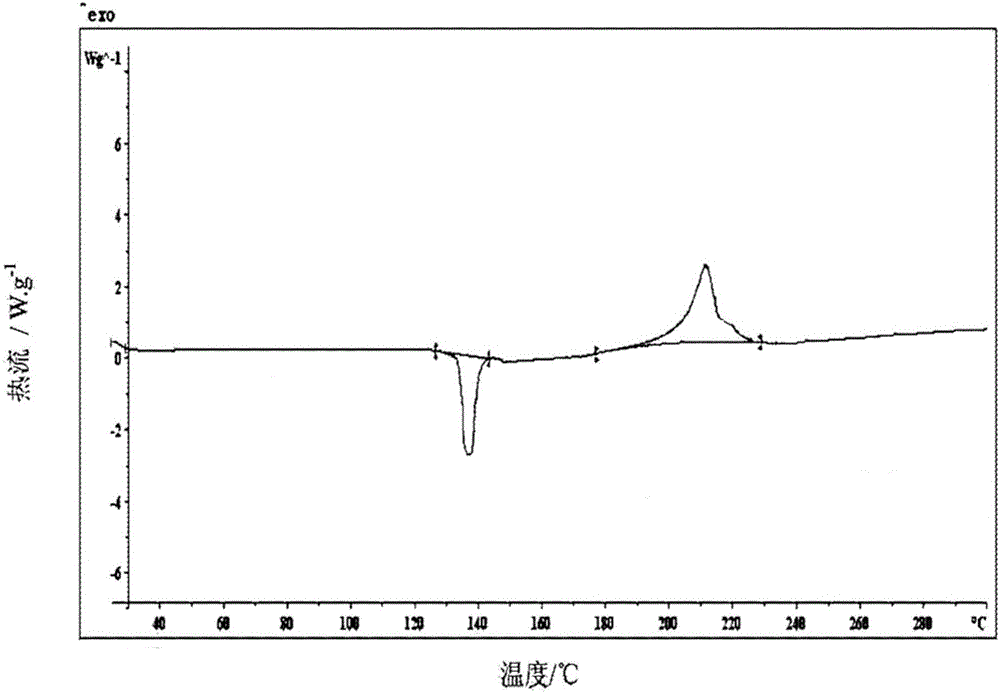
[0088] The new crystal form A of dexlansoprazole sodium according to the present invention has an endothermic characteristic peak at 132.4±1°C in its DSC spectrum and an exothermic characteristic peak at 204.6±1°C. The spectrum is as attached to the descript...
Embodiment 2
[0092] Example 2 Comparative preparation of crystal form 1
[0093] Refer to Example 5 on page 15 of the specification in PCT patent WO2012095859A1 for preparation. Dissolve 25g of dexlansoprazole in 250mL of absolute ethanol, add 32.5g of sodium isooctanoate, stir for 30min, remove the solvent under reduced pressure, add 250mL of n-heptane to the residue, stir at room temperature for 3h, filter, and dry to obtain a comparative Form 1 is about 18.5g.
Embodiment 3
[0094] Example 3 Comparative preparation of crystal form 2
[0095] Refer to Example 8 on page 16 of the specification in PCT patent WO2012095859A1 for preparation.
[0096] Dissolve 10 g of dexlansuprazole in a mixed solvent of 100 mL of absolute ethanol and 5 mL of water, cool down to -5 °C, add 2.2 g of sodium hydroxide, stir at -5 °C for 30 min, add 80 mL of n-heptane to the reaction solution, Stirring was continued for 30 min, the solid was collected and dried to obtain about 6 g of comparative crystal form 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com