Method for preparing amorphous dexlansoprazole
A technology of dexlansoprazole and amorphous is applied in the field of preparation of amorphous dexlansoprazole, which can solve the problems that crystalline raw materials are not as good as amorphous raw materials, unfavorable scale-up production, complex operation, etc., so as to omit complicated process and can The effect of strong repeatability and simplified process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
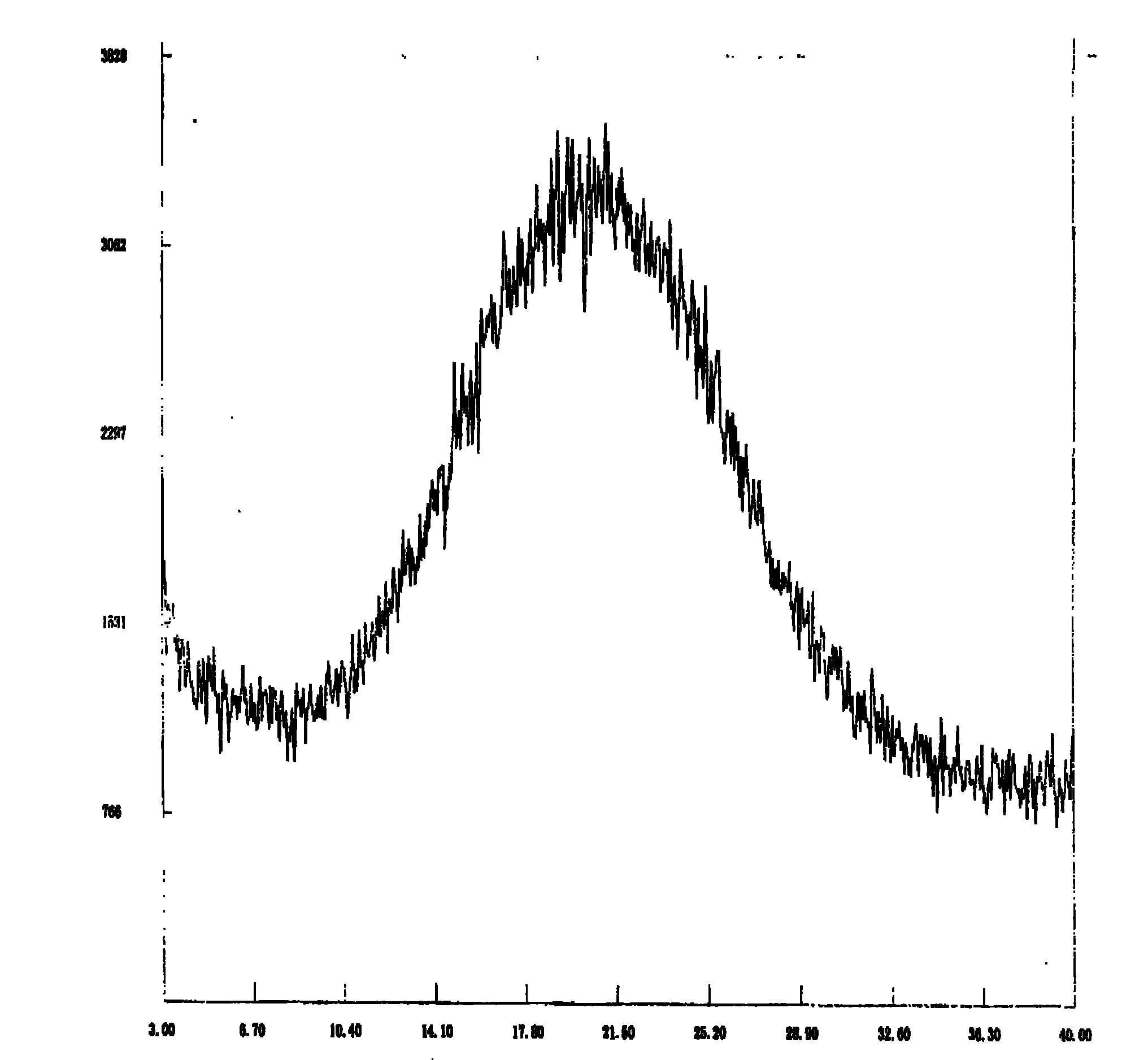
[0031] The crude dexlansoprazole (235.6g) prepared in the following step 6 was dissolved in ammonia water (12.5%, 5040ml) at room temperature, filtered to remove a little black insoluble matter, and washed with dichloromethane (1000ml×3). The pH of the water layer was adjusted to about 9 with glacial acetic acid (1290 ml) at -10°C, and a white solid precipitated. Stir for 5 min, filter, wash the solid with ice water (1000 ml), and dry under vacuum at room temperature for 24 h to obtain dexlansoprazole (off-white solid, 150.0 g). By figure 1 It is confirmed that the product is amorphous dexlansoprazole.
Embodiment 2
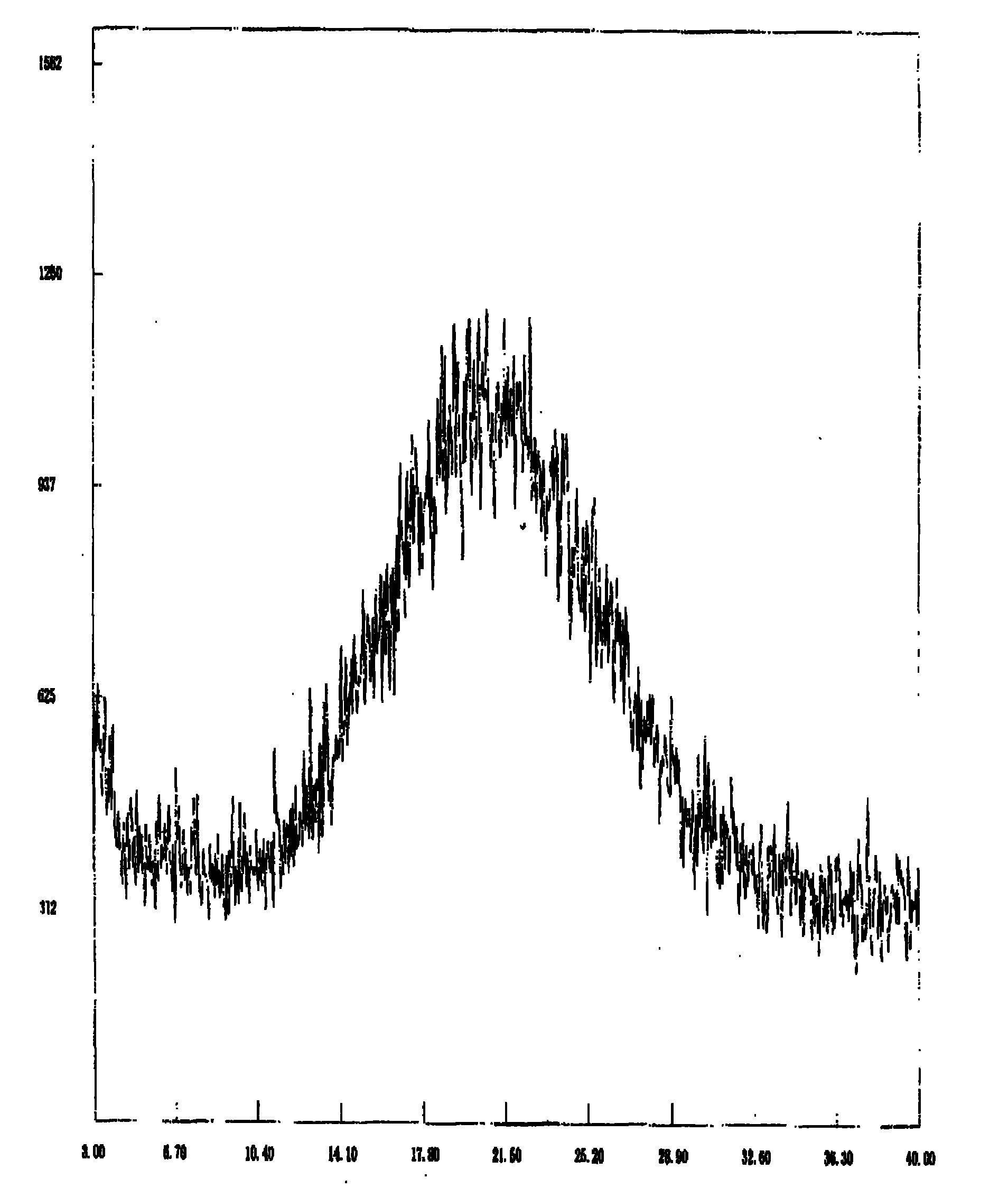
[0033] As described in Example 1, the temperature is -5°C and the pH is 8.
[0034] Attached figure 2 It is confirmed that the product is amorphous dexlansoprazole.
Embodiment 3
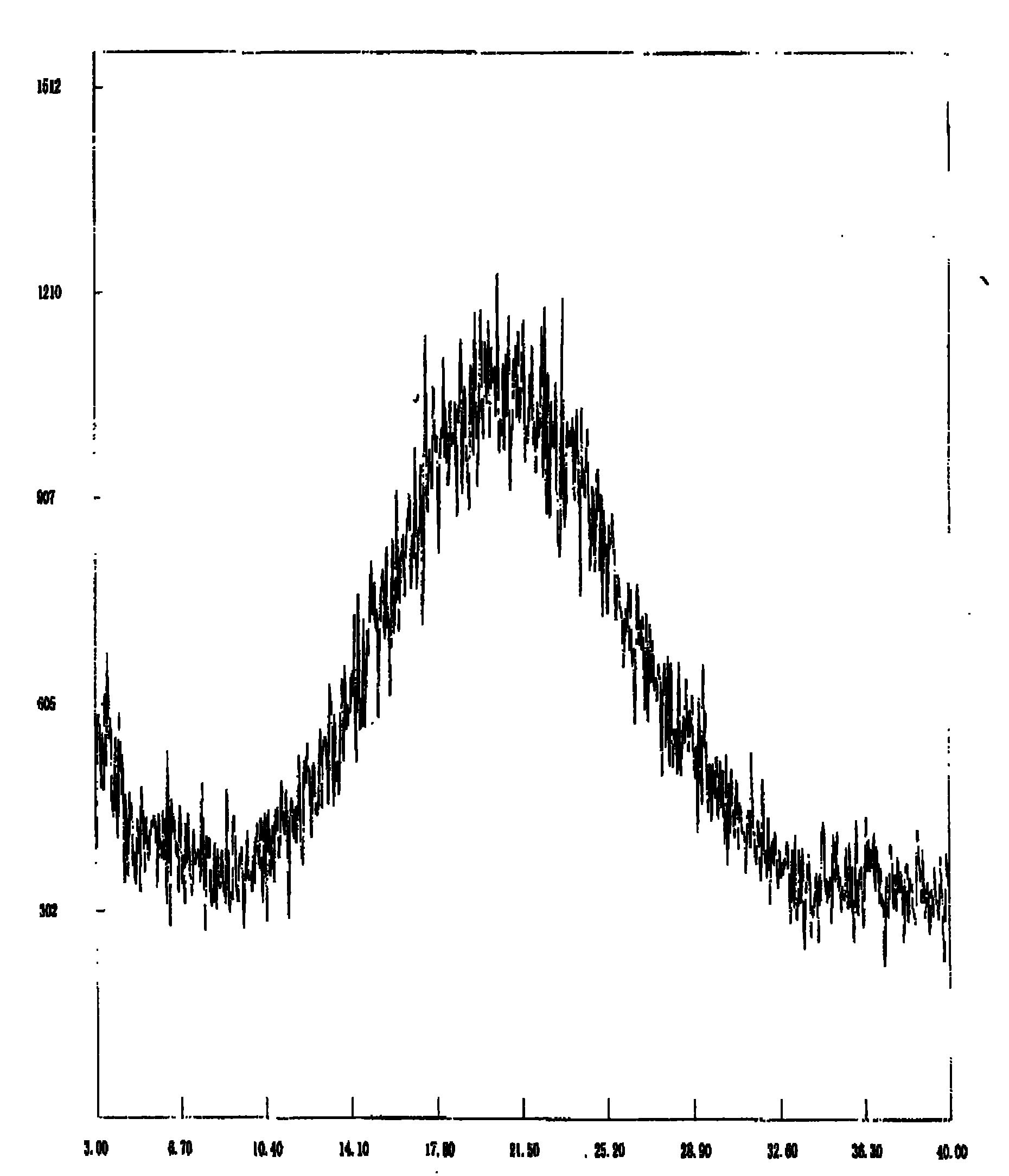
[0036] As described in Example 1, the temperature is -25°C and the pH is 10.
[0037] Attached image 3 It is confirmed that the product is amorphous dexlansoprazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com