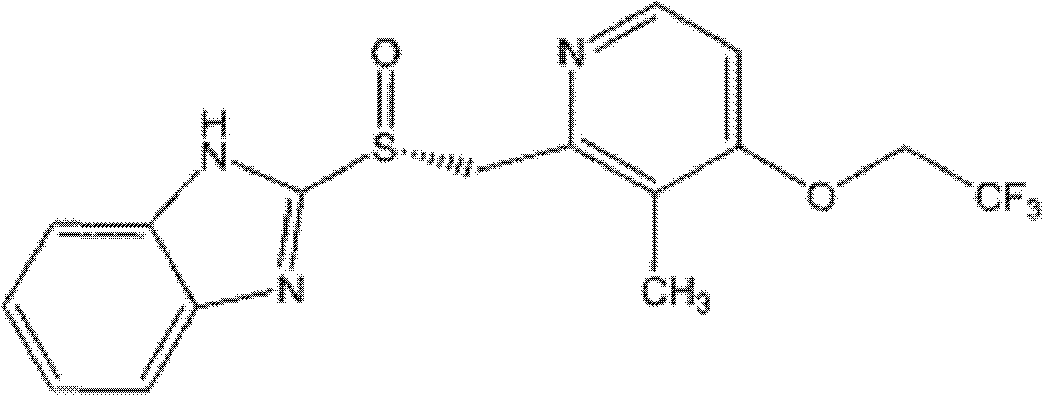
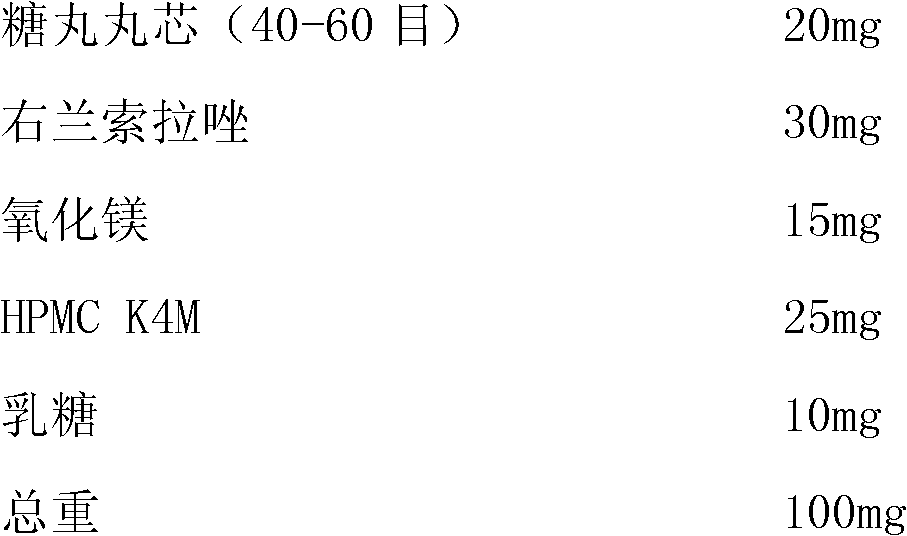Dexlansoprazole enteric-coated slow controlled-release pellet tablets
A technology of lansoprazole enteric and pellet tablets, applied in the preparation of dexlansoprazole sustained and controlled release preparations, dexlansoprazole enteric-coated sustained and controlled release pellets, dexlansoprazole sustained and controlled release In the field of pellet preparation, it can solve problems such as low cost, large output, and difficult swallowing of capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of drug-containing pill core I (blank pill core medicine method):
[0035]
[0036] Preparation Process:
[0037] 90g dexlansoprazole, 45g magnesium oxide, 75g HPMC K4M, 30g lactose are uniformly dissolved or dispersed in 1500ml water, and the blank sugar ball core (sucrose, made by starch) is filled in the fluidized bed coater, adjusted Coating parameters: Spray the drug-containing suspension on the blank pellet core. After the drug application is completed, continue to dry until the moisture content of the pellets is below 3%.
Embodiment 2
[0039] Preparation of drug-containing pill core I (blank pill core medicine method):
[0040]
[0041] The preparation process is the same as in Example 1.
Embodiment 3
[0043] Preparation of drug-containing pill core I (blank pill core medicine method):
[0044]
[0045] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com