Solid dispersion composition as well as preparation method and application thereof
A technology of solid dispersion and composition, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, etc., can solve the problems of taste masking of the undisclosed dextromethorphan quinidine composition, and achieve Satisfy the requirements of finished medicine, improve the experience of medication, and the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
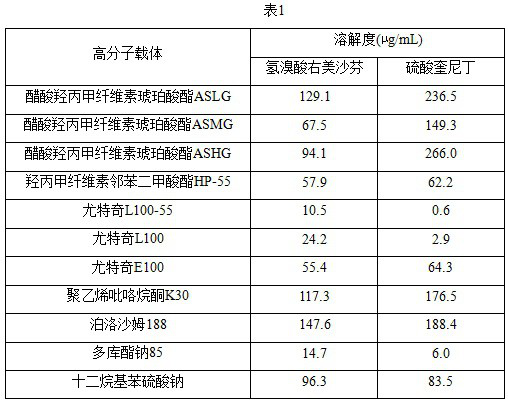
[0030] Dextromethorphan hydrobromide, quinidine sulfate and polymer carrier dissolved in ethanol to prepare a solution of 5mg / mL, Pipette 0.2 mL of polymer carrier solution into the wells of the high-throughput experimental apparatus, and then aspirate 0.1 mL of dextromethorphan hydrobromide solution and 0.1 mL of quinidine sulfate solution respectively and add them to the wells containing the polymer carrier solution, shake on the high-throughput instrument, place the well plate in a vacuum oven to dry, respectively, to obtain a solid dispersion of dextromethorphan hydrobromide containing a polymer carrier and a solid dispersion of quinidine sulfate containing a polymer carrier.
[0031] The prepared solid dispersion was added to 0.5 mL of water, 100 μL of the solution was aspirated at 60s, diluted after centrifugation, and the detection sample was obtained. The solubility of the sample was measured to obtain a solubility (μg / mL) of a solid dispersion containing different polymer...
Embodiment 2
[0036]Weigh dextromethorphan hydrobromide and quinidine sulfate respectively added utchi L100-55 and an appropriate amount of ethanol, stirred, sonicated until dissolved and clarified, sprayed dry in the spray dryer, after the end of the collection of samples into a glass bottle, placed in a vacuum drying box to dry, respectively to obtain dextromethorphan hydrobromide solid dispersion and quinidine sulfate solid dispersion.
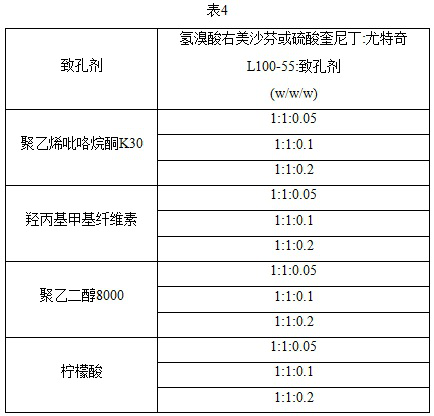
[0037] According to the above method, prepare solid dispersions of different ratios of dextromethorphan or quinidine and utchi L100-55 ratios, and investigate the dissolution of different proportions of solids in pH 1.2 sodium chloride buffer (37 °C), the results are shown in Table 2 below.
[0038]
[0039] The results show that with the increase of the proportion of polymer materials, the dissolution of dextromethorphan and quinidine increased first and then decreased. When the mass ratio of the polymer carrier to dextromethorphan hydrobromide is 1:1, an...
Embodiment 3
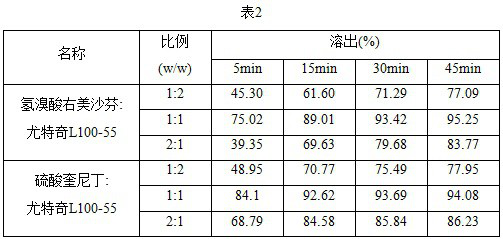
[0044]Dextromethorphan hydrobromide, quinidine sulfate, and Utechi L100-55 were dissolved in ethanol, respectively, and prepared into a solution of 20mg / mL, and the pore-causing agent was dissolved in ethanol to prepare a solution of 10mg / mL. Take the same volume of Yutechi L100-55 solution, dextromethorphan hydrobromide solution or quinidine sulfate solution to the wells of the high-throughput experimental device, add different volumes of porosity solutions, oscillate on the high-throughput instrument, and dry the well plates in a vacuum oven to obtain dextromethorphan hydrobromide solid dispersions and quinidine sulfate solid dispersions with different pore-causing agent contents. Using different porous agents, the resulting solid dispersions are shown in Table 4 below.
[0045]
[0046] Using the method of artificial taste in Example 1, each solid dispersion is tasted. The bitterness, numbness, sourness, sweetness, dryness, and grit sensation of solid dispersions are evaluate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com