Dipyridamole solid dispersion, orally disintegrating tablet and preparation method thereof
A technology of pyridamole solid and dispersion, applied in the field of pharmaceutical preparations, can solve the problems of inconvenient medication for patients, storage aging, low bioavailability, etc., achieve good physical and chemical stability, avoid high temperature heating, and simplify preparation The effect of craft
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 3.75g of dipyridamole, dissolve in 600ml 80%v / v ethanol aqueous solution, obtain drug solution, slowly add 11.25g of the carrier macromolecule as shown in table 1 respectively in drug solution under the action of magnetic stirring The polymer is dissolved until the carrier is completely dissolved to form a uniform drug-containing carrier solution, and the drug-containing carrier solution is spray-dried at a liquid feed rate of 10 mg / min to obtain a dipyridamole solid dispersion. The process parameters of spray drying are as follows: the outlet air temperature is 79℃~80℃, and the wind speed is 0.6m 3 / min, the atomization pressure is 10Mpa.
[0043] Table 1 is used to prepare the carrier of dipyridamole solid dispersion
[0044]
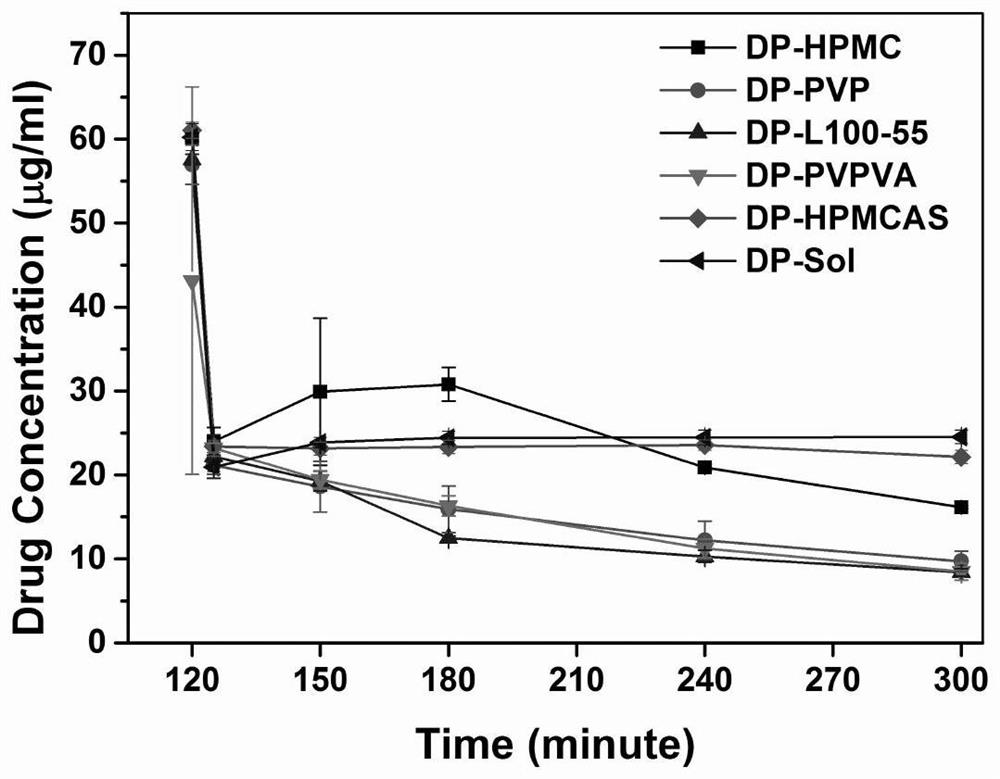
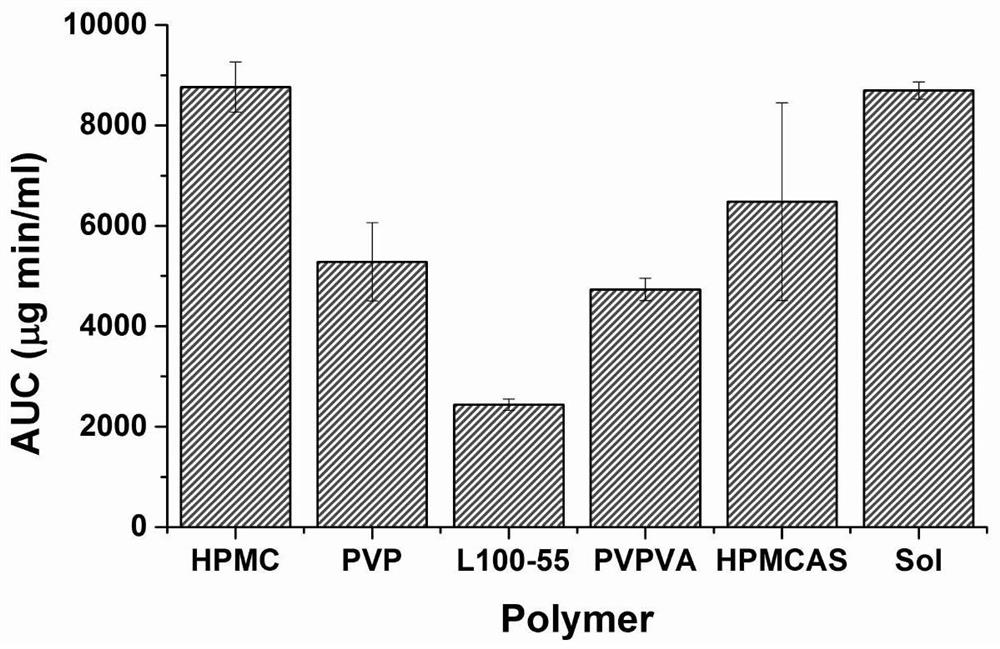
[0045] The dipyridamole solid dispersion prepared from each recipe in this example was subjected to an in vitro supersaturated dissolution test. Precisely weigh the dipyridamole solid dispersion of each prescription to make it contai...
Embodiment 2
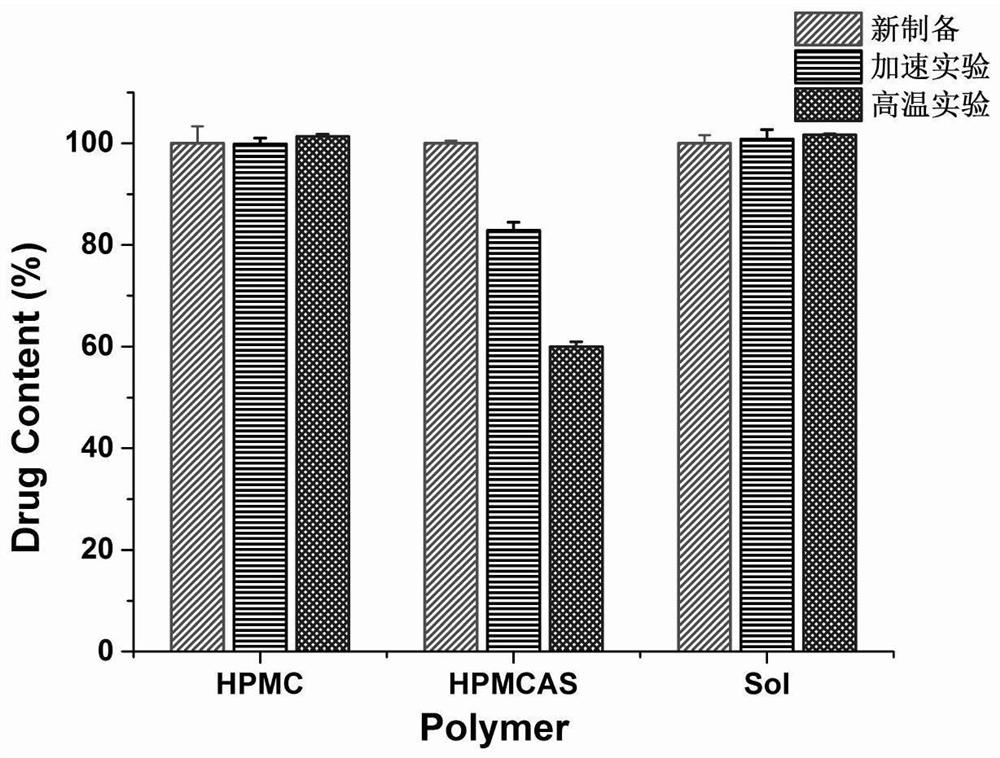
[0049] Stability test of dipyridamole solid dispersion: (1) high temperature test, the dipyridamole solid dispersion newly prepared in Example 1 was spread on a watch glass, and sealed with a cover. Place the watch glass in a constant temperature oven at 60°C, and take samples on the 60th day; (2) Accelerated test, take the dipyridamole solid dispersion newly prepared in Example 1 and place it in a constant temperature and constant humidity at 40°C / 75%RH Under box conditions, samples were taken on the 60th day. The samples taken were characterized as follows.
[0050] (1) Observation of crystal changes by polarizing microscope: take an appropriate amount of sample and place it on a glass slide, gently grind the sample with another clean cover glass to spread it in a thin layer on the glass slide, and then observe the sample under a polarizing microscope Whether there is crystal precipitation.
[0051] (2) Determination of drug content: Accurately weigh 5 mg each of the newly...
Embodiment 3
[0057] Take dipyridamole 3.75g, be dissolved in the ethanol aqueous solution of 600ml 60% v / v, obtain drug solution, slowly add 11.25g HPMC in the drug solution under the action of magnetic stirring, until the carrier material is dissolved to form uniform containing drug carrier solution. The drug-containing carrier solution was spray-dried according to the process parameters described in Example 1 to prepare dipyridamole solid dispersion. The dipyridamole solid dispersion prepared in this example was placed under high temperature and accelerated test conditions (same as Example 2) for stability test, and the storage period was 60 days. The newly prepared and stability tested samples were subjected to supersaturated dissolution according to the method of Example 1, to investigate whether the dissolution behavior of the drug changed. In addition, X-ray powder diffractometer (XRD) is used to determine the crystal form of the drug, and to investigate whether the crystal form of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com