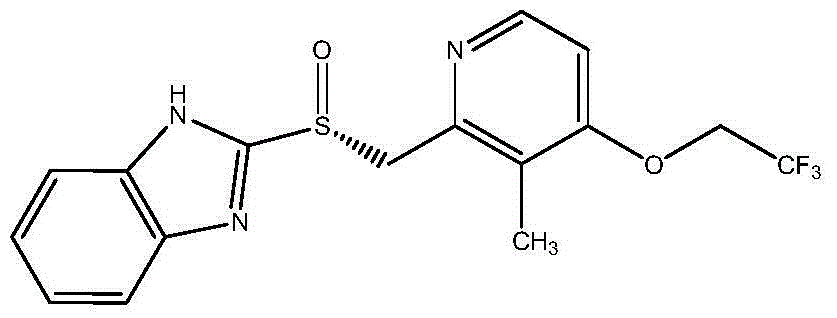
Dexlansoprazole sustained release capsule and preparation method thereof
A technology for dexlansoprazole and sustained-release capsules is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, and can solve the problems that the preparation method is unfavorable to safe production, environmental protection, toxic and side effects high, poor stability of dexlansoprazole, etc., to avoid damage, simplify the production process, and reduce production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
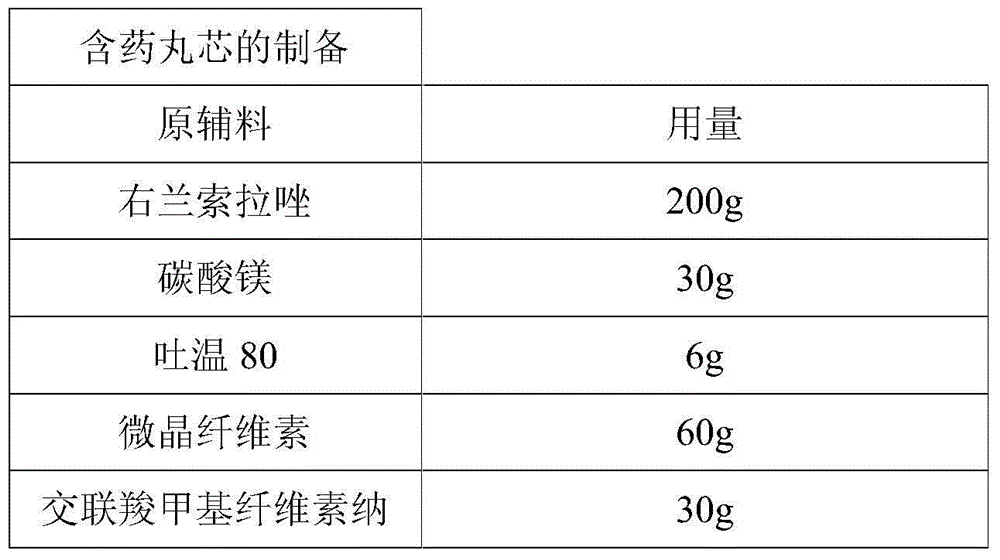
[0055]
[0056] Preparation Process:
[0057] Weigh the raw and auxiliary materials according to the prescription amount, pass through an 80-mesh sieve three times and mix evenly. Put 200g sucrose ball cores (30 mesh-40 mesh) in the centrifugal granulation coating pan, spray 3% hypromellose aqueous solution, adjust machine parameters and centrifugally powder. After the powdering is completed, the pellets are dried in a fluidized bed at 40°C for 2 hours, and then sieved to obtain the product.
Embodiment 2
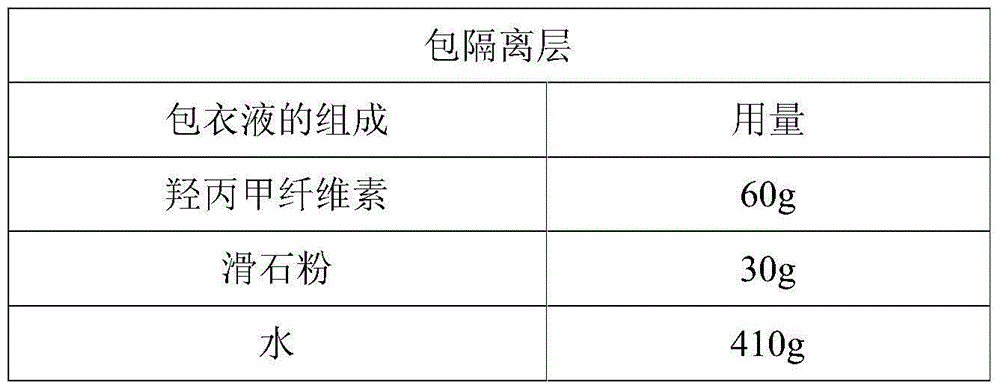
[0059]
[0060] Preparation Process:
[0061]Weigh the prescribed amount of hypromellose and fully swell it in water, add talcum powder, and stir evenly to obtain the coating solution. 500g of the pellets obtained in Example 1 were placed in a fluidized bed for coating. After the coating is completed, the pellets are dried in a fluidized bed at 40°C for 1 hour to obtain the final product.
Embodiment 3
[0063]
[0064] Preparation Process:
[0065] Weigh Eudragit S100, add a small amount of water and stir for 5 minutes, slowly add ammonia water (about 10 minutes), continue stirring for 30 minutes after the addition, add triethyl citrate and talcum powder for later use. Get 200g embodiment 2 gained pellets and place in fluidized bed, carry out coating. After the coating is completed, the pellets are dried in a fluidized bed at 40°C for 2 hours to obtain the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com