Dexlansoprazole and its preparation method and use
A technology of dexlansoprazole and lansoprazole essence, which is applied in the field of drug synthesis, can solve the problems of low optical purity, low overall yield, and high content of L-isomers, and achieve good results and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
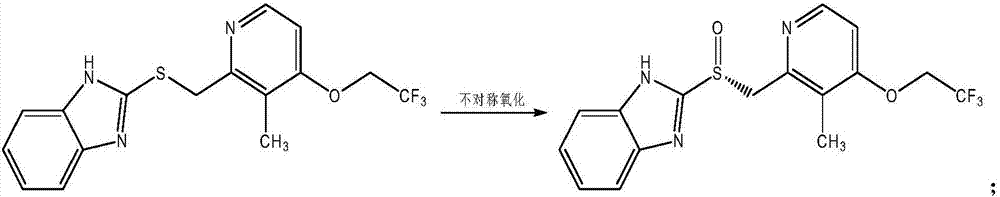
[0086] The present invention provides a method for preparing dexlansoprazole. The lansoprazole sulfide prepared by lansochloride hydrochloride and 2-mercaptobenzimidazole is asymmetrically oxidized to obtain dexlansoprazole The crude product is refined by optical purity to obtain a refined product of dexlansoprazole, and further purified to obtain dexlansoprazole.
[0087] The preparation method of the dexlansoprazole of the present invention synthesizes crude dexlansoprazole through a simple process, and can obtain a high-purity dexlansoprazole product with an optical purity of nearly 100% through further optical purity refining and purification.
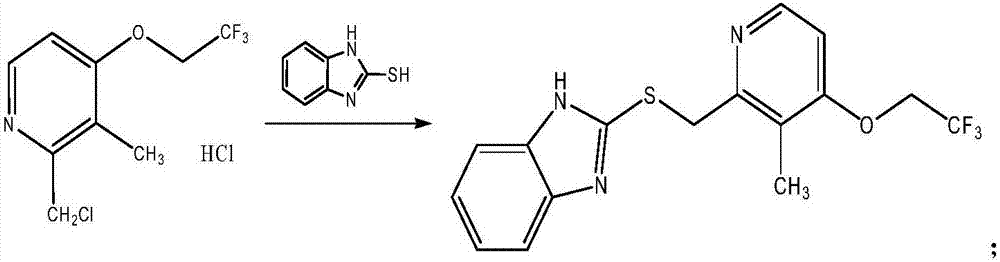
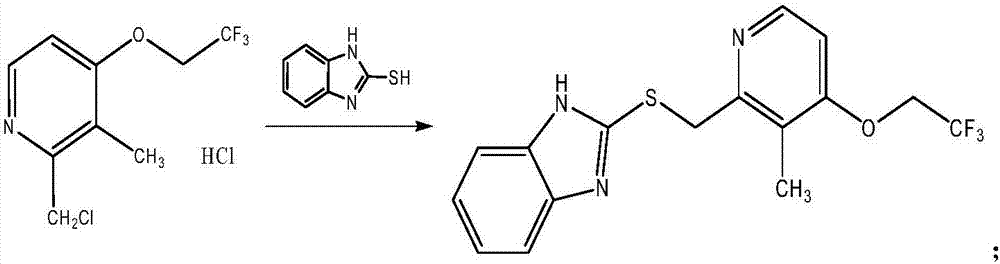
[0088] In a preferred embodiment of the present invention, the reaction route for the preparation of the lansoprazole sulfide is as follows:
[0089]
[0090] Including the addition of 2-mercaptobenzimidazole and lanso chloride hydrochloride in a solvent with alkali, and the reaction of 2-mercaptobenzimidazole and lanso chloride hydrochlo...
Embodiment 1
[0178] A preparation method of dexlansoprazole includes the following steps:
[0179] Step 1: Preparation of Lansoprazole Sulfide:
[0180]
[0181] Add 16675 mL of methanol to a 50L glass reactor, and turn on a stirring ice bath to cool down. Slowly add 1256 g of sodium hydroxide and stir to dissolve completely. The temperature is controlled below 35℃. Add 1256 g of 2-mercaptobenzimidazole and 2300 g of lanso chloride hydrochloride sequentially, and heat to reflux. At 60-70°C, start the heat preservation reaction for 2 hours. HPLC detects the completion of the reaction and starts to cool down. When the temperature is lower than 30°C, filtration starts. After the filtration is completed, the filtrate starts to be distilled under reduced pressure (50-60°C). When the vaporized methanol is 1 / 2 of the feed volume, the temperature is reduced to 25-30°C. 34500mL of water was added, a white solid precipitated, and the mixture was stirred for 30 minutes. Shake filtered water and ri...
Embodiment 2
[0190] A preparation method of dexlansoprazole adopts the same method as in Example 1, except that:
[0191] In the first step:
[0192] The mass ratio of the lanso chloride hydrochloride and 2-mercaptobenzimidazole is 1:0.5;
[0193] After the reaction, the temperature is lowered to 20°C and filtered;
[0194] After vacuum distillation, the temperature is reduced to 20°C, and then water is added to precipitate the product;
[0195] The temperature of the vacuum drying is 60°C;
[0196] The vacuum drying time is 6h.
[0197] In the first step, the yield is about 127%, the content is about 79.4%, and the loss on drying (%) is less than or equal to 0.5%.
[0198] In the second step:
[0199] The volume ratio of toluene and water is 1:1;
[0200] The dosage ratio of the lanso sulfide to the chiral ligand is preferably 1g:1mL;
[0201] The temperature of the heating and heat preservation is 50°C;
[0202] The heating and heat preservation time is 10 minutes;
[0203] The dosage ratio of the lanso s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com