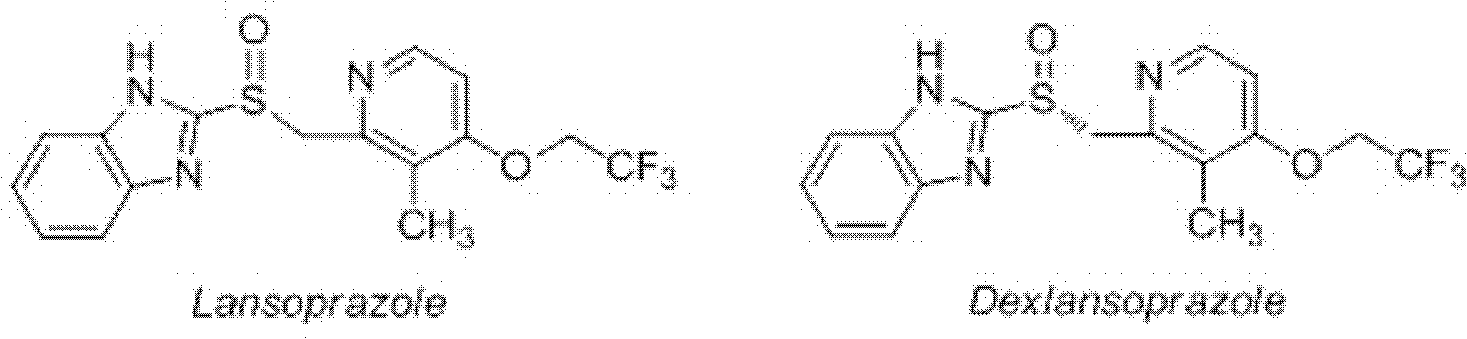
Method for synthesizing and purifying dexlansoprazole
A technology of dexlansoprazole and an oxidizing agent, which is applied in organic chemistry and other fields, can solve the problems of poor product stability, complicated process, waste of resources and energy, etc., and achieve the effect of simple and easy-to-control preparation process, high purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
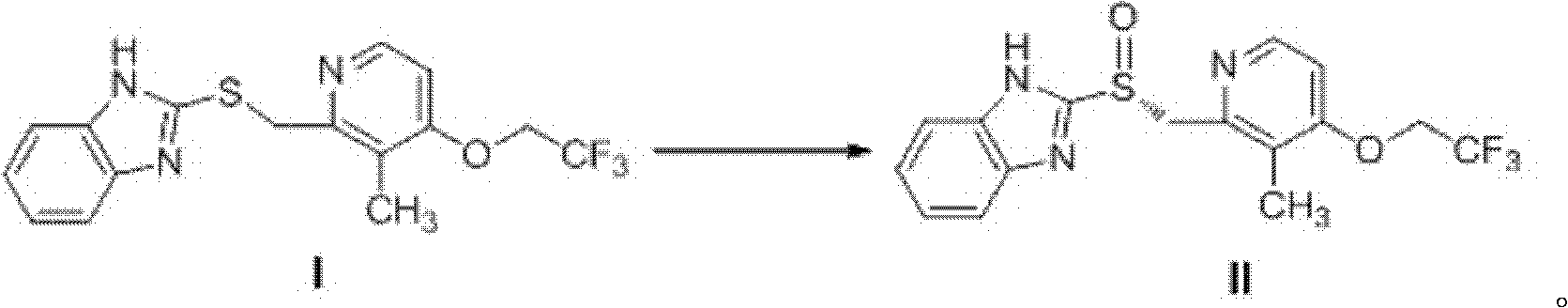
[0026] The preparation method of embodiment 1 dexlansoprazole
[0027]
[0028] 2.70kg of the compound shown in formula I (Cas No.: 103577-40-8, purchased from Zhejiang Xinsanhe Pharmaceutical Chemical Co., Ltd.) and 1.50L of L-diethyl tartrate were suspended in 10L of toluene, Add catalytic amount of purified water dropwise, stir mechanically evenly, then add 1.5L of tetraisopropyl titanate, at this time the solution turns into a tea-red clear liquid, keep the temperature at 50-55°C and stir for 1 hour, then add 1.3L after cooling to room temperature Put the diisopropylethylamine in the cooling bath to lower the temperature. When the internal temperature drops to -5~0℃, slowly add 3.5L of cumene hydroperoxide dropwise, keep the temperature constant, and continue to stir for 1h. TLC monitoring (CH 2 Cl 2 : MeOH=25: 1, the same below) the reaction is completed, and the dexlansoprazole shown in the formula II is obtained.
[0029] Slowly pour the above reaction liquid int...
Embodiment 2
[0031] The preparation method of embodiment 2 dexlansoprazole
[0032]
[0033] Suspend 2.0 kg of the compound represented by formula I and 1.50 L of L-diethyl tartrate in 12 L of methanol, dropwise add a catalytic amount of purified water, and stir mechanically, then add 1.5 L of tetraisopropyl titanate, At this time, the solution turns into a tea-red clear liquid, keep the temperature at 40-45°C and stir for 1 hour, add 1.0L triethylamine after cooling to room temperature, put it in a cooling bath to cool down, and when the internal temperature drops to -10--5°C, Start to slowly add 3.5L cumene hydroperoxide dropwise, keep the temperature constant, continue to stir for 1h, and TLC monitors the completion of the reaction to obtain the dexlansoprazole shown in formula II.
[0034] Slowly pour the above reaction liquid into 30L of petroleum ether, a large amount of white milky matter is formed, let it stand for 0.5~1h, after the stratification is complete, separate the bro...
Embodiment 3
[0036] The preparation method of embodiment 3 dexlansoprazole
[0037]
[0038] Suspend 3.0 kg of the compound represented by formula I and 2.0 L of L-diethyl tartrate in 12 L of toluene, add a catalytic amount of purified water dropwise, and stir mechanically, then add 2.0 L of tetraisopropyl titanate At this time, the solution turns into a tea-red clear liquid. Keep the temperature at 60-70°C and stir for 1 hour. After cooling to room temperature, add 1.5L diisopropylethylamine, put it in a cooling bath to cool down, and wait until the internal temperature drops to -15-15°C. At -10°C, start to slowly add 4.0L cumene hydroperoxide dropwise, keep the temperature constant, continue to stir for 1h, and monitor the completion of the reaction by TLC to obtain dexlansoprazole shown in formula II.
[0039] Slowly pour the above reaction liquid into 43L of n-heptane, a large amount of white milky substance is formed, let it stand for 1.5h, wait for the stratification to complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com