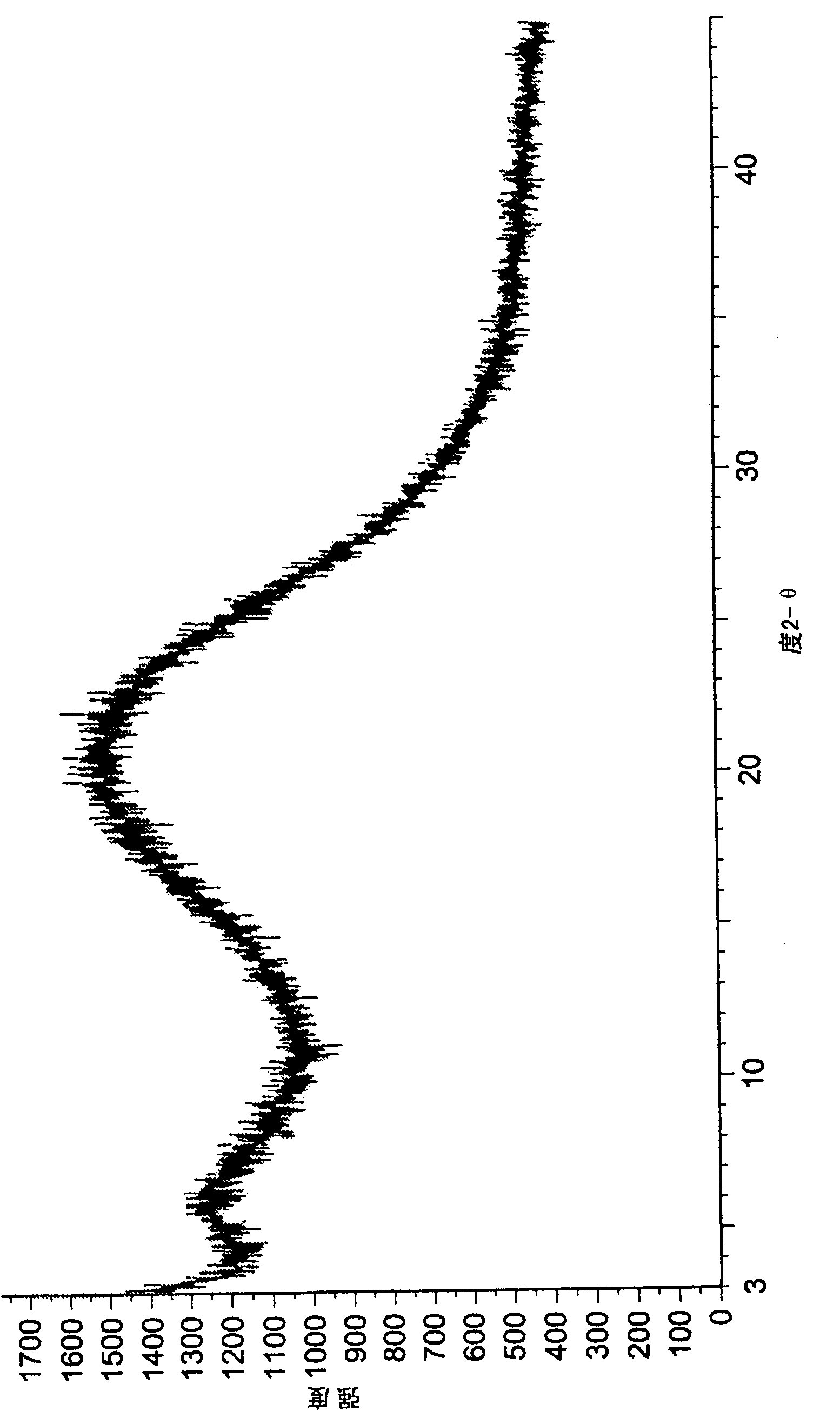
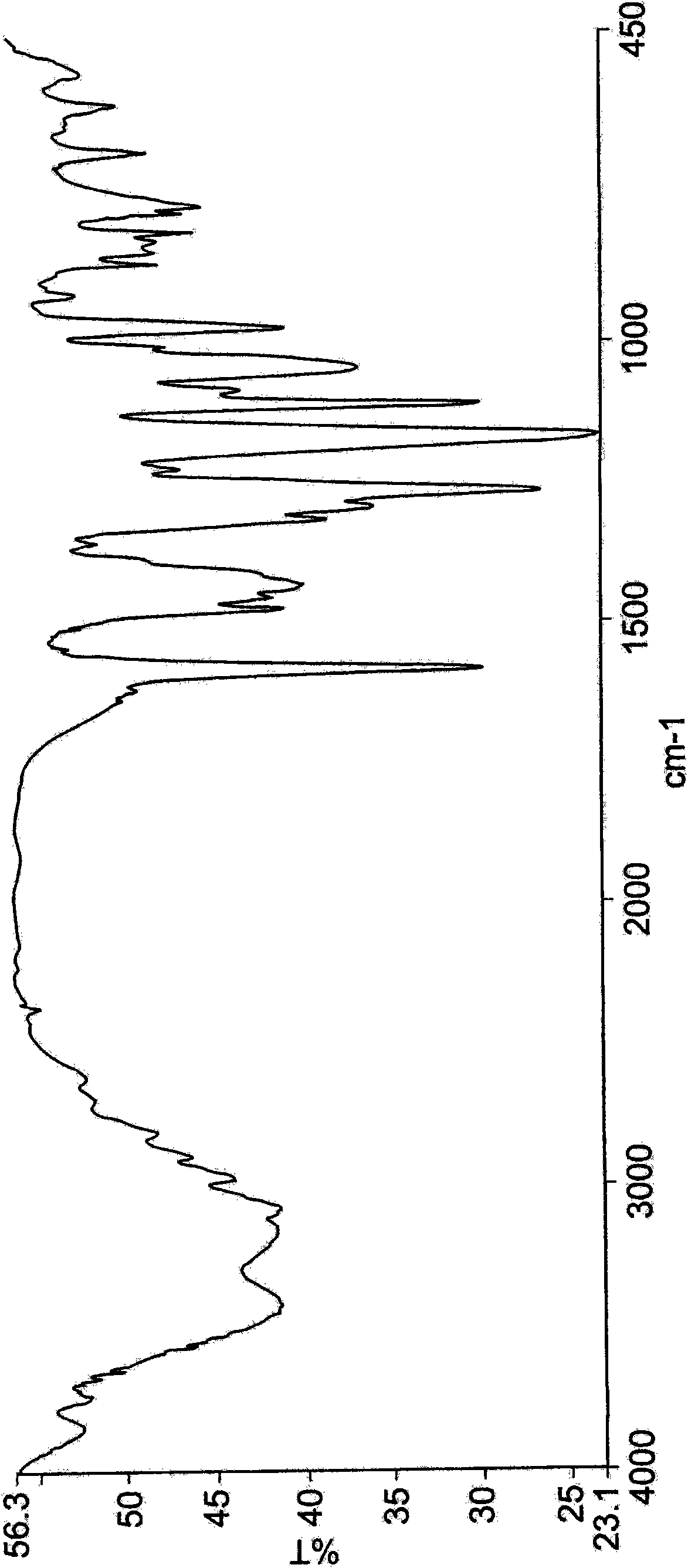
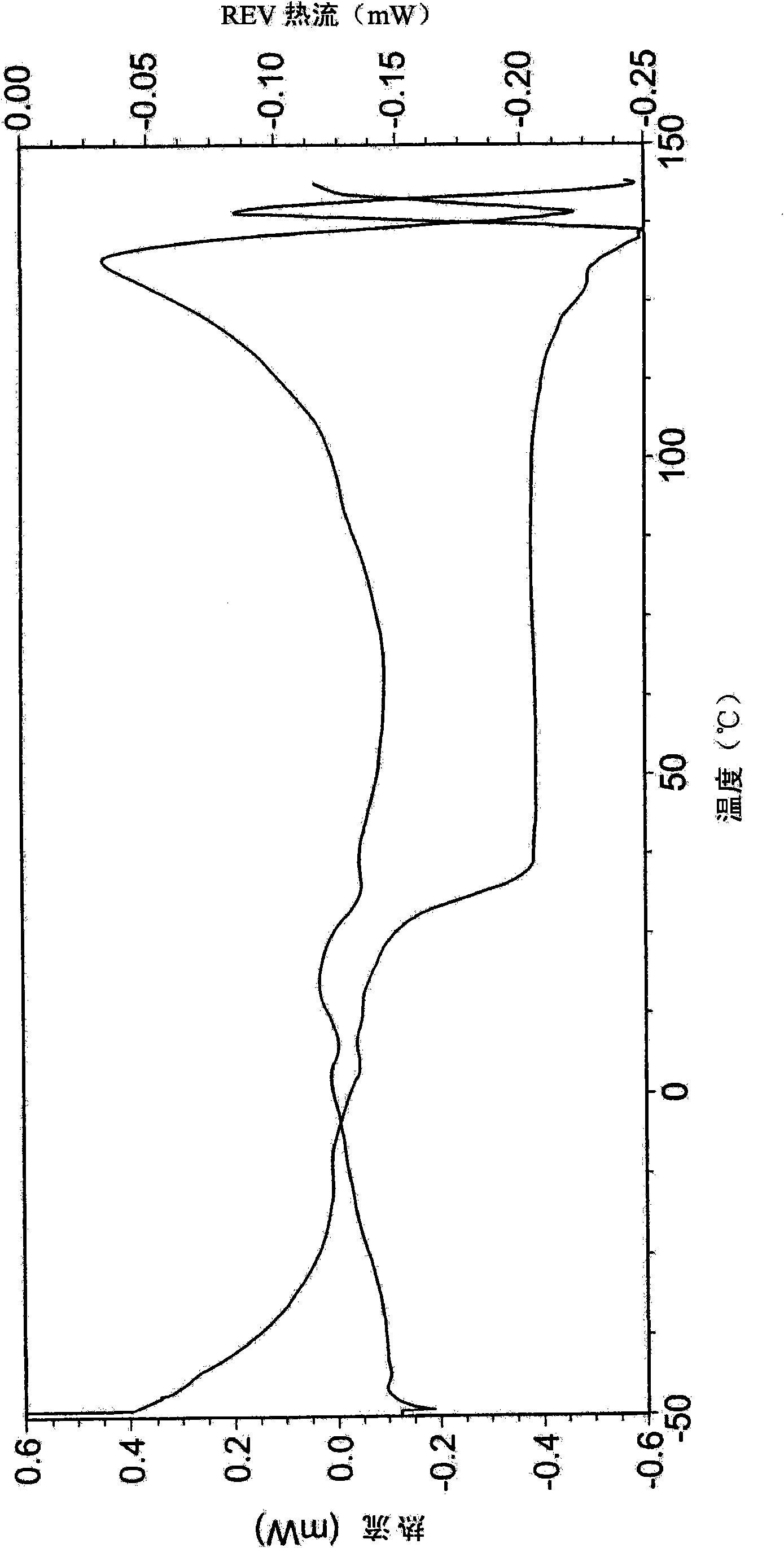
Dexlansoprazole process and polymorphs
A dexlansoprazole and crystallization technology, applied in the field of preparing dexlansoprazole, can solve the problems such as yield loss, unenvironmental protection, uneconomical method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0358] Example 1: Preparation of 2-[(R)-[(4-nitro-3-methyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole
[0359] Under a nitrogen atmosphere, 2-[[(4-nitro-3-methyl-2-pyridyl)methyl]thio]-1H-benzimidazole (10.2 g) and toluene (300 mL) were added to a round bottom flask and stirred at 25-35°C for 5-10 minutes. Water (0.09 mL) and (+)-diethyl tartrate (2.5 mL) were added and stirred at 25-35°C for 5-10 minutes. This mixture was heated to 65-70°C for 30 minutes. Titanium isopropoxide (11.68 mL) was added to the mixture at 65-70°C for 1-2 hours. The mixture was cooled to 15-20°C, then diisopropylethylamine (5.73 mL) was added and stirred for 5-10 minutes. The mixture was cooled to 0-5°C and cumene hydroperoxide (8.22 mL) was added over 20-30 minutes. The reaction mixture was maintained at 0-5°C for 4-5 hours. The mixture was extracted with 12.5% piperidine solution (2 x 100 mL) and 12.5% ammonia solution (2 x 100 mL), then the combined aqueous layers were washed with toluen...
Embodiment 2
[0360] Example 2: Preparation of 2-[(R)-[(4-nitro-3-methyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole
[0361] 2-[[(4-Nitro-3-methyl-2-pyridyl)methyl]thio]-1H-benzimidazole (10.6 g) and toluene (300 mL) were charged in a Dean-Stark apparatus Place in a round bottom flask and stir for 5-10 minutes. The mixture was heated to 110°C and allowed to azeotropically reflux for 1-2 hours to completely remove the water. The mixture was cooled to 70°C, then water (0.36 mL), (+)-diethyltartrate (12.58 mL) and titanium isopropoxide (11.71 mL) were added and stirred at 65-70°C for 1 hour. The mixture was cooled to 15-25°C, and diisopropylethylamine (5.73 mL) was added, then the mixture was cooled to 0-5°C. At 0-5°C, cumene hydroperoxide (10.38 mL) was added within 30-45 minutes, and the mixture was maintained at 0-5°C for 4-5 hours. The reaction was quenched with 12.5% piperidine (200 mL), and the organic and aqueous layers were separated. The organic layer was extracted with 12.5% ...
Embodiment 3
[0362] Example 3: Optical Purification of 2-[(R)-[(4-nitro-3-methyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole
[0363] 2-[(R)-[(4-Nitro-3-methyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole (5.0 g) and acetone (125 mL) were added to the round bottom flask and stir for 5-10 minutes. This mixture was heated to 50-55°C and maintained to completely dissolve 2-[(R)-[(4-nitro-3-methyl-2-pyridyl)methyl]sulfinyl]-1H-benzo imidazole. The solution was cooled to 25-35°C, further cooled to 5-10°C, then held at 5-10°C for 1-2 hours, then the solid formed was filtered and washed with acetone (10 mL). The filtrate was evaporated under reduced pressure at 40-45°C to give 3.2 g of the title compound. The chiral purity of the input material: 86.12%, the chiral purity of the product determined by HPLC was 99.49%, and the chemical purity of the product determined by HPLC was 98.14%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com