Dextrorotation lansoprazole diphasic spansule preparation and preparing method thereof
A dual- and sustained-release technology for dexlansoprazole, which is applied in pharmaceutical formulations, drug delivery, and the digestive system, and can solve the problems of low process feasibility, instability of dexlansoprazole in acid, and unstable release rate And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
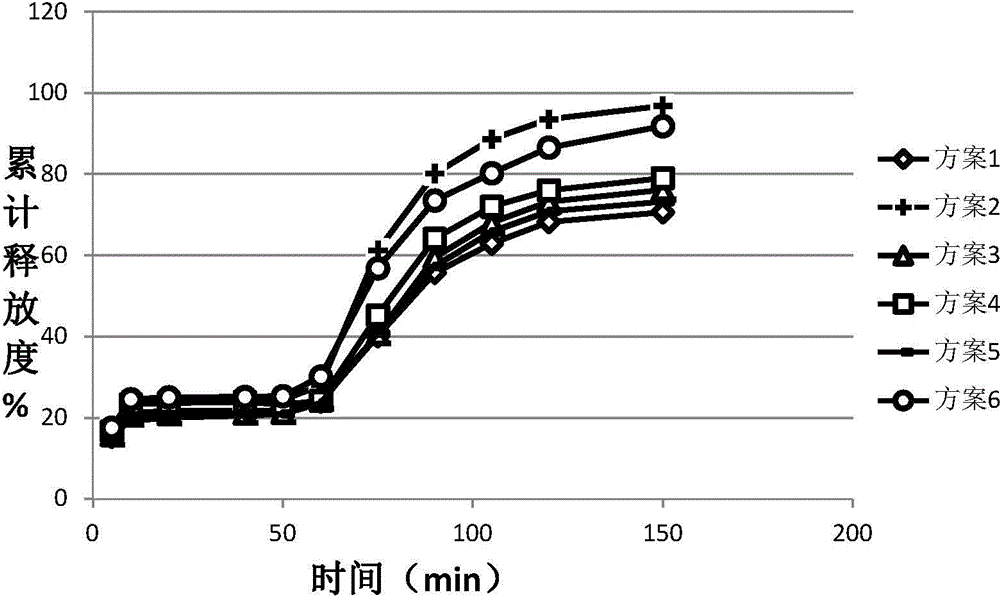
Image
Examples
Embodiment 1
[0066] Embodiment 1: Formulation 1
[0067]
[0068]
[0069] Preparation method:
[0070] 1. According to formula 1, mix dexlansoprazole with sucrose and starch and ball mill it at a speed of 20 rpm for 3 hours, and pass through a 200-mesh sieve to obtain the main drug mixed powder;
[0071] 2. Mix the ball-milled main drug mixed powder with low-substituted hydroxypropyl cellulose and magnesium carbonate according to formula 1 to obtain the drug mixed powder;
[0072] 3. Prepare the adhesive solution according to formula 1 and set aside;
[0073] 4. Using a centrifugal granulator, the speed of feeding the powder and the speed of the binder solution are 2:1, while inhaling the mixed powder of the drug and spraying the binder solution on the side to apply the drug to the blank pellet core, and then drying and sieving to increase the weight 340%, get the pill core;
[0074] 5. Prepare the isolation layer solution according to formula 1, use the fluidized bed bottom spra...
Embodiment 2
[0078] Embodiment 2: Recipe 2
[0079]
[0080]
[0081] Preparation method:
[0082] 1. Mix dexlansoprazole with sucrose and microcrystalline cellulose according to formula 2 and ball mill it at a speed of 30 rpm for 4 hours, and pass through a 200-mesh sieve to obtain the main drug mixed powder;
[0083] 2. Mix the ball-milled main drug mixed powder with low-substituted hydroxypropyl cellulose and sodium bicarbonate evenly according to formula 2 to obtain the drug mixed powder;
[0084] 3. Prepare the adhesive solution according to formula 2, and set aside;
[0085] 4. Use a centrifugal granulator to apply medicine to the blank pill core at a rate of 2:1 between the powder feeding speed and the binder solution feeding speed, dry and sieve, and increase the weight by 320%, and the pill core is obtained;
[0086] 5. Prepare the isolation layer solution according to formula 2, use the fluidized bed bottom spray method to coat the isolation layer on the upper pill core, ...
Embodiment 3
[0090] Embodiment 3: formula 3
[0091]
[0092]
[0093] Preparation method:
[0094] 1. According to formula 3, mix dexlansoprazole with lactose and microcrystalline cellulose and ball mill for 5 hours at a speed of 40 rpm, and pass through a 200-mesh sieve to obtain the main drug mixed powder;
[0095] 2. Mix the ball-milled main drug mixed powder with low-substituted hydroxypropyl cellulose and sodium bicarbonate evenly according to formula 3 to obtain the drug mixed powder;
[0096] 3. Prepare the adhesive solution according to formula 3 and set aside;
[0097] 4. Use a centrifugal granulator to apply medicine to the blank pellet core at a rate of 2:1 between the powder feed rate and the binder solution feed rate, dry and sieve, increase the weight by 250%, and get the pill core;
[0098] 5. Prepare the isolation layer solution according to formula 3, use the fluidized bed bottom spray method to coat the isolation layer on the upper pill core, dry and sieve to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com