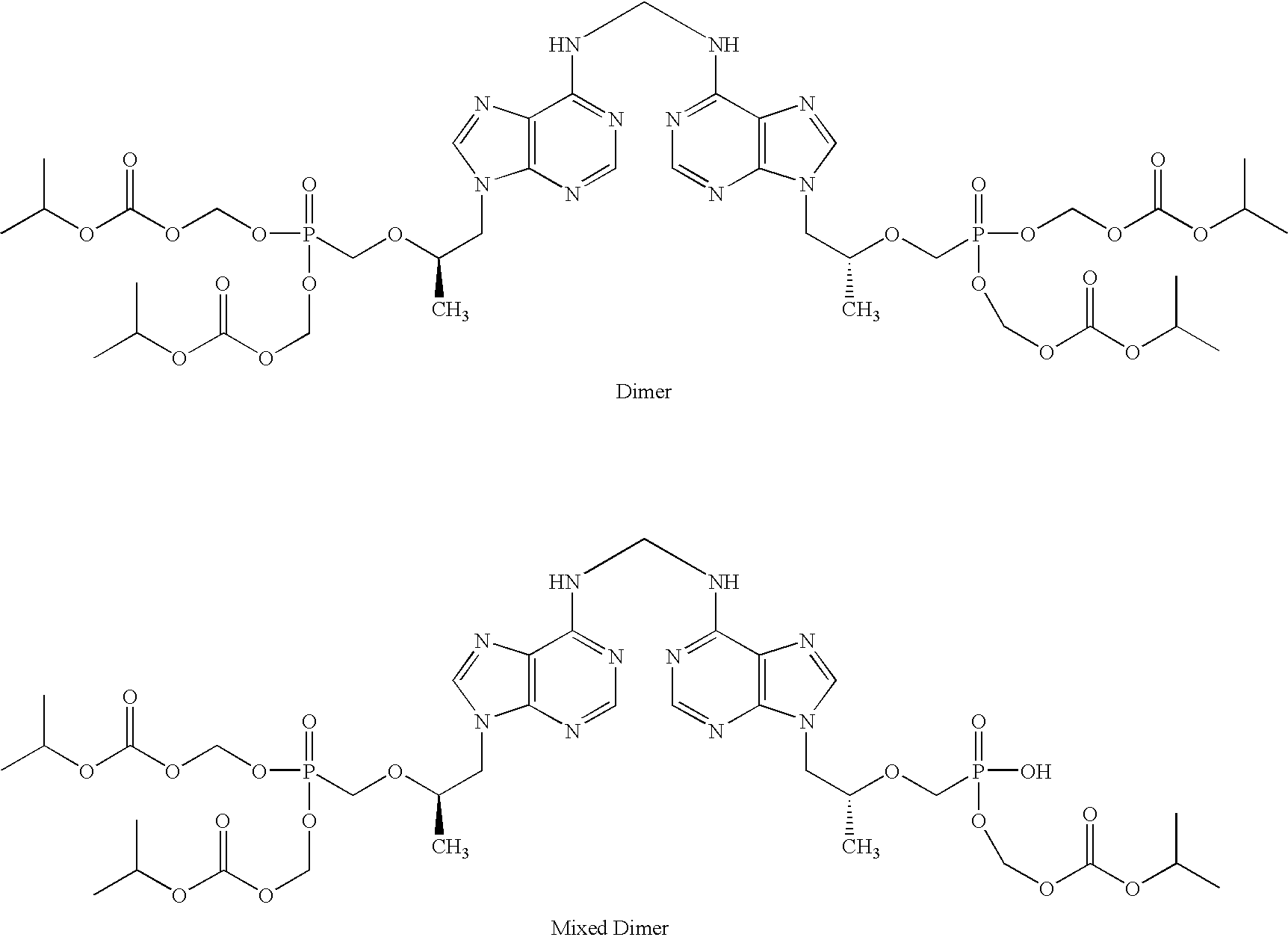
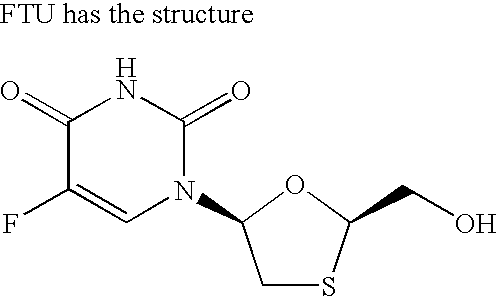
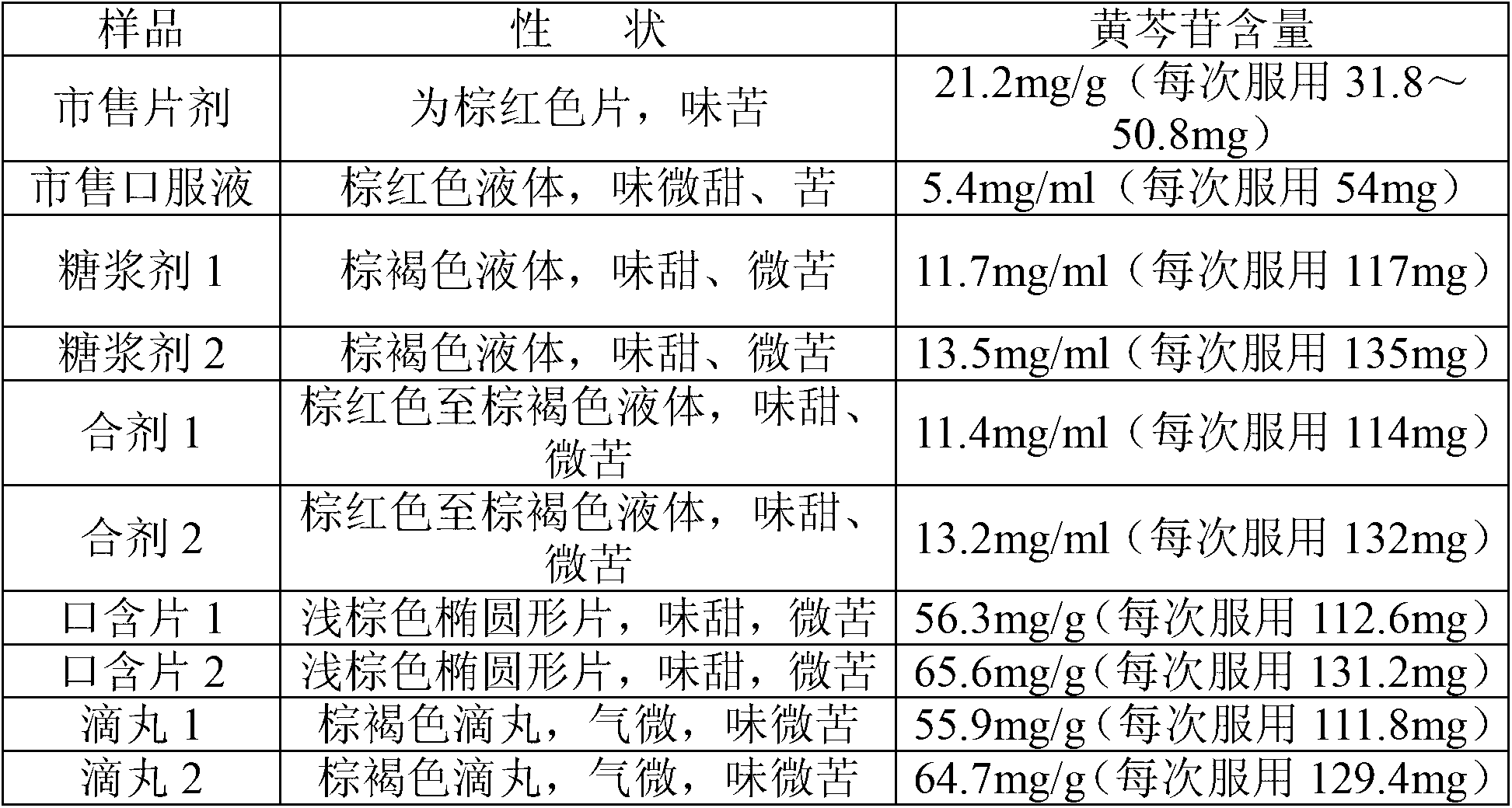
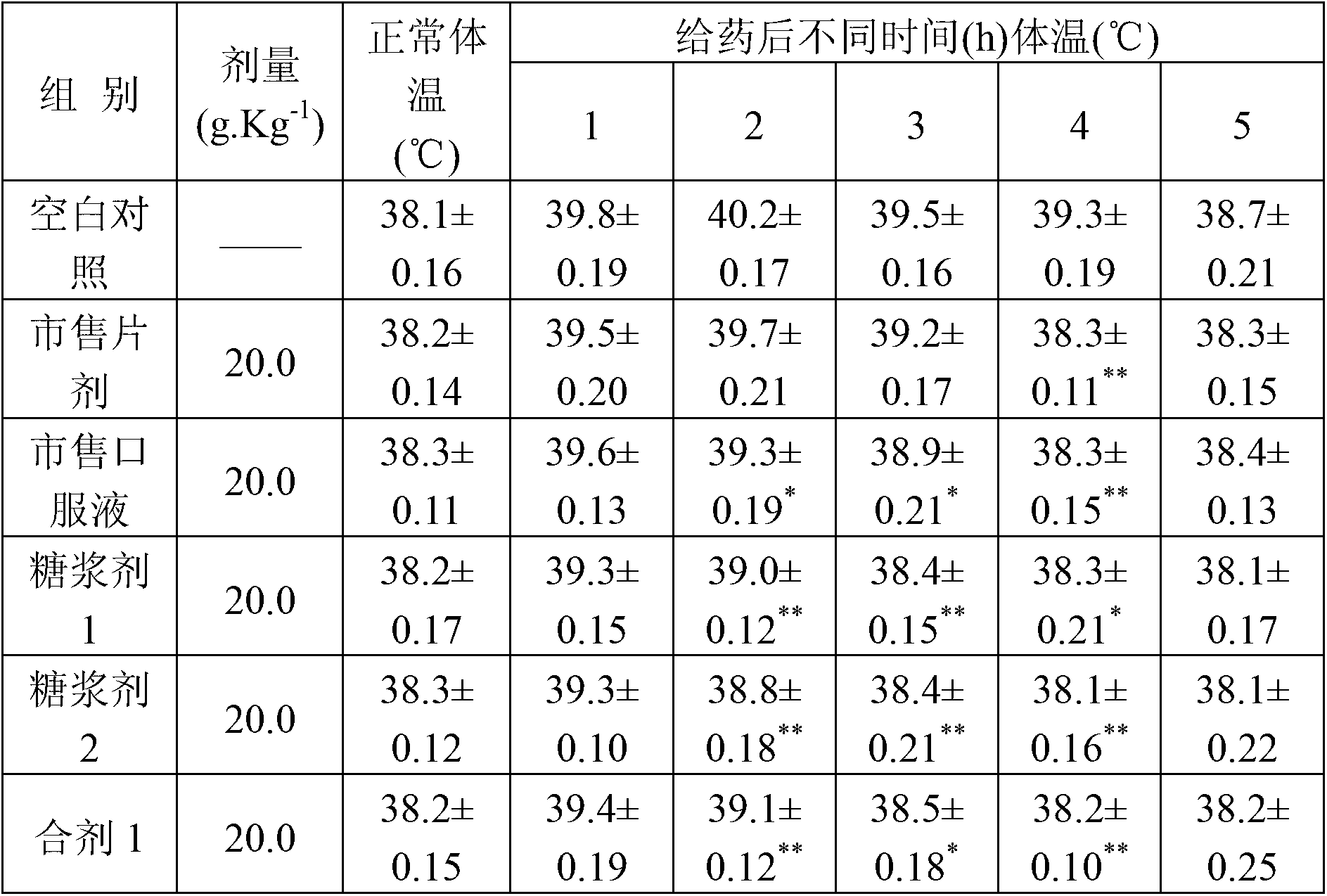
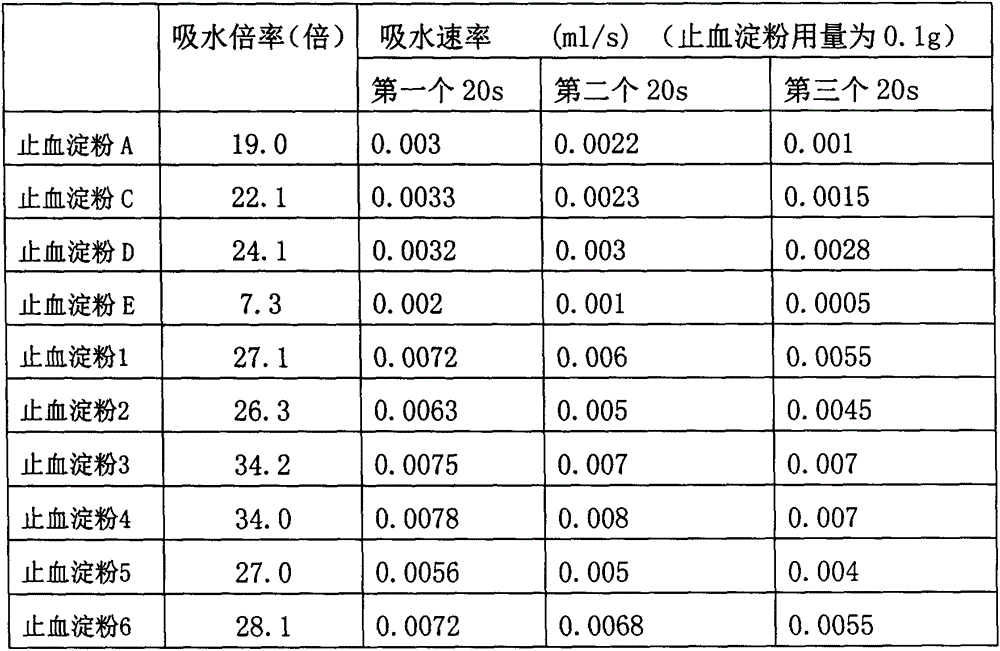
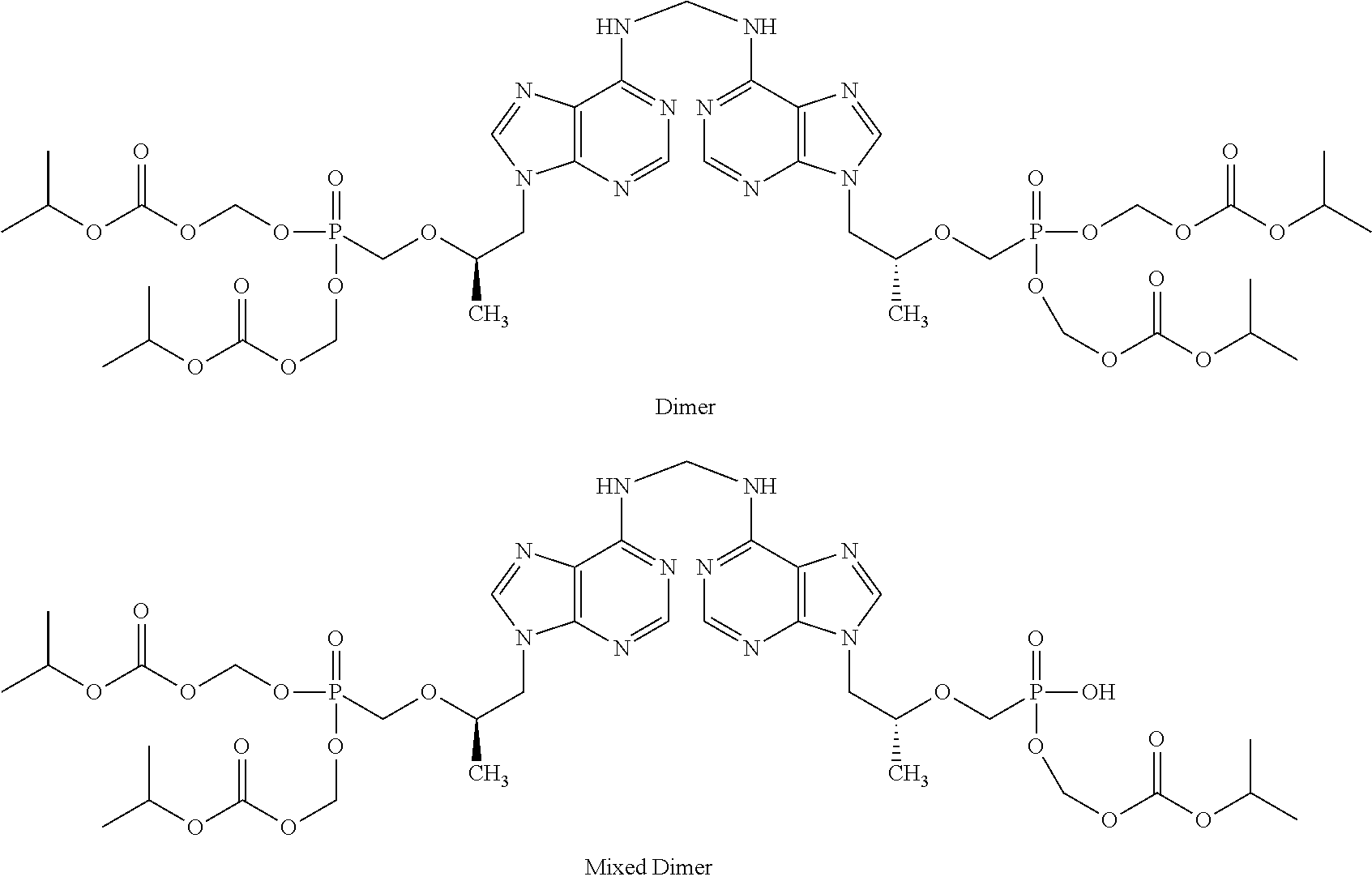
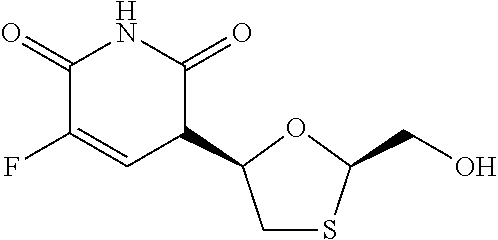
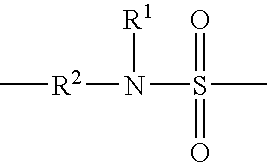Patents
Literature
181results about How to "Formulation stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
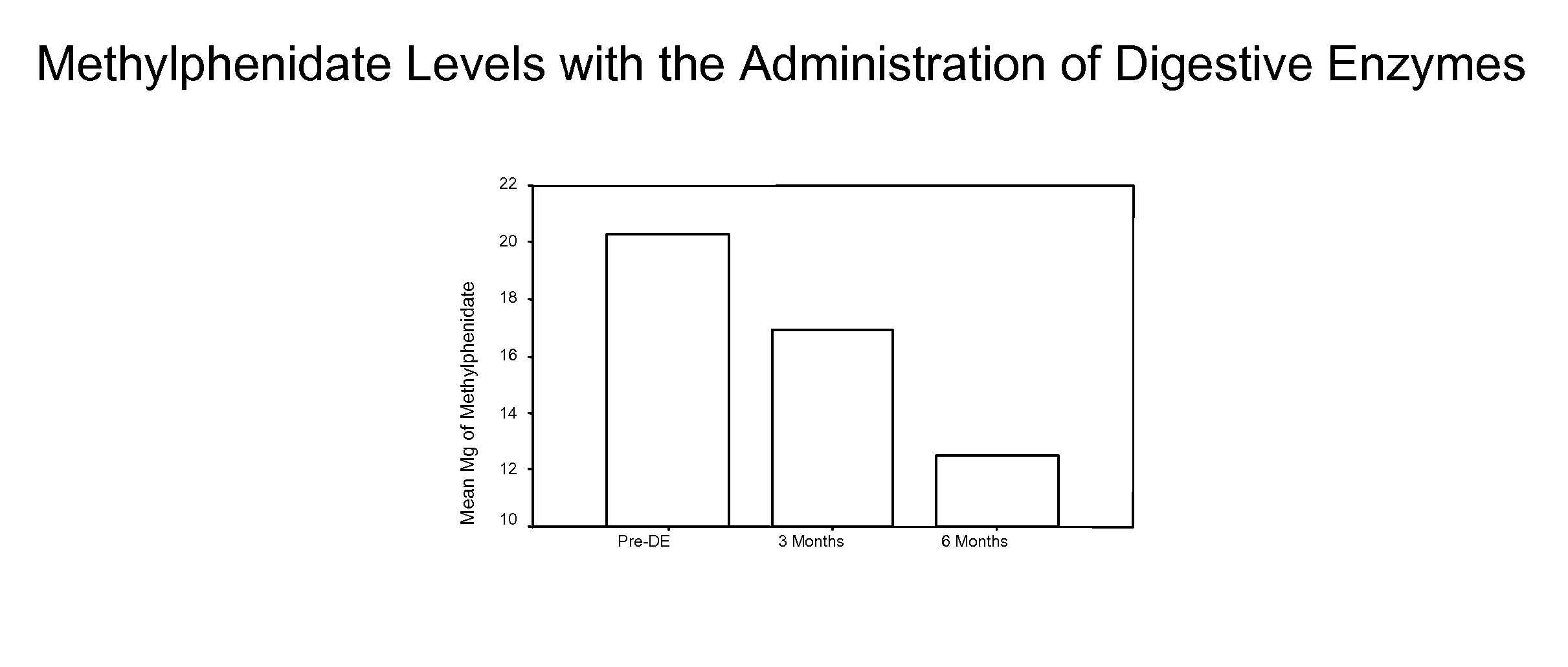
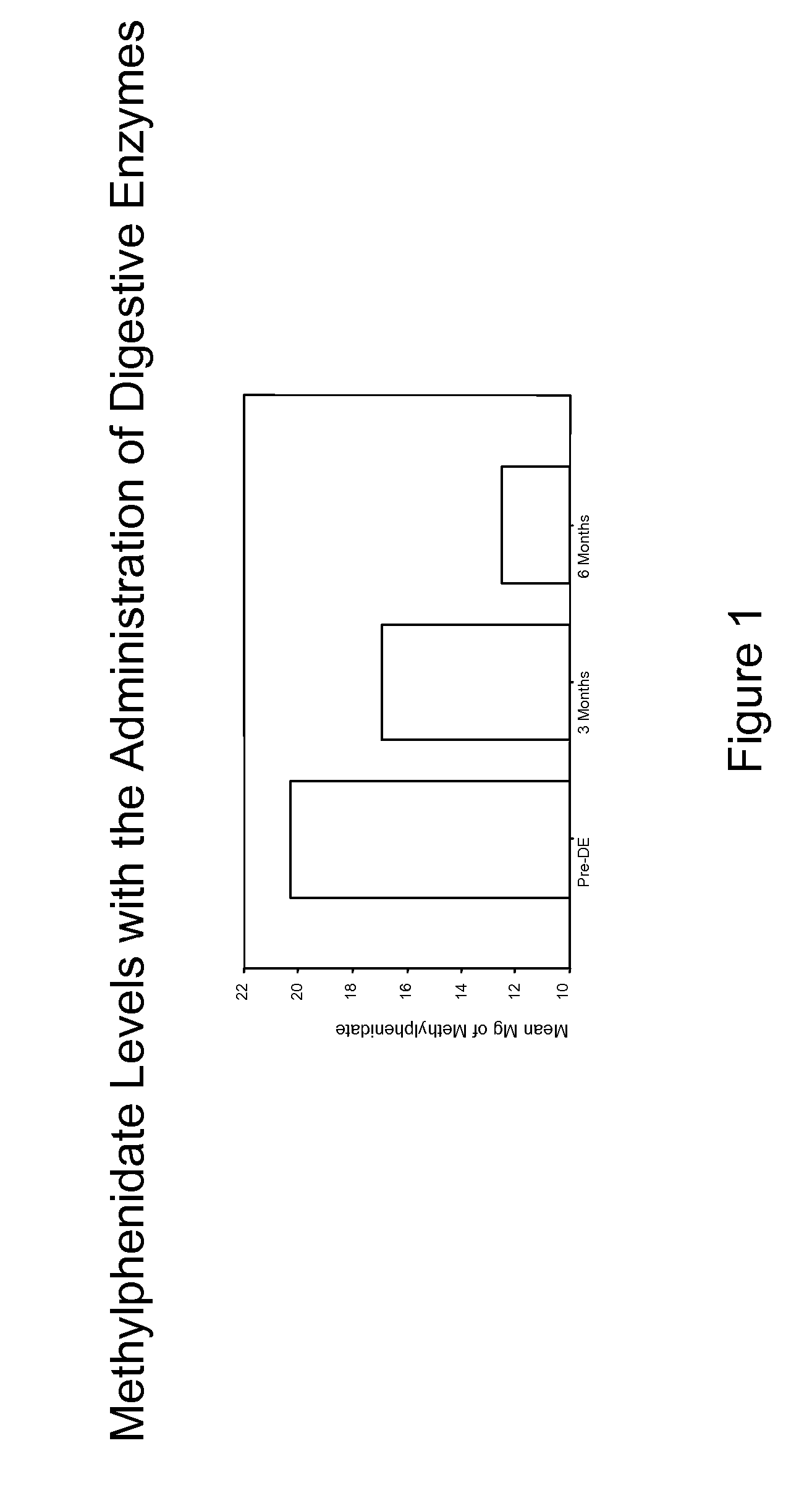
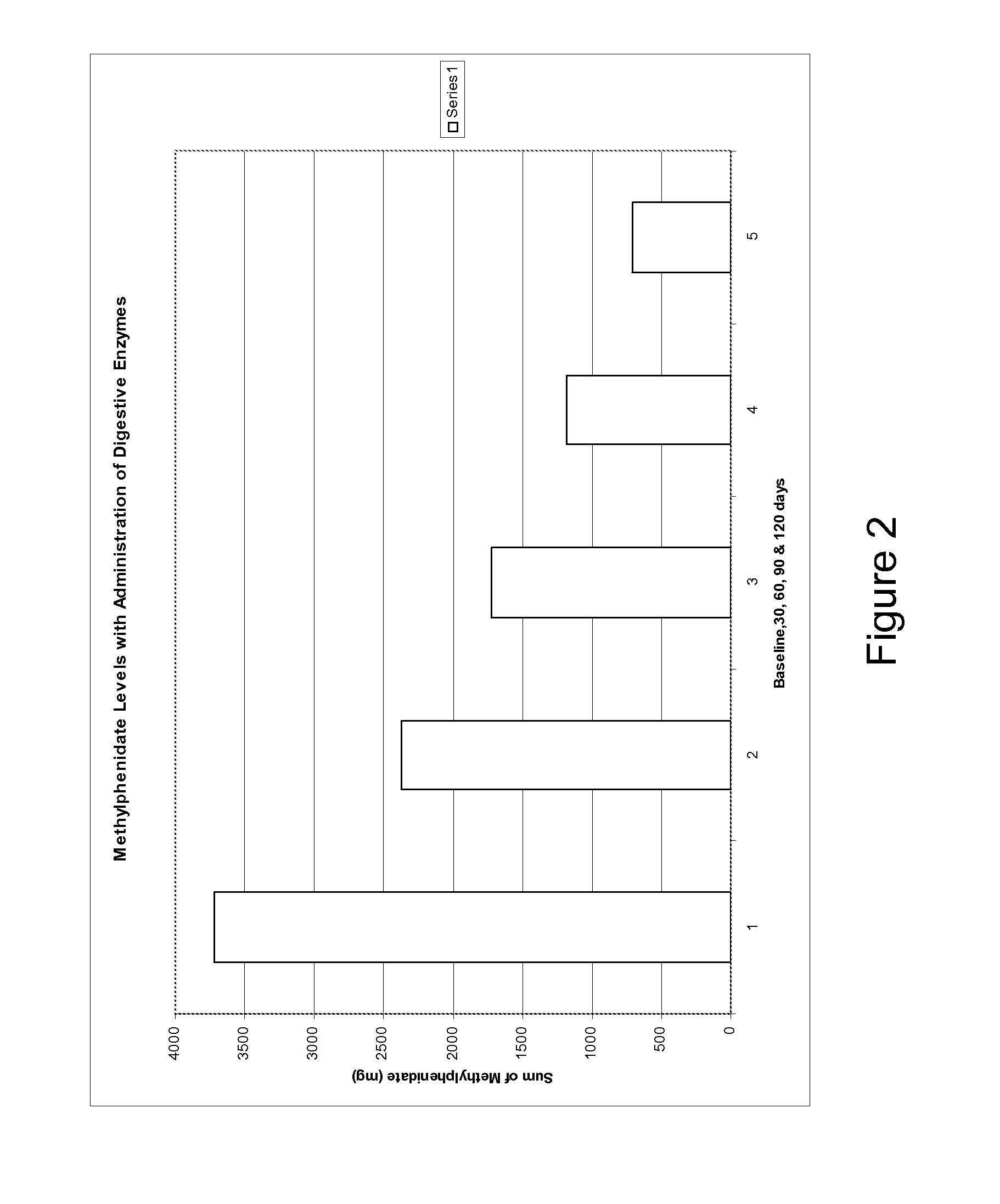
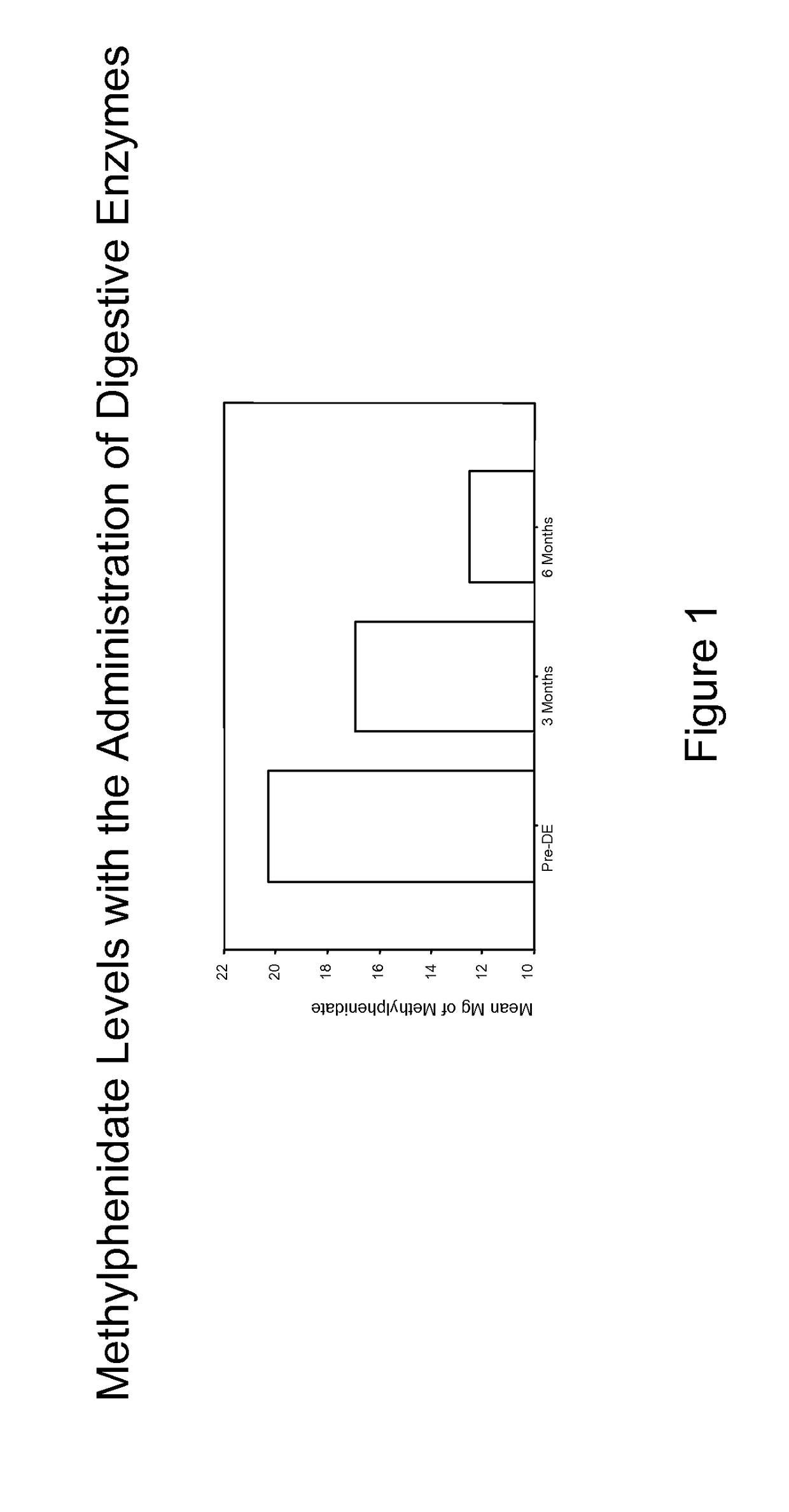
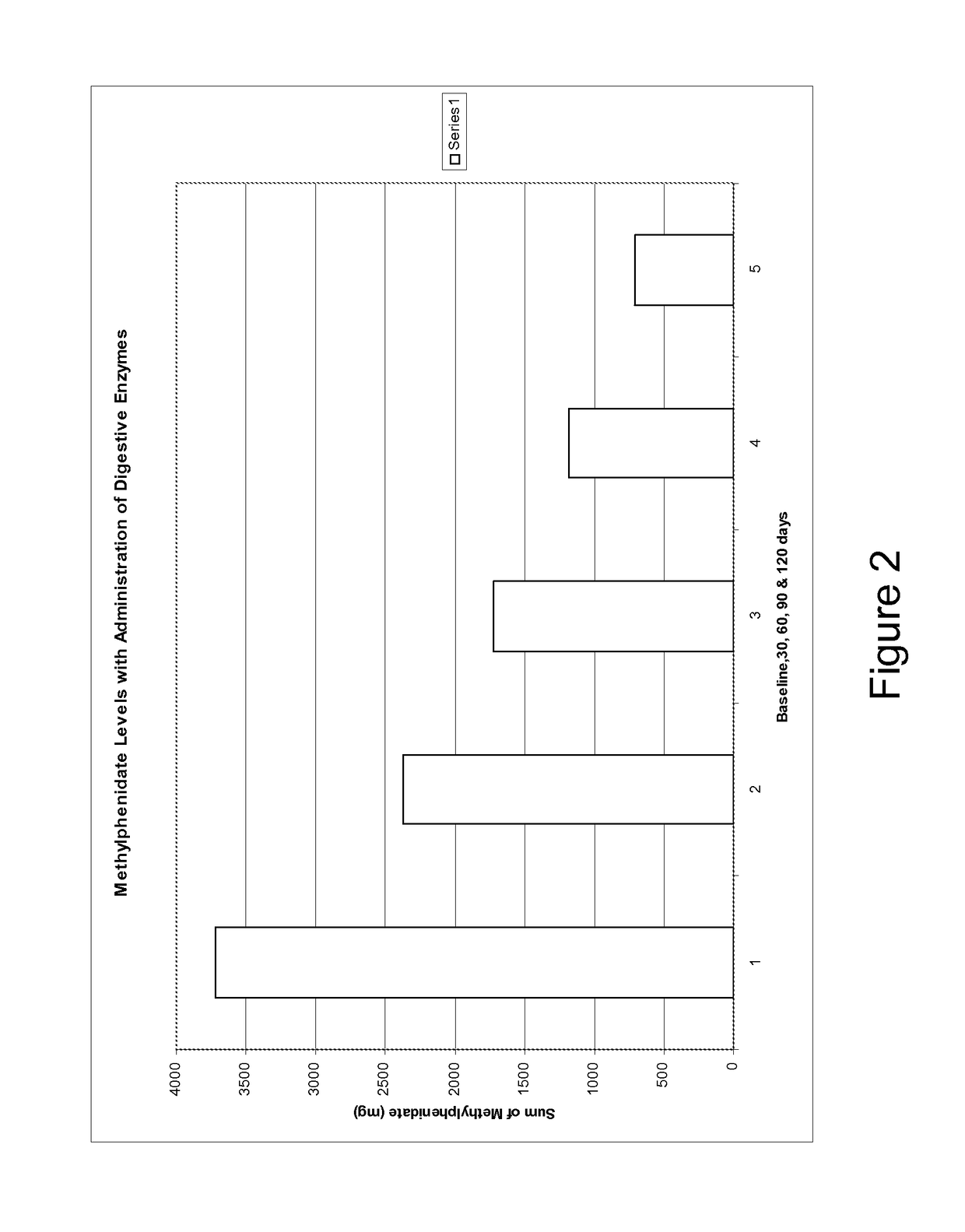
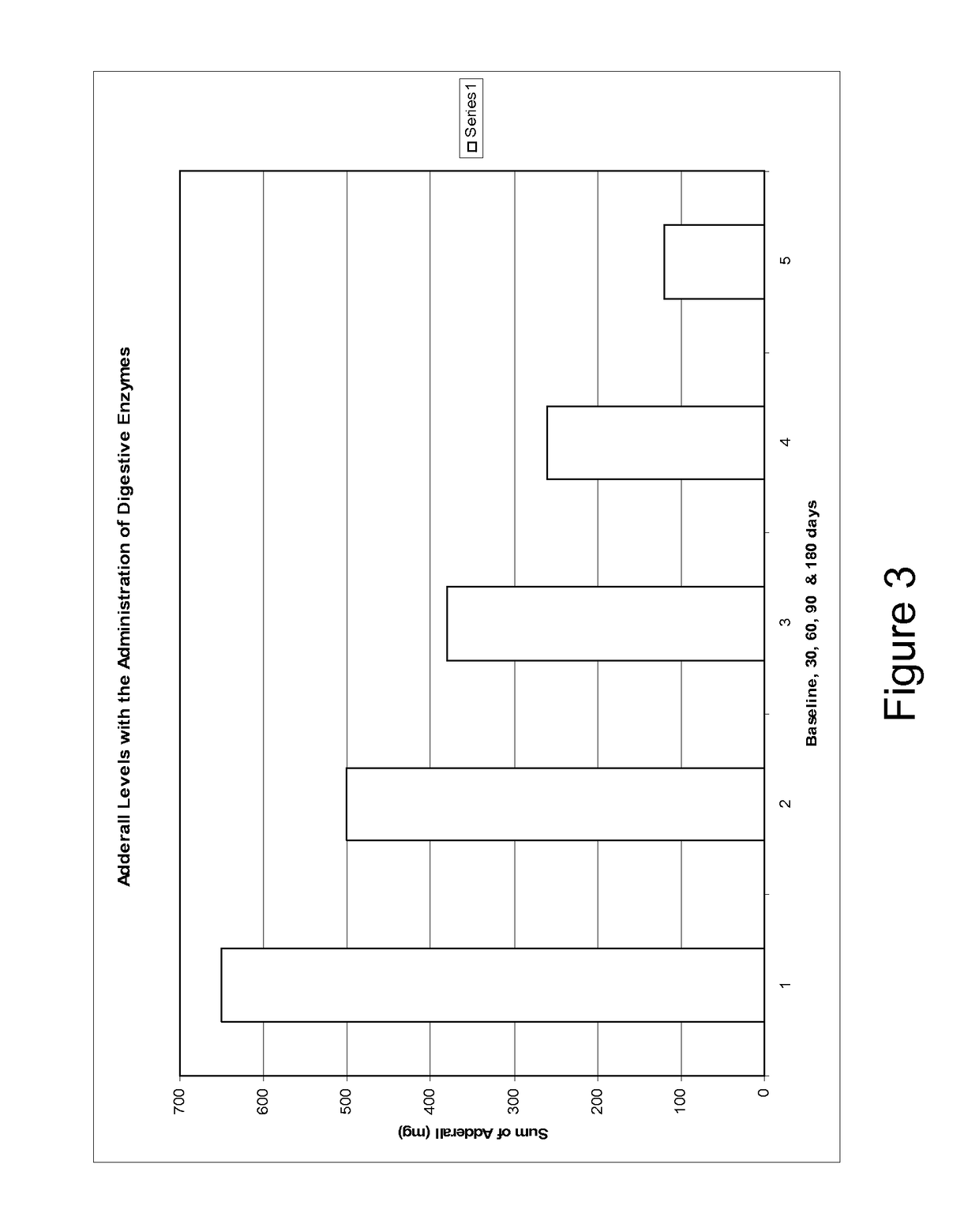
Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders
InactiveUS20070116695A1Well formedFormulation stabilityBiocidePeptide/protein ingredientsChymotrypsinAttention deficits
A pharmaceutical preparation for the treatment of attention deficit disorders combines a therapeutically effective amount of digestive enzymes, such as chymotrypsin, and medication used to treat attention deficit disorders, such as Ritalin®, Concert®, Adderall® and Strattera®. The preparation may be in the form of a tablet, capsule or time released formula in order to reduce the amount of pills per dosage. The pharmaceutical preparation ameliorates the symptoms of the attention deficit disorder. The preparation has a stabilizing matrix containing a solidified microcrystalline cellulose which captures and protects therapeutically effective amounts of digestive enzyme particles within the stabilizing matrix.
Owner:CUREMARK
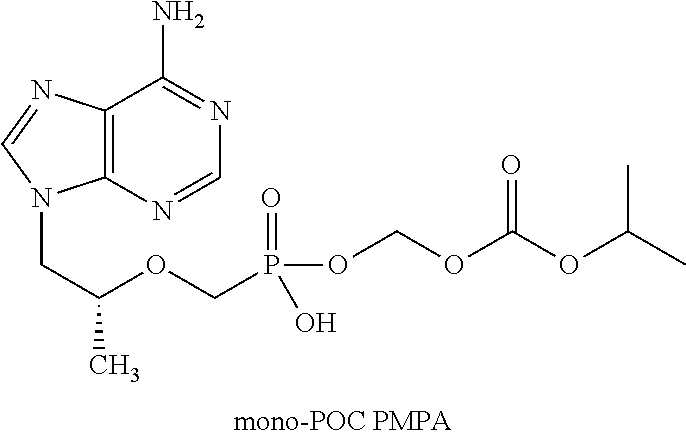
Method and composition for pharmaceutical product
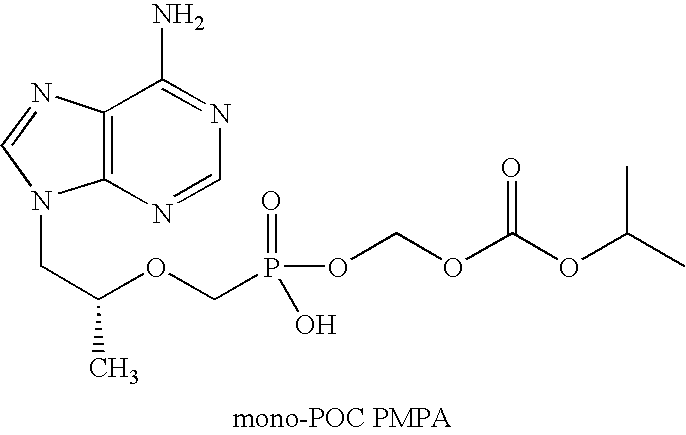
InactiveUS20070077295A1Improve stabilityFormulation stabilityOrganic active ingredientsBiocideMedicineEmtricitabine
This invention is directed to a composition comprising dry granulated tenofovir DF and emtricitabine, and a method for making same. Dry granulation was unexpectedly found to be important in preparing a tenofovir DF containing composition suitable for inclusion in a combination dosage form containing emtricitabine, efavirenz and tenofovir DF.
Owner:GILEAD SCI INC
Combination enzyme for cystic fibrosis
InactiveUS20080166334A1Well formedFormulation stabilityPeptide/protein ingredientsDigestive systemExocrine pancreatic insufficiencyCystic fibrosis lungs
A stable preparation of digestive / pancreatic enzymes which can be readily formed into a dosage formulation is provided as a treatment of pancreatic insufficiency in persons having cystic fibrosis. The dosage formulation can be administered either by an oral preparation including, but not limited to, a microcapsule, mini-capsule, time released capsule, sprinkle or other methodology. A further object of this invention is to provide a stabilized preparation of a combination medicant which resists degradation by light, heat, humidity or association with commonly used excipients.
Owner:CUREMARK
Copolymer for cosmetic preparation
The polymer for cosmetics produced by polymerizing (A) a fluorine-containing (meth)acrylate, and (B) at least one silicon-containing polymerizable compound selected from the group consisting of a mercapto-modified silicone, an azo group-containing silicone and a polymerizable silane can be blended easily in cosmetic preparations and can form a film excellent in a water proofing property, a water- and oil-repellency, feelings in use and safety. This copolymer for cosmetics can improve the drawbacks of fluorine compound-treated powders.
Owner:DAIKIN IND LTD
Topical composition
InactiveUS20050074474A1Formulation stabilityGood skin feelCosmetic preparationsMake-upSolubilityChemical composition
Disclosed is a topical composition comprising: (1) a porous spherical disintegrative silica impregnated with a water-insoluble skin benefit agent, wherein: (a) the porous spherical disintegrative silica has an average volume particle size of from about 3 μm to about 20 μm, a maximum particle size of no more than about 50 μm, and a pore volume of from about 1.5 cm3 / g to about 3.0 cm3 / g; and provides a certain dynamic viscoelasticity when sheared; (b) the water-insoluble skin benefit agent having a solubility in water at less than about 0.1 g / l at 25° C. and having a molecular weight of no more than about 5,000, selected from the group consisting of liquid water-insoluble skin benefit agents, solid water-insoluble skin benefit agents which dissolve in liquid water-insoluble skin benefit agents, solid water-insoluble skin benefit agents which dissolve in emollients and / or volatile solvents, and mixtures thereof; and (2) a suitable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
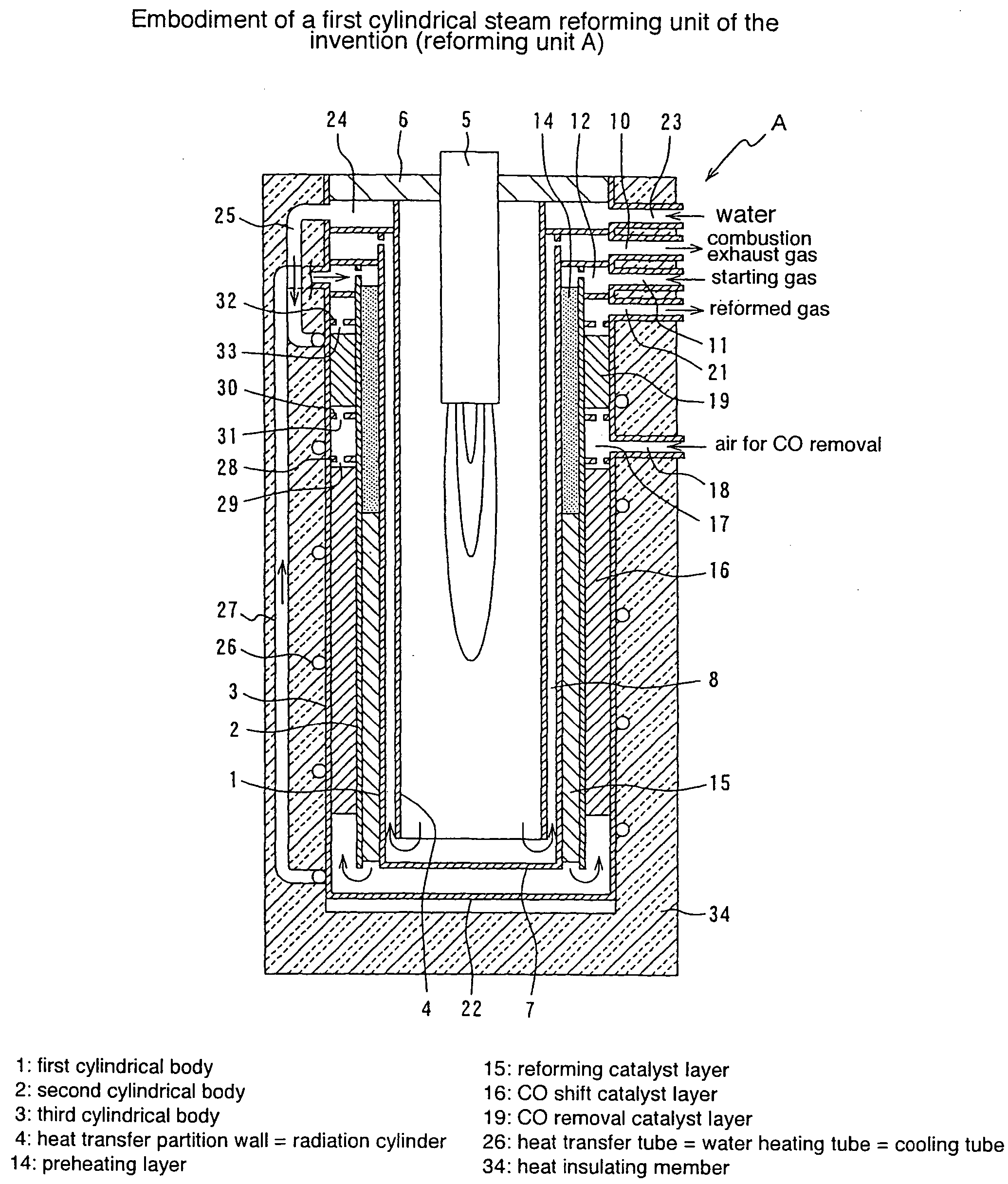
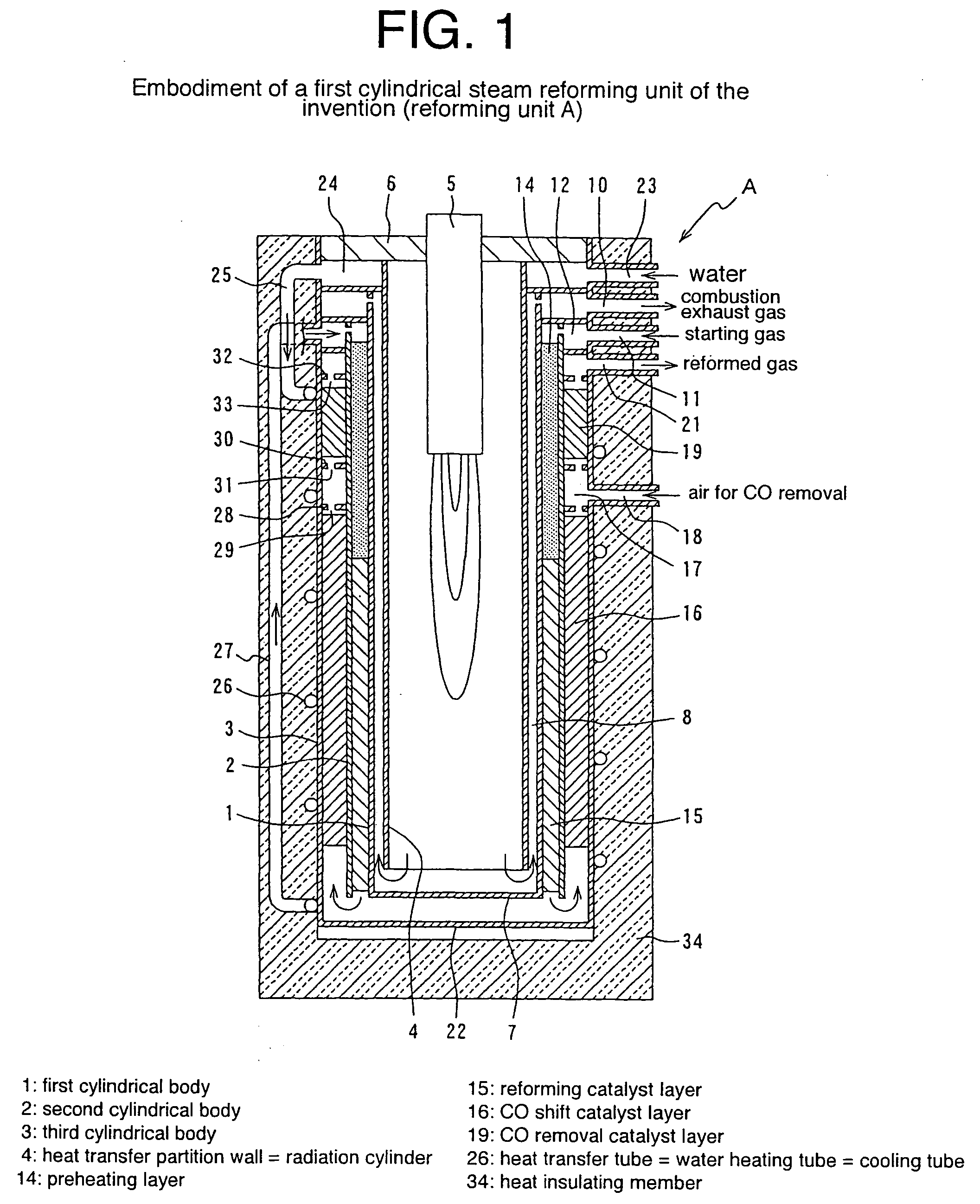
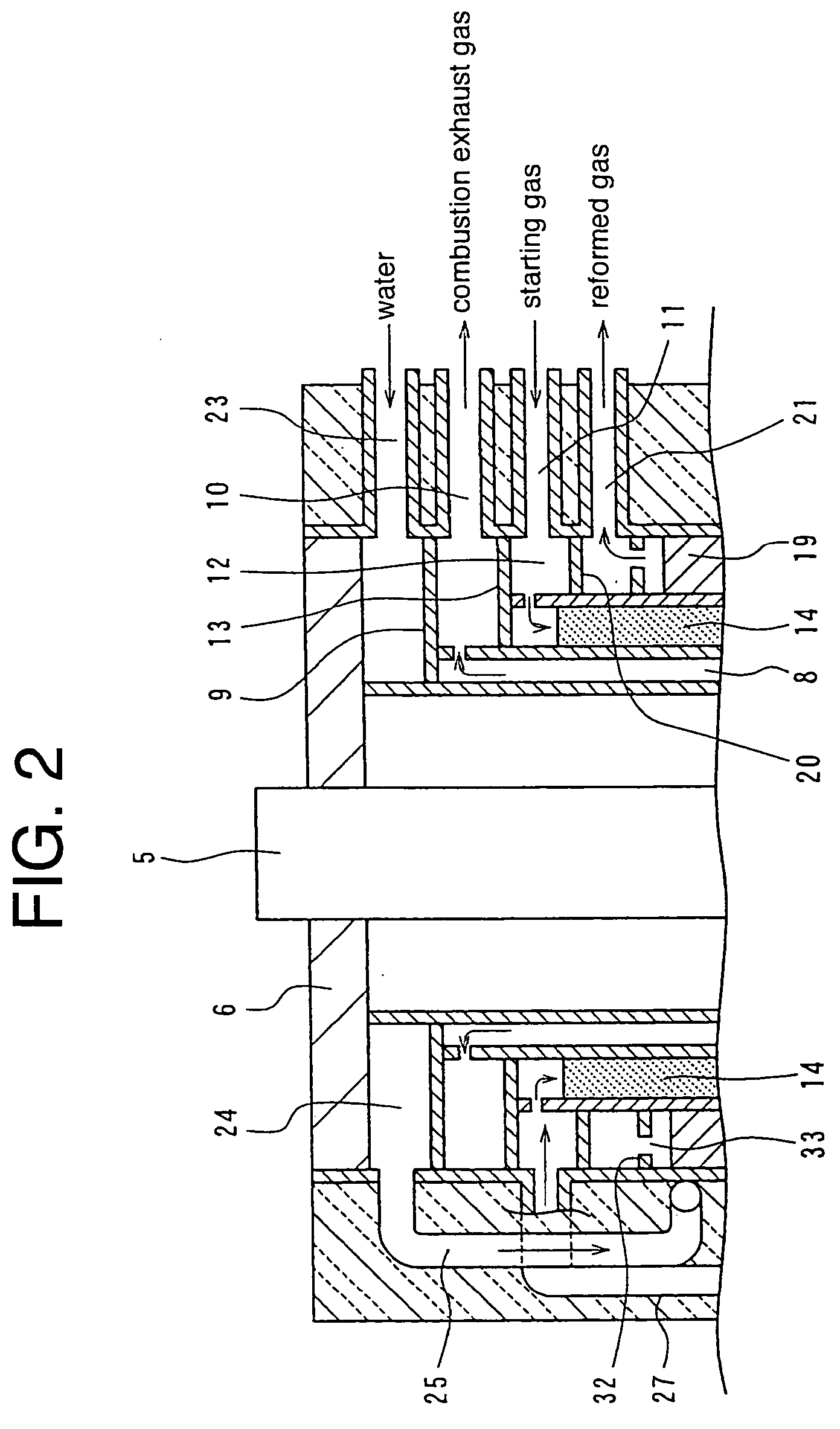
Cylindrical water vapor reforming unit
InactiveUS20040144029A1Small sizePass smoothlyHydrogen/synthetic gas productionChemical/physical/physico-chemical processesSteam reformingInsulation layer
A cylindrical steam reforming unit comprising a plurality of cylindrical bodies consisting of a first cylindrical body, a second cylindrical body and a third cylindrical body of successively increasing diameters disposed in a concentric spaced relation, a radiation cylinder disposed within and concentrically with the first cylindrical body, a burner disposed in the radial central portion of the radiation cylinder, and a reforming catalyst layer with a reforming catalyst filled in a gap between the first and second cylindrical bodies, wherein a CO shift catalyst layer and a CO removal catalyst layer are disposed in a gap between the second and third cylindrical bodies, the CO shift catalyst layer being formed in a gap with the direction of flow reversed at one axial end of the reforming catalyst layer and through a heat recovery layer of predetermined length. According to this reforming unit, without internally disposing a heat insulation layer, a cooling mechanism or the like, the reforming catalyst layer, CO shift catalyst layer, and CO removal catalyst layer can be integrated, achieving various useful effects, including size and weight reductions and the shortening of startup time.
Owner:TOKYO GAS CO LTD
Sulfonated derivative of andrographolide and combination of medication
ActiveCN1687049AClear structureGood antibacterialAntibacterial agentsPowder deliveryDiseaseTonsillitis
The present invention discloses II kinds of andrographolide sulfonated derivatives with the actions of resisting bacteria, relieving inflammation and reducing fever and medicine composition containing them. They can be used for preparing freeze-dried powder, injection or oral preparation, and can be used for curing the diseases of pneumonia, bronchitis, tonsillitis and bacillary dysentery, etc.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Rapidly disintegrating tablet comprising an acid-labile active ingredient
InactiveUS7147869B2Improve stabilityUniform deliveryBiocideDigestive systemOral medicationTriglyceride
A rapidly disintegrating tablet for oral administration of acid-labile active ingredients is described. The rapidly disintegrating tablet for oral administration of an acid-labile active ingredient comprises a plurality of individual active ingredient units together with pharmaceutical excipients, where the acid-labile active ingredient is present in the individual active ingredient units in a matrix composed of a mixture comprising at least one solid paraffin and one or more substances from the group of fatty alcohol, triglyceride and fatty acid ester, and where excipients which, on oral intake of the tablet, bring about rapid disintegration of the tablet are present.
Owner:TAKEDA GMBH
Pharmaceutical preparation comprising an active dispersed on a matrix
InactiveUS7175854B2Improve stabilityUniform deliveryBiocidePowder deliveryEngineeringBULK ACTIVE INGREDIENT
The present invention relates to the field of pharmaceutical technology and describes a novel advantageous preparation for an active ingredient. The novel preparation is suitable for producing a large number of pharmaceutical dosage forms. In the new preparation an active ingredient is present essentially uniformly dispersed in an excipient matrix composed of one or more excipients selected from the group of fatty alcohol, triglyceride, partial glyceride and fatty acid ester.
Owner:ASTRAZENECA AB
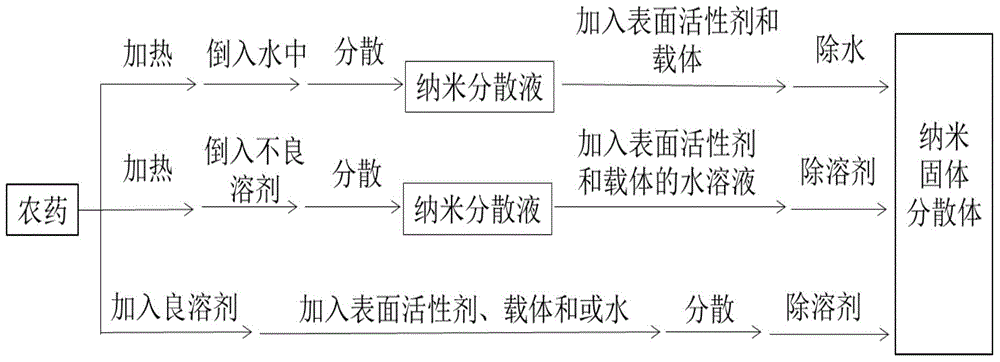
Pesticide nano-solid dispersion and preparation method thereof
ActiveCN104798772ASolution to short lifeGood coating effectBiocideAnimal repellantsOrganic solventEcological safety
The invention relates to a pesticide nano-solid dispersion and a preparation method thereof. The pesticide nano-solid dispersion comprises 0.001-90 parts of a pesticide by weight, 0.001-50 parts of a surfactant and 5-99.9 parts of a water-soluble carrier, wherein the pesticide is selected from one or more insoluble insecticides, bactericides, herbicides or plant growth regulators; the particle size of the solid dispersion is smaller than 1 mu m. Compared with the prior art, the dispersion and the preparation method have the benefits as follows: the pesticide dispersion particle size is smaller and more uniform, the coating effect of the surfactant on the dispersion is better, the content of the surfactant can be lower than 1% and even lower than 0.1%, the good dispersion property and the good stability are kept, organic solvents can be completely avoided, and the production cost is significantly saved; the pesticide usage amount can be reduced by increasing the effective utilization rate, agricultural product residues such as pesticide residues, harmful solvents and aids as well as environmental pollution can be significantly reduced, and grain, food and ecological safety can be guaranteed.
Owner:INST OF ENVIRONMENT & SUSTAINABLE DEV IN AGRI CHINESE ACADEMY OF AGRI SCI
Lipid preparation, particularly cosmetic preparation
InactiveUS20060165645A1Simple processFormulation stabilityBiocideCosmetic preparationsLipid formationMedicine
A lipid-bearing preparation and method for preparing same in particular in the form of a stick or a workable paste, which is suitable for cosmetic uses, in particular in the field of decorative cosmetics for coloring and improving the appearance of the skin, the lips and the eyelids. The lipid-bearing preparation is in water-free form and contains in the lipid phase exclusively vegetable-base raw materials.
Owner:SCHWAN STABILO COSMETICS
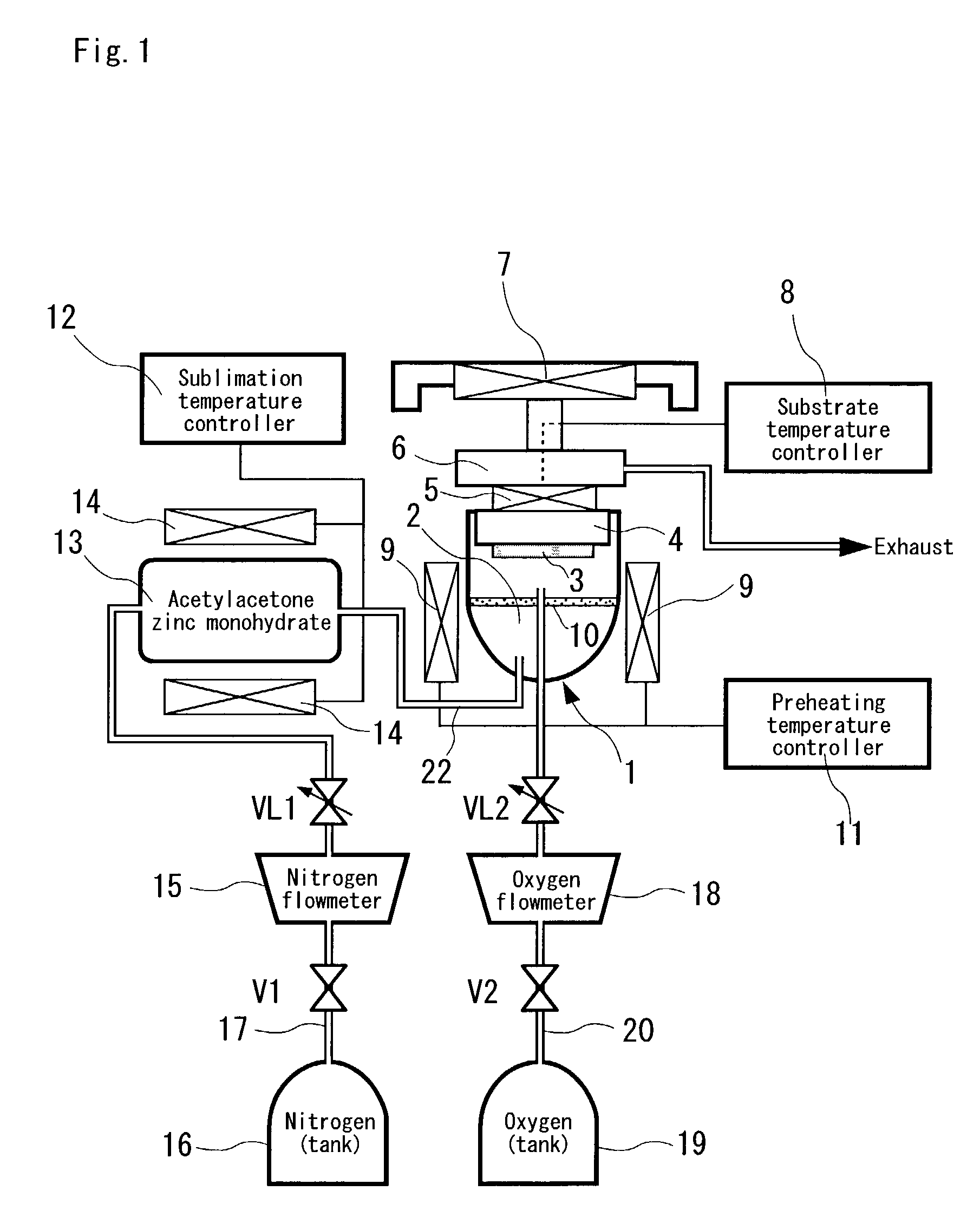
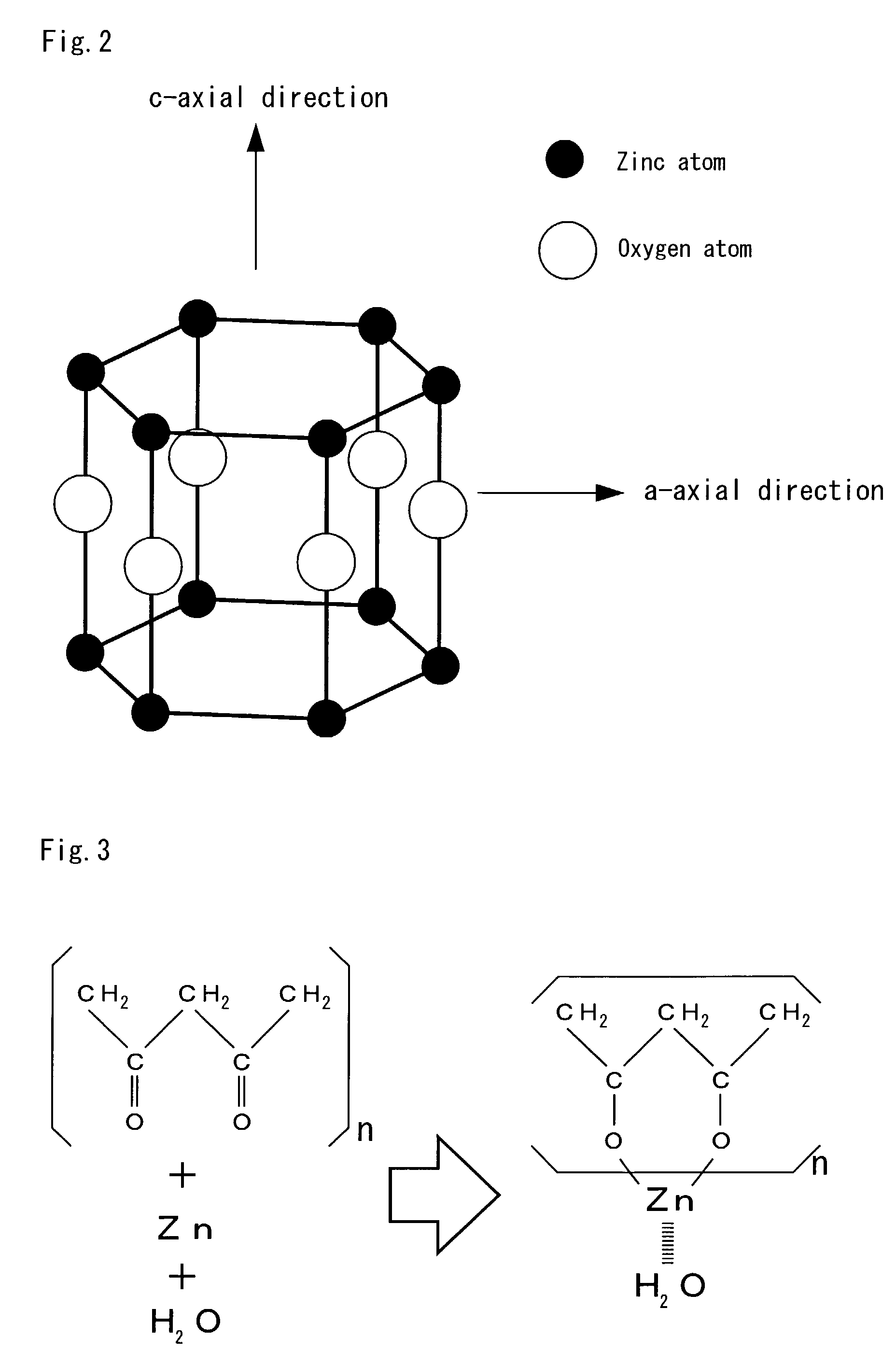
Zinc oxide semiconductor material
InactiveUS6936188B1Excellent in doping characteristicFormulation stabilityLight-sensitive devicesFrom solid stateCrystal orientationOxygen
A zinc oxide semiconductor material comprising at least zinc and oxygen as constituent elements, which can be deterred with respect to the deterioration of doping characteristic, luminous characteristic and the like, compared with a conventional c-axial oriented one by orienting the crystal orientation plane to a-axis of the wurtzite structure.
Owner:TOHOKU TECHNO ARCH CO LTD +1
Melt-Coated Dosage Forms
InactiveUS20110217289A1High active ingredient loadingGood stabilityBiocidePretreated surfacesSolventFoam Dosage Form
Formulations of sparingly water-soluble active ingredientscomprising carrier particles provided with active ingredient-containing coatings, the sparingly soluble active ingredients being embedded in coatings composed of amphiphilic copolymers, and the coatings being applied in the form of a solvent-free melt.
Owner:BASF SE
Pharmaceutical preparation comprising an active dispersed on a matrix
InactiveUS20070122474A1Improve stabilityUniform deliveryPowder deliveryOrganic non-active ingredientsTriglycerideEngineering
The present invention relates to the field of pharmaceutical technology and describes a novel advantageous preparation for an active ingredient. The novel preparation is suitable for producing a large number of pharmaceutical dosage forms. In the new preparation, an active ingredient is present essentially uniformly dispersed in an excipient matrix composed of one or more excipients selected from the group of fatty alcohols, triglycerides, partial triglycerides and fatty acid esters.
Owner:ASTRAZENECA AB
Ointment with clindamycin and metronidazole and method for preparing the same
InactiveCN1732969ANon-irritatingNovel compositionAntibacterial agentsOrganic active ingredientsParaffin waxPolyethylene glycol
Disclosed is an ointment using the salts or esters of clindamycinum and metronidazole as the active constituents, the constituents of the ointment include (by weight percent): clindamycinum or its medicinal salts or its esters 0.25-5%, metronidazole 0.2-4%, oleaginous base 10-40%, water soluble base 5-50%, and balancing water, wherein the oleaginous base is selected from stearinic acid, glyceryl monostearate, paraffin wax, fluid wax, Vaseline, lanoline, cetyl alcohol, stearyl alcohol, Span series, bee wax, animal or vegetable fat, the water soluble base is selected from glycerin, propylene glycol, sorbitol, polyethylene glycol series, Tween series, sodium dodecylsulfate, dimethyl sulfoxide, triethanolamine, and ethanol.
Owner:王卫阳
Chinese pulsatilla root granules for preventing and treating livestock and poultry bacterial diseases and preparation method and premix thereof
ActiveCN102028795AEasy to useGood curative effectAntibacterial agentsClimate change adaptationAilanthusAdditive ingredient
The invention relates to Chinese pulsatilla root granules for preventing and treating livestock and poultry bacterial diseases and a preparation method, application and a premix thereof, and belongs to the field of veterinary medicines and premixes. The Chinese pulsatilla root granules consist of medicinal components such as Chinese pulsatilla root, golden thread, amur corktree bark, parslane herb, longhairy antenoron herb, common cephalanoplos herb, ash bark and tree-of-heaven ailanthus bark. The granules are suitable for preventing and treating the livestock and poultry bacterial diseases, has the characteristics of definite curative effect, stable preparation and simple preparation process and the like, and is suitable for industrial production. The Chinese pulsatilla root granules also can be added into a feed to prepare a feed for promoting the growth of livestock and poultry, such as the premix, a batch and the like.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
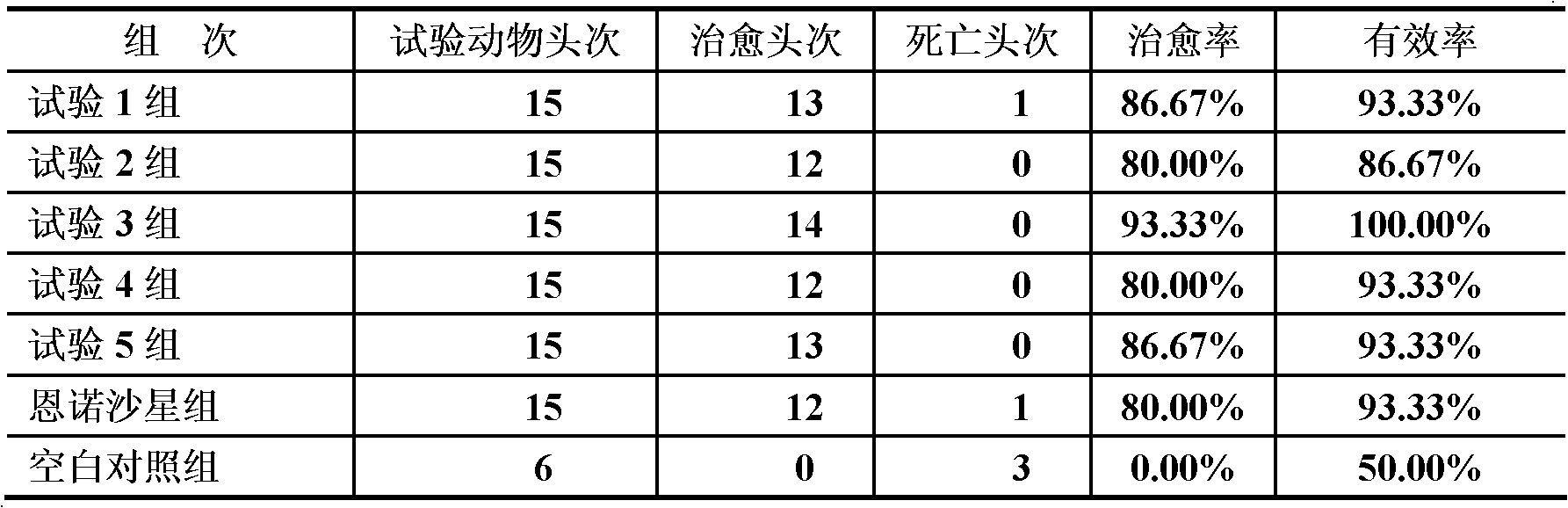
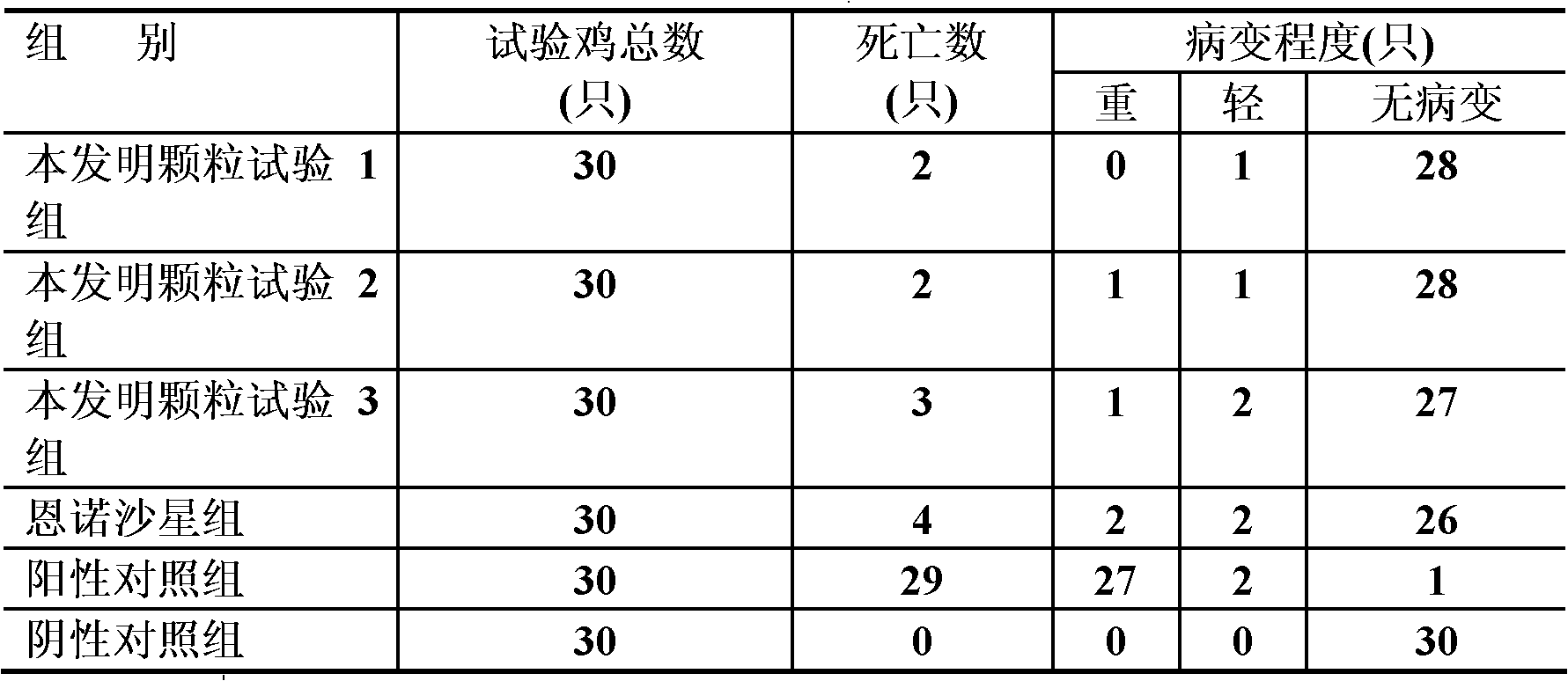
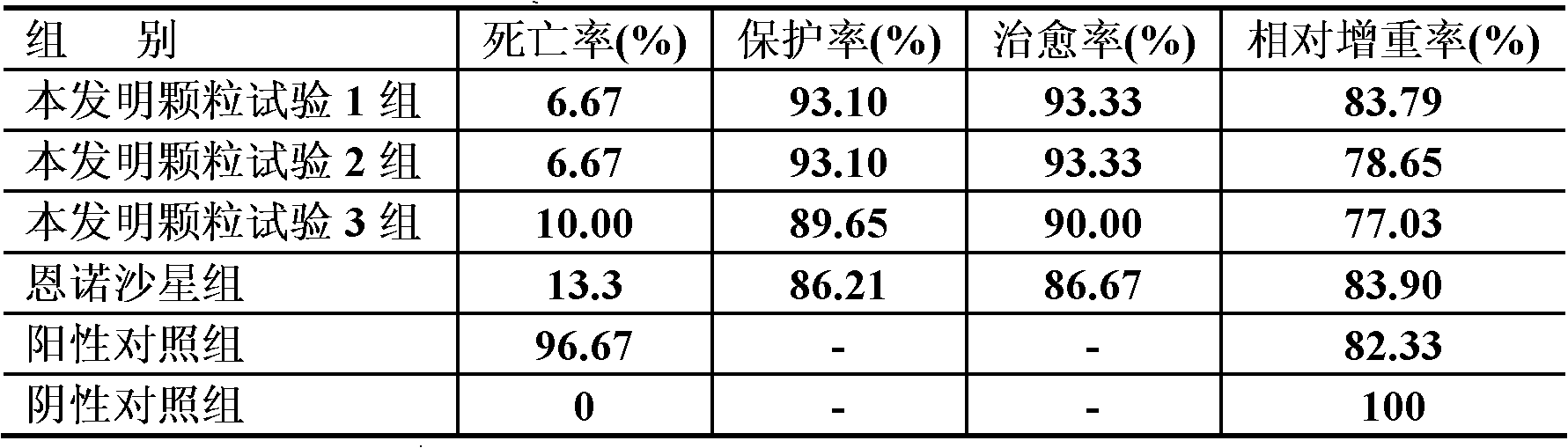
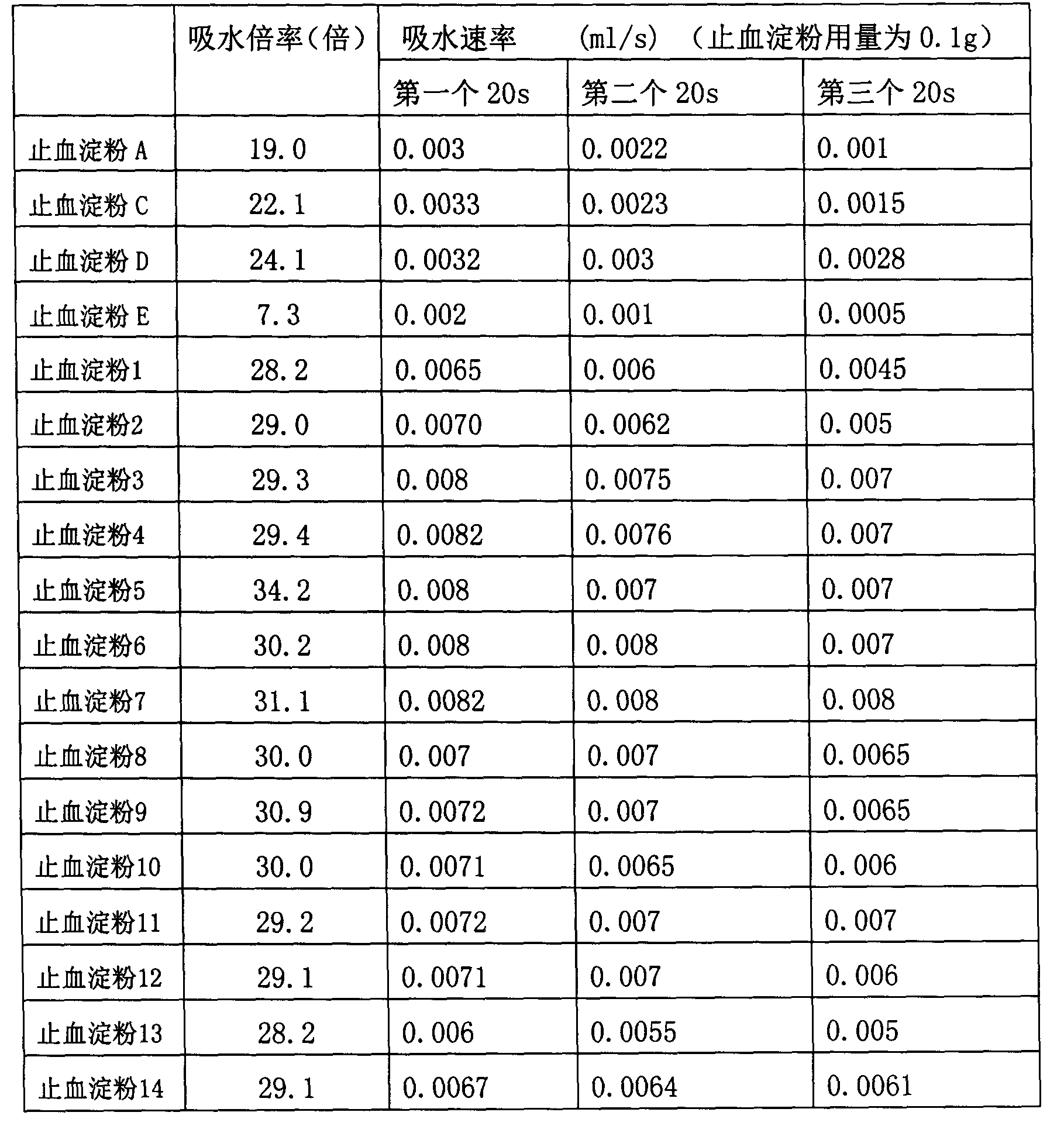
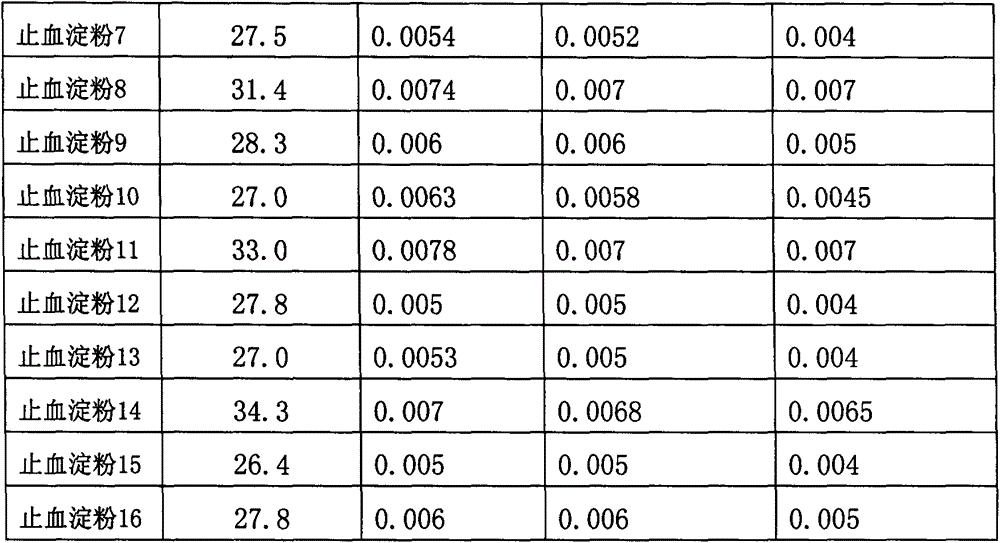
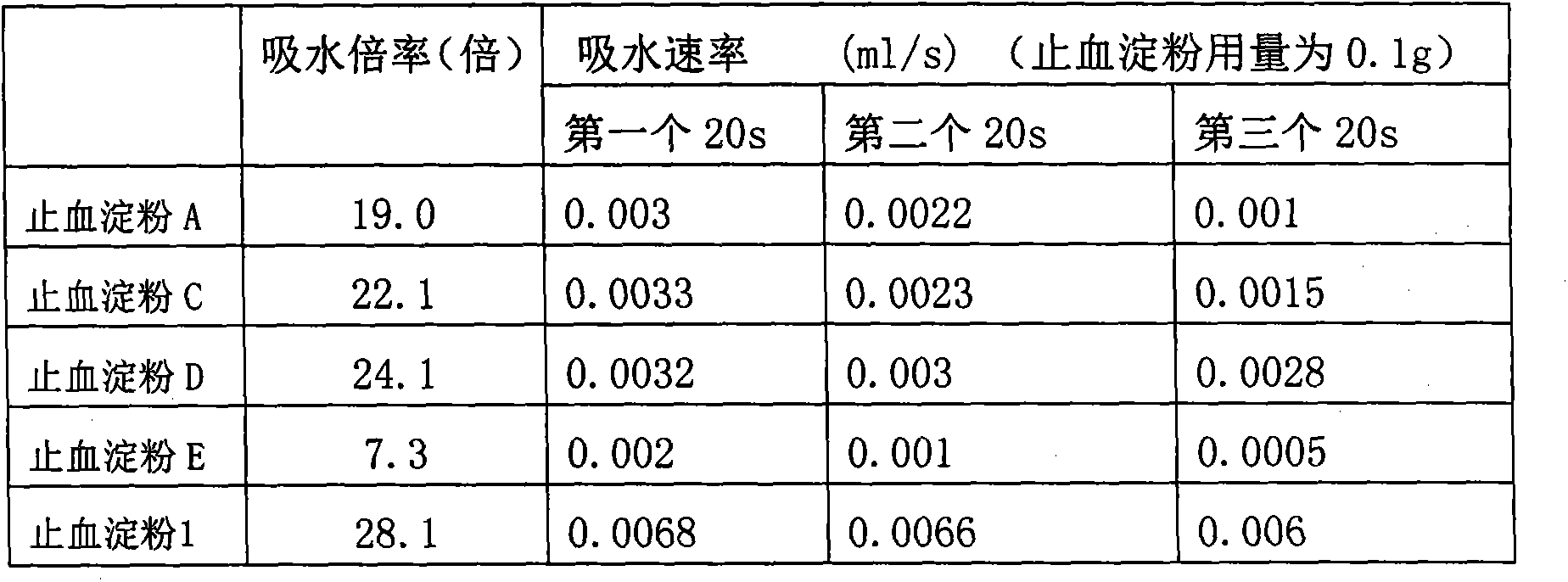
Hemostasis starch and preparation method thereof
ActiveCN103265641AImprove water absorptionImprove water absorption rateSurgeryAbsorbent padsBiocompatibility TestingWound surface
The present invention relates to hemostasis starch and applications thereof, wherein modified starch in the existing technology is subjected to denaturation again, and then is subjected to a ray irradiation treatment to finally prepare the hemostasis starch. The hemostasis starch has characteristics of excellent water absorption effect, safety, stability and biocompatibility, and can be directly used for a wound surface having blood.
Owner:江苏德威兰医疗器械股份有限公司 +1
Long-acting slow release preparation for treating keratomycosis as well as preparation method and application thereof
ActiveCN104940936AHigh drug loadingFormulation stabilityOrganic active ingredientsSenses disorderAntifungalSide effect
The invention relates to a preparation for treating treating keratomycosis, and particularly relates to a long-acting slow release preparation for treating keratomycosis as well as a preparation method and application thereof. The substrate of the long-acting slow release preparation is formed through electrostatic bonding of a graphene material and a drug-carried chitosan material, drugs are carried on the sheet structure of the graphene material, the structure is stable, and the loading rate is high. The preparation provided by the invention has excellent bacteriostatic activity on fungus and bacterium, and is excellent in cytocompatibility, toughness, and tensile strength. The preparation can be simply and conveniently applied onto cornea, and has no toxic effects on normal tissue while achieving excellent bacteriostatic activity. The long-acting slow release preparation is taken as a dosage form in ophthalmology, can be adhered onto a cornea of a patient for long-acting slow release, so as to achieve effective drug concentration, and the preparation has the advantages that the preparation is simple and convenient, the drug-carried material self is excellent in bacteriostatic activity and mechanical property, stimulation and toxic or side effects on orbital tissue can be avoided, and the use is convenient.
Owner:SOUTH CHINA AGRI UNIV
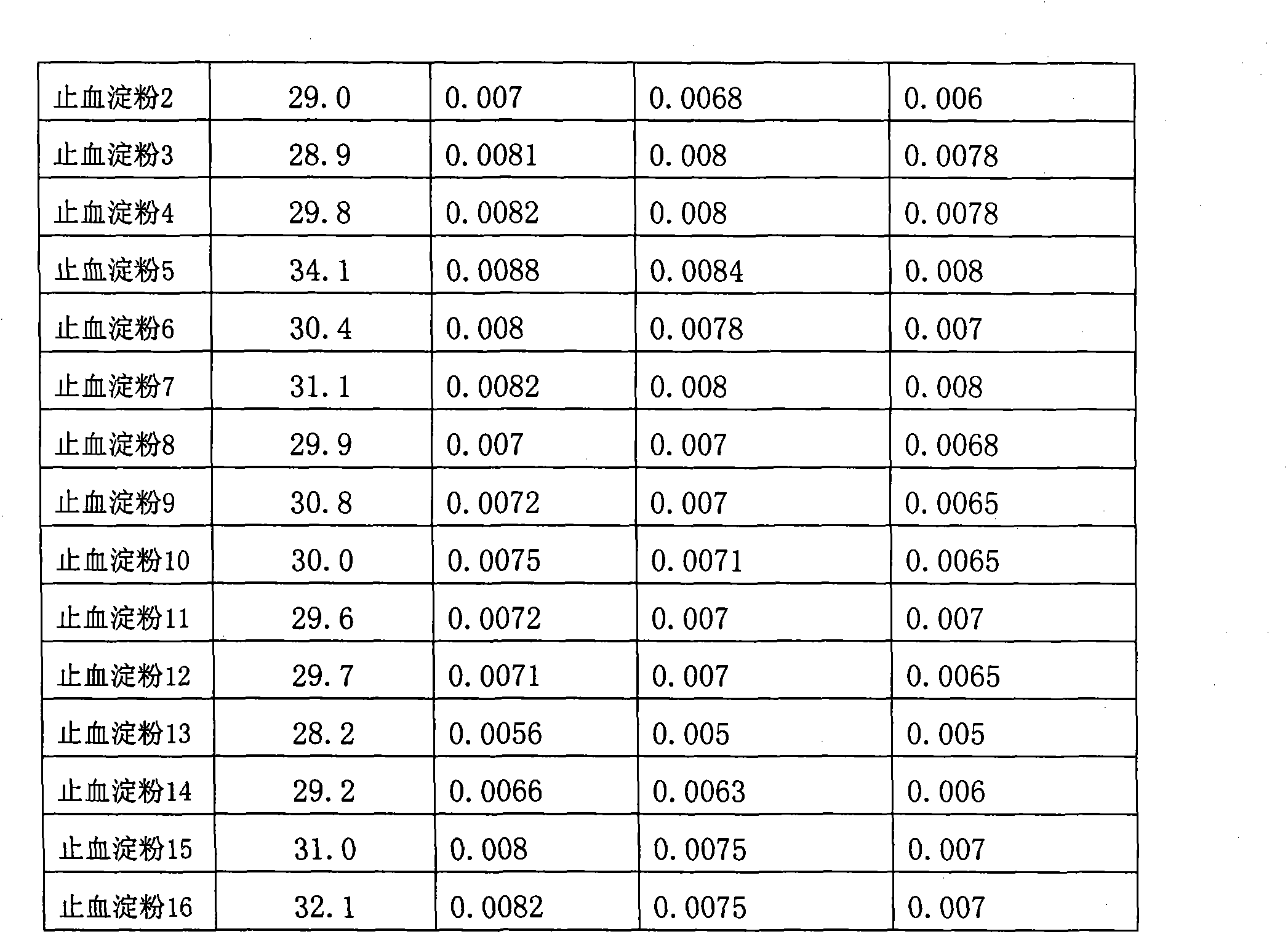
Hemostatic sponge and preparation method thereof
ActiveCN103275344AImprove water absorptionImprove water absorption rateSurgeryAbsorbent padsWound surfaceAbsorption effect
The invention relates to hemostatic sponge and applications of the hemostatic sponge. The hemostatic sponge is prepared from the modified starch in the prior art, which is re-modified and subjected to ultrasonic treatment. The hemostatic sponge is excellent in water absorption effect, safe and stable, and is biocompatible and can be directly applied to bloody wound surfaces.
Owner:江苏德威兰医疗器械股份有限公司 +1
External-use ointment and its preparing method
ActiveCN1813804AFormulation stabilityOvercome the disadvantages of external useAmphibian material medical ingredientsHeavy metal active ingredientsExternal applicationMedicine
The present invention discloses a kind of six spirits ointment for external application, including six spirits adhesive plaster, six spirits rubber plaster, six spirits babu preparation and six spirits ointment. It is made up by using six spirits pill powder as main medicine and adding matrix through a certain preparation process, and has the functions of clearing away heat and toxic material, relieving inflammation and stopping pain and can obtain good therapeutic effect for curing furuncle in children, large carbuncle, acute mastitis, innominate inflammatory swelling, herpes and mammitis, etc.
Owner:雷允上药业集团有限公司
Traditional Chinese medicine extract with heat-clearing, detoxifying, antibacterial and anti-inflammatory functions and preparations and quality control method thereof
InactiveCN103070931AReduce dosageFormulation stabilityAntibacterial agentsComponent separationMedicinal herbsDisease
The invention aims to provide a traditional Chinese medicine extract with heat-clearing, detoxifying, antibacterial and anti-inflammatory functions. The traditional Chinese medicine extract with the heat-clearing, detoxifying, antibacterial and anti-inflammatory functions is prepared by extracting the following medicine raw materials of radix scutellariae, dandelion, herba corydalis bungeanae and isatis root through a scientific and reasonable process method, wherein the mass ratio of radix scutellariae to dandelion to herba corydalis bungeanae to isatis root is (1-3): (3-5):(0.5-2):(1-3). The traditional Chinese medicine extract with the heat-clearing, detoxifying, antibacterial and anti-inflammatory functions has the advantages the impurity ingredients in the medicine materials are maximumly removed, the effective ingredients are fully remained, and the heat-clearing, detoxifying, antibacterial and anti-inflammatory effects are realized. The invention also discloses four preparations containing the traditional Chinese medicine extract with the heat-clearing, detoxifying, antibacterial and anti-inflammatory functions, i.e. syrup, mixture, buccal tablet and dripping pill. The four preparations have the advantages that the heat-clearing, detoxifying, antibacterial and anti-inflammatory functions are realized, the preparations can be clinically used for preventing and controlling diseases, such as furuncle, pharyngitis, parotitis, lymphadenitis and tonsillitis, the content of effective ingredients is high, the dosage is small, the preparations are stable, and the therapeutic effect is good. The invention also provides a quality control method of the preparations containing the traditional Chinese medicine extract with the heat-clearing, detoxifying, antibacterial and anti-inflammatory functions.
Owner:朱柏丞
Hemostasis starch and preparation method thereof
ActiveCN103333261AImprove water absorptionImprove water absorption rateSurgeryAbsorbent padsBiocompatibility TestingWound surface
The present invention relates to hemostasis starch and applications thereof, wherein modified starch in the existing technology is subjected to denaturation again, and then is subjected to an ultrasonic treatment to finally prepare the hemostasis starch. The hemostasis starch has characteristics of excellent water absorption effect, safety, stability and biocompatibility, and can be directly used for a wound surface having blood.
Owner:江苏德威兰医疗器械股份有限公司 +1
Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders
InactiveUS20170246265A1Well formedFormulation stabilityPeptide/protein ingredientsPill deliveryChymotrypsinAttention deficits
A pharmaceutical preparation for the treatment of attention deficit disorders combines a therapeutically effective amount of digestive enzymes, such as chymotrypsin, and medication used to treat attention deficit disorders, such as Ritalin®, Concerta®, Adderall® and Strattera®. The preparation may be in the form of a tablet, capsule or time released formula in order to reduce the amount of pills per dosage. The pharmaceutical preparation ameliorates the symptoms of the attention deficit disorder. The preparation has a stabilizing matrix containing a solidified microcrystalline cellulose which captures and protects therapeutically effective amounts of digestive enzyme particles within the stabilizing matrix.
Owner:CUREMARK
Bleeding stopping starch and preparation method thereof
InactiveCN103224568AImprove water absorptionImprove water absorption rateSurgeryAbsorbent padsBiocompatibility TestingAbsorption effect
The invention relates to a bleeding stopping starch and an application thereof. The bleeding stopping starch is prepared through redenaturation of denaturated starch in the prior art, and microwave processing. The bleeding stopping starch has the advantages of excellent water absorption effect, safety and stability, has biocompatibility, and can be directly applied to bleeding wounds.
Owner:BEIJING JIADE SUNSHINE TECH
A compound suspension of molluscicidal drug niclosamide ethanolamine salt and its preparation method
ActiveCN101006778AGrind evenlyReduce interfacial tensionBiocideMolluscicidesNiclosamideSuspending Agents
The invention discloses a snail control insecticide of compound niclosamide ethanolamine salt suspending agent which comprises the constituents (by weight portion) of niclosamide ethanolamine salt 1-50 parts, methaldehyde 0.01-5 parts, dispersing agent 1-20 parts, wetting agent 0.5-20 parts, and thickening agent 0.05-10 parts, and balancing water. The invention also discloses the process for preparing the suspending agent.
Owner:JIANGSU ESSENCE AGROCHEM
High-content toltrazuril soluble powder, as well as preparation method and application thereof
InactiveCN103142487AHigh drug contentImprove bioavailabilityPowder deliveryAntiparasitic agentsSolubilityFreeze-drying
The invention provides a high-content toltrazuril soluble powder, as well as a preparation method and application of the high-content toltrazuril soluble powder, relates to the field of the soluble powders, preparation methods and application of the soluble powders, and aims at solving the problems of the existing preparation method that the toltrazuril preparation is low in water solubility, low in bioavailability, poor in stability, and easy to generate precipitate and discolor so that the treatment effects and application of the toltrazuril preparation are greatly limited. The high-content toltrazuril soluble powder is prepared from toltrazuril, an alkaline pH (Potential of Hydrogen) regulator, a cosolvent and a soluble filler in parts by weight. The preparation method comprises the following steps of: 1, weighing; 2, mixing and preparing solution; and 3 preparing the powder by the spray drying process. The application is the application of the high-content toltrazuril soluble powder serving as the raw material in tablet preparations, granular preparations and freeze-dried powder preparations. The preparation method is simple in preparation process; the prepared powder contains the toltrazuril up to 60% and is quick to dissolve; and the preparations can be placed for three years at the room temperature without packing and discoloring. The high-content toltrazuril soluble powder is applicable to the field of veterinary drug preparations.
Owner:HEILONGJIANG UNIV
Probiotics preparation for conditioning infant intestinal tract and preparation method thereof
InactiveCN110037127AFull of nutritionLight tasteLipidic food ingredientsEdible oils/fatsDeoxygenationDiarrhea
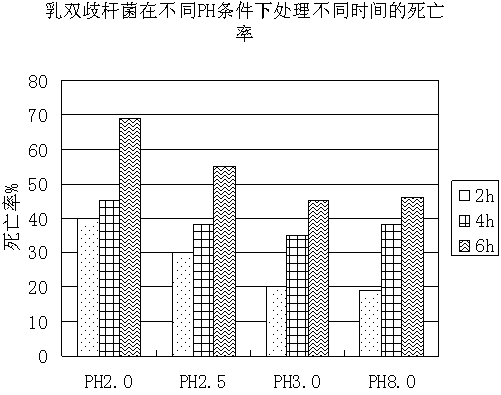
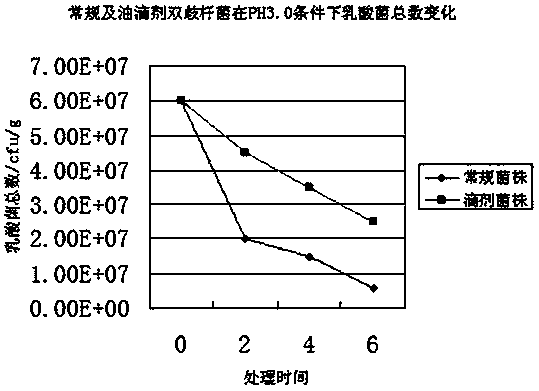
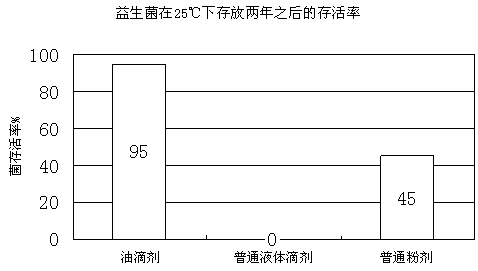
The invention discloses a probiotics preparation for conditioning infant intestinal tracts. The preparation is prepared by the following steps: taking walnut oil or plant blend oil taking walnut oil as a main component as a carrier, adds probiotics and other blend components into the carrier, performing blending at low temperature, and carries out negative-pressure deoxygenation to enable the probiotics to be embedded in the carrier so as to obtain drops. The method is simple, the process is easy to control, and the drops are stable in performance, do not solidify at low temperature, and are uniform in components. The viable bacteria in the probiotic drops are always in a dormant state, and the drops are stable after long-time preservation, light in taste, and natural and safe. After the drops are taken, the drops can withstand the acid and alkali environment in a human body, the survival rate of probiotics is high, the probiotics are rapidly planted and propagated in the intestinal tract of the human body, and the balance of microecology is adjusted, so that various intestinal tract problems are safely, effectively and efficiently solved. The method is suitable for preparing the probiotic preparation, and the preparation is further used for conditioning symptoms such as constipation and diarrhea in intestinal tracts of infants under the age of 3 years.
Owner:彤博士健康产业河北有限公司
Glass compositions
Disclosed is a glass composition which can be suitably used as a glass filler to be blended into a polycarbonate resin. This glass composition contains, in mass %, 50≦SiO2≦60, 8≦Al2O3≦15, 0≦MgO≦10, 10≦CaO≦30, 0≦Li2O+Na2O+K2O<2, and 5<TiO2≦10, and does not substantially contain B2O3, F, ZnO, SrO, BaO and ZrO2.
Owner:NIPPON SHEET GLASS CO LTD
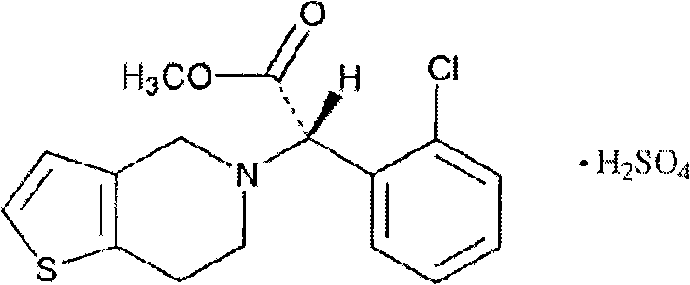
Clopidogrel hydrogen sulfate tablet and preparation method thereof
InactiveCN102247333AImprove liquiditySimple processOrganic active ingredientsBlood disorderAdhesiveMedicine
The invention relates to a preparation for preventing and treating adverse events of atherosclerosis and cardio-cerebrovascular embolism as well as the complications thereof, i.e. a clopidogrel hydrogen sulfate tablet and a preparation method thereof. The tablet comprises clopidogrel hydrogen sulfate, a filler, a disintegrating agent, a lubricating agent, an adhesive, and a film coating premixing agent. The preparation method is characterized by the steps of: weighing the filler, the disintegrating agent in a proportion specified in the prescription, mixing them well, making wet granules with the right amount of the adhesive, drying the wet granules at a temperature of 40-60DEG C, sieving the granules through a sieve of 24 meshes and finishing the granules, adding clopidogrel hydrogen sulfate and the lubricating agent and mixing well, then conducting tabletting, coating and packaging, thus obtaining the tablet. The tablet of the invention has the characteristics of simple prescription and process, good stability, high bioavailability, low cost, high production efficiency and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Method and composition for pharmaceutical product
ActiveUS20140037732A1Improve stabilityFormulation stabilityPowder deliveryOrganic active ingredientsEmtricitabineMedicine
This invention is directed to a composition comprising dry granulated tenofovir DF and emtricitabine, and a method for making same. Dry granulation was unexpectedly found to be important in preparing a tenofovir DF containing composition suitable for inclusion in a combination dosage form containing emtricitabine, efavirenz and tenofovir DF.
Owner:GILEAD SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com