Chinese pulsatilla root granules for preventing and treating livestock and poultry bacterial diseases and preparation method and premix thereof
A Pulsatilla and granule technology, applied in the direction of antibacterial drugs, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the opportunity of expanding the spread of drug resistance, affecting animal food safety, and the mortality rate of sick livestock and poultry. To prevent bacterial diseases of livestock and poultry, avoid drug residues and drug resistance, and achieve good effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
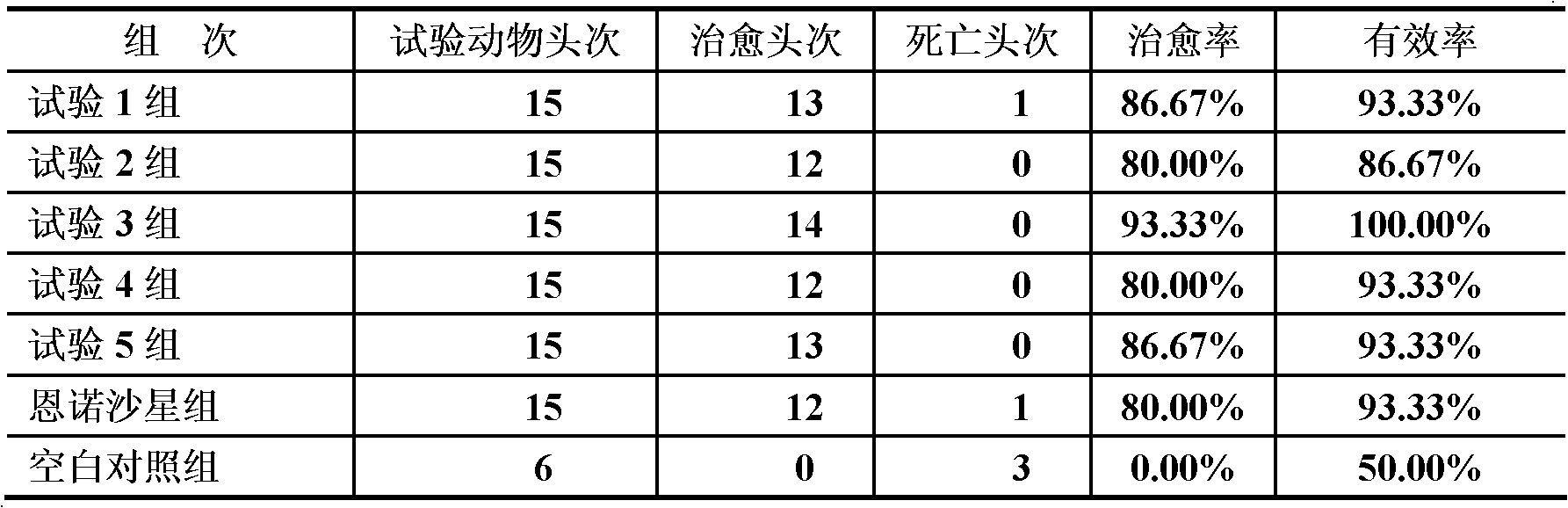
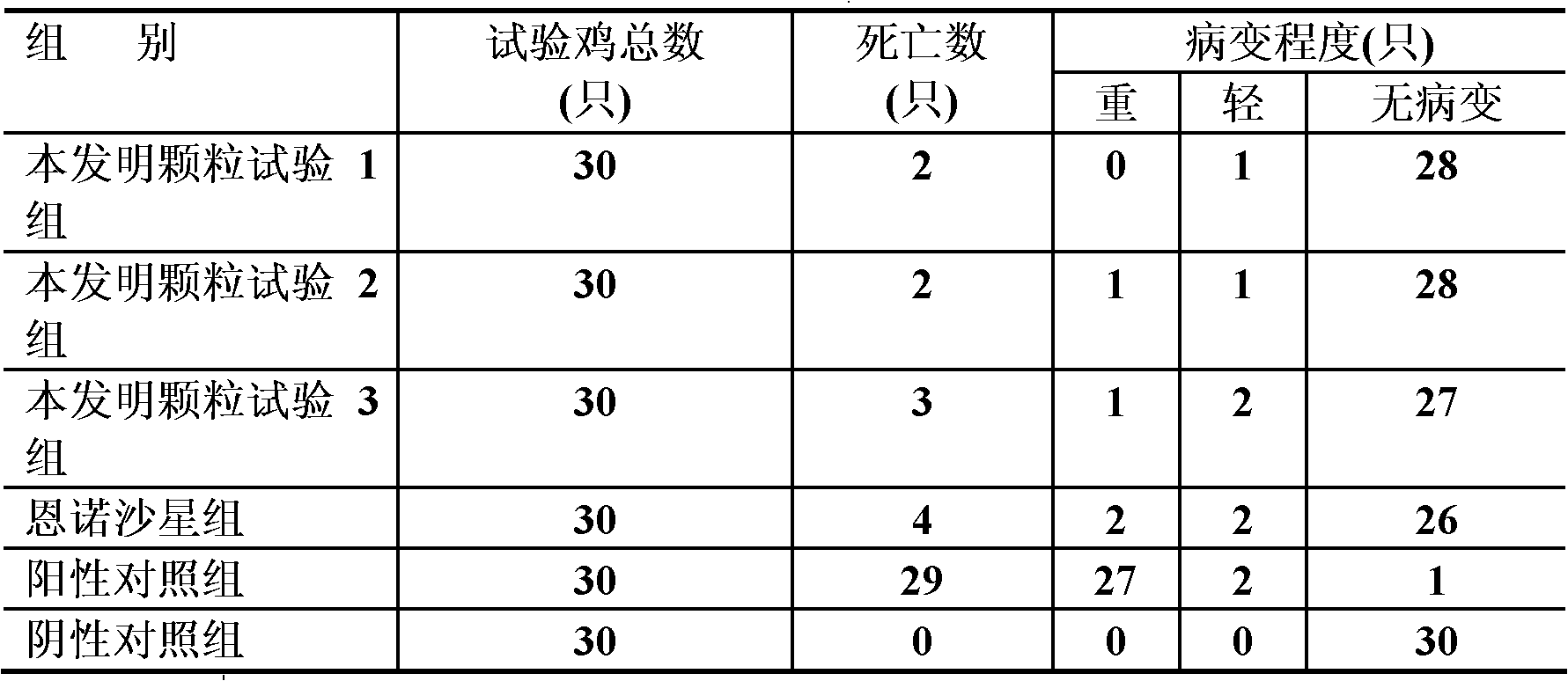
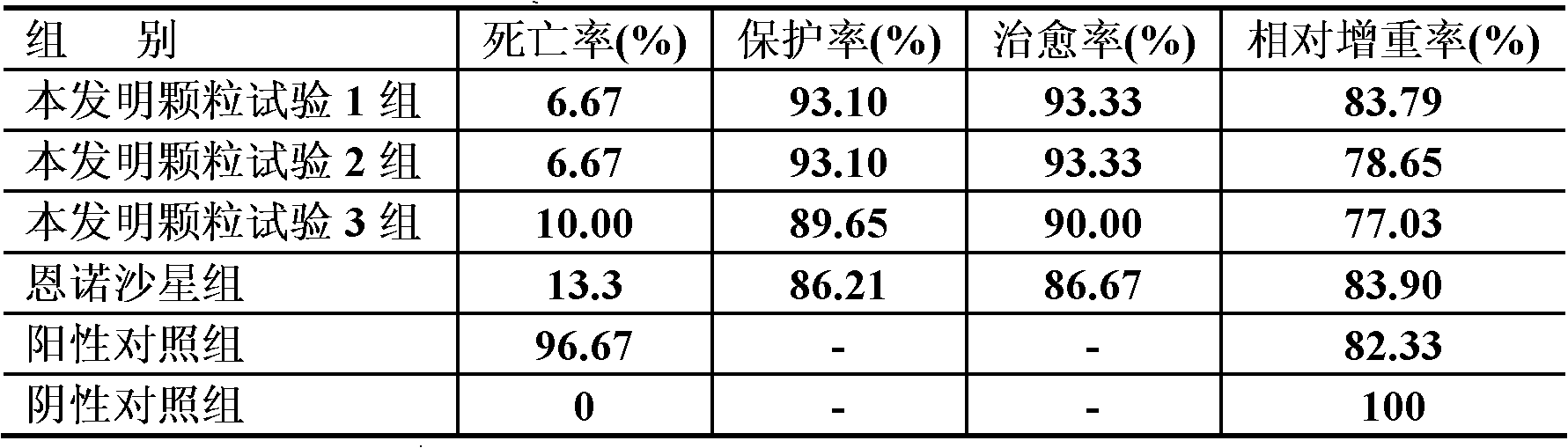
Examples
Embodiment 1
[0027] Embodiment 1 Medicine preparation and use of the present invention
[0028] (1) Weigh raw materials containing the following weights:
[0029] 60 parts of Pulsatilla, 40 parts of Coptidis, 60 parts of Cortex Phellodendri, 60 parts of Purslane, 60 parts of Desmodium, 60 parts of Thistle, 40 parts of Qinpi, and 40 parts of Chunpi.
[0030] (2) add 95% ethanol of 10 times the amount of Pulsatilla chinensis, Coptidis Rhizoma, Cortex Phellodendri, Cortex Chinensis, Cortex Chinensis to 10 times of amount, soak for 30 minutes, reflux and extract twice, each time for 1.5 hours, filter, and the combined filtrate is concentrated to a relative density of 1.05 (60 ℃) clear paste. Add water to decoct the remaining purslane, desmodium, and thistle twice, add 12 times the amount of water for the first time, decoct for 2 hours, add 10 times the amount of water for the second time, decoct for 1.5 hours, filter After passing, the combined filtrate was concentrated to a clear paste with...
Embodiment 2
[0032] Embodiment 2 Medicine preparation and use of the present invention
[0033] (1) Weigh raw materials containing the following weights:
[0034] 20 parts of Pulsatilla, 20 parts of Coptidis, 20 parts of Cortex Phellodendri, 20 parts of Purslane, 20 parts of Desmodium, 20 parts of Thistle, 20 parts of Qinpi, and 20 parts of Chunpi.
[0035] (2) add 95% ethanol of 10 times the amount of Pulsatilla chinensis, Coptidis Rhizoma, Phellodendron Phellodendri, Cortex Chinensis, Cortex Chinensis, soak for 30 minutes, reflux and extract twice, each time for 1.5 hours, filter, and the combined filtrate is concentrated to a relative density of 1.10 (60 ℃) clear paste. Add water to decoct the remaining purslane, desmodium, and thistle twice, add 12 times the amount of water for the first time, decoct for 2 hours, add 10 times the amount of water for the second time, decoct for 1.5 hours, filter After passing, the combined filtrate was concentrated to a clear paste with a relative den...
Embodiment 3
[0037] Embodiment 3 Medicine preparation and use of the present invention
[0038] (1) Weigh raw materials containing the following weights:
[0039] 40 parts of Pulsatilla, 30 parts of Coptidis, 40 parts of Cortex Phellodendri, 40 parts of Purslane, 40 parts of Desmodium, 40 parts of thistle, 30 parts of Qinpi, and 30 parts of Chunpi.
[0040] (2) add 95% ethanol of 10 times of amount to Pulsatilla, Coptidis Rhizoma, Phellodendron Phellodendron, Cortex Chinensis, Cortex Chinensis, soak for 30 minutes, reflux and extract twice, each time for 1.5 hours, filter, and the combined filtrate is concentrated to a relative density of 1.08 (60 ℃) clear paste. Add water to decoct the remaining purslane, desmodium, and thistle twice, add 12 times the amount of water for the first time, decoct for 2 hours, add 10 times the amount of water for the second time, decoct for 1.5 hours, filter After passing, the combined filtrate was concentrated to a clear paste with a relative density of 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com