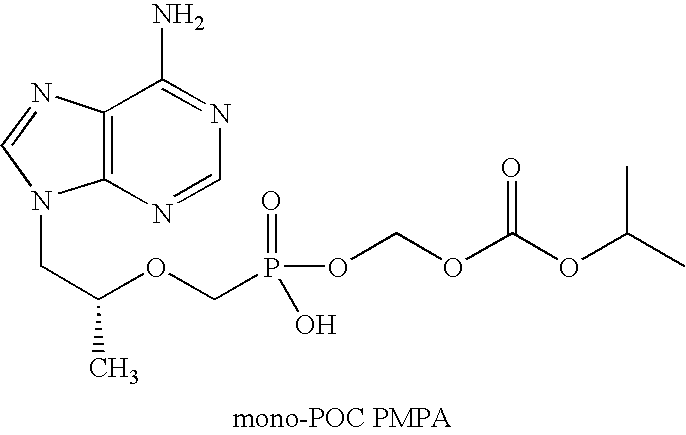
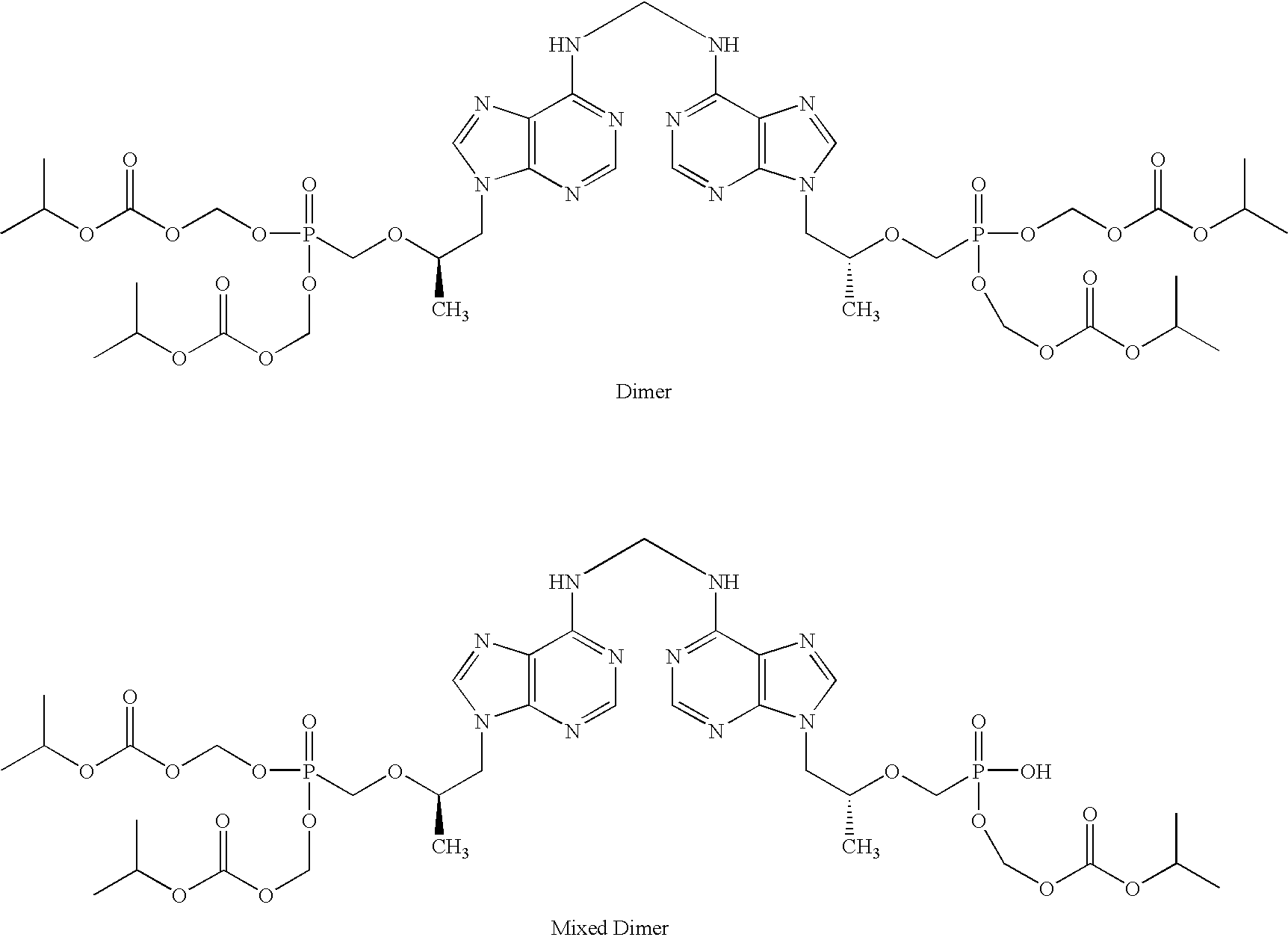
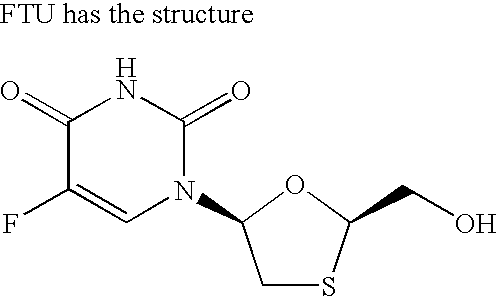
Method and composition for pharmaceutical product
a technology for pharmaceutical products and compositions, applied in the field of viral infection products, can solve the problems of high instability of tenofovir df in this combination tablet, inability to produce chemically stable tablets, and rapid deformation in stability studies, and achieve the effect of improving the stability of the resulting pharmaceutical produ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] Dry granulation is a well-known pharmaceutical manufacturing process per se. In general, API is combined with excipients and lubricant excipient and then compressed to form a mass. This mass typically is then comminuted or milled, then sieved to obtain the desired size of particle. The granular product is compressed into tablets, filled into capsules or otherwise formed into a unitary dosage form in conventional fashion. This invention at least in part is directed to the products produced by this process.
[0012] Compression into a mass is accomplished by conventional equipment. Typically, the API and excipients are passed through a roller compactor or chilsonator apparatus for compaction. However, other means for compacting, e.g., compaction into slugs (or “slugging”), the API / excipient mixture optionally are used. This in turn is comminuted or milled, and then optionally sieved to produce the desired size granules.
[0013] A dry granulated composition comprising emtricitabine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| geometric mean diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com