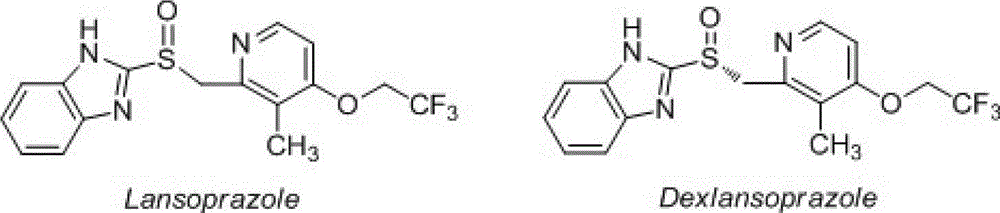
Dextral lansoprazole freeze-drying preparation and preparation method thereof
A technology of dexlansoprazole sodium and freeze-dried preparations, which is applied in the field of pharmaceutical preparations to achieve the effects of enhancing convenience and safety, good resolubility and solution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 dexlansoprazole sodium freeze-dried preparation
[0021] Prescription 1
[0022]
[0023]
[0024] Preparation:
[0025] (1) Take about 140ml of water for injection, add 3.0g of hydroxypropyl-β-cyclodextrin, stir until completely dissolved, add 1mol / L sodium hydroxide solution, cool down to 10°C, and adjust the pH to about 11.0;
[0026] (2) Add 3.0 g of dexlansoprazole sodium raw material again, stir until fully dissolved, and make up water for injection to 200 ml;
[0027] (3) Add 0.05% activated carbon for needles, stir at room temperature for 15min to 30min, coarsely filter out carbon with a titanium rod, filter with a 0.45μm filter membrane, and fine filter with two 0.22μm filter membranes. degree, content, pH, endotoxin), filled after passing the test, and freeze-dried in a freeze dryer (LGJ-18S freeze dryer, Beijing Songyuan Huaxing Technology Development Co., Ltd., the same below).
Embodiment 2
[0028] Embodiment 2 dexlansoprazole sodium freeze-dried preparation
[0029] Prescription 2
[0030]
[0031] Preparation:
[0032] (1) Take about 140ml of water for injection, add 6.0g of hydroxypropyl-β-cyclodextrin, stir until completely dissolved, add 1.5mol / L sodium carbonate solution, cool down to 15°C, and adjust the pH to about 11.5;
[0033] (2) Then add 3.0g of dexlansoprazole sodium raw material, stir until completely dissolved, and add water for injection to 200ml;
[0034] (3) Add 0.05% activated carbon for needles, stir at room temperature for 15min to 30min, remove carbon by coarse filtration with titanium rod, filter with 0.45μm filter membrane, and fine filter with two 0.22μm filter membranes. degree, content, pH, endotoxin), filled after passing the test, and freeze-dried in a freeze dryer.
Embodiment 3
[0035] Embodiment 3 dexlansoprazole sodium freeze-dried preparation
[0036] Prescription 3
[0037]
[0038] Preparation:
[0039] (1) Take about 140ml of water for injection, add 12.0g of hydroxypropyl-β-cyclodextrin, stir until completely dissolved, add 1mol / L sodium hydroxide solution, cool down to 15°C, and adjust the pH to about 11.5;
[0040] (2) Then add 3.0g of dexlansoprazole sodium raw material, stir until completely dissolved, and add water for injection to 200ml;
[0041] (3) Add 0.05% activated carbon for needles, stir at room temperature for 15min to 30min, remove carbon by coarse filtration with titanium rod, filter with 0.45μm filter membrane, and fine filter with two 0.22μm filter membranes. degree, content, pH, endotoxin), filled after passing the test, and freeze-dried in a freeze dryer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com