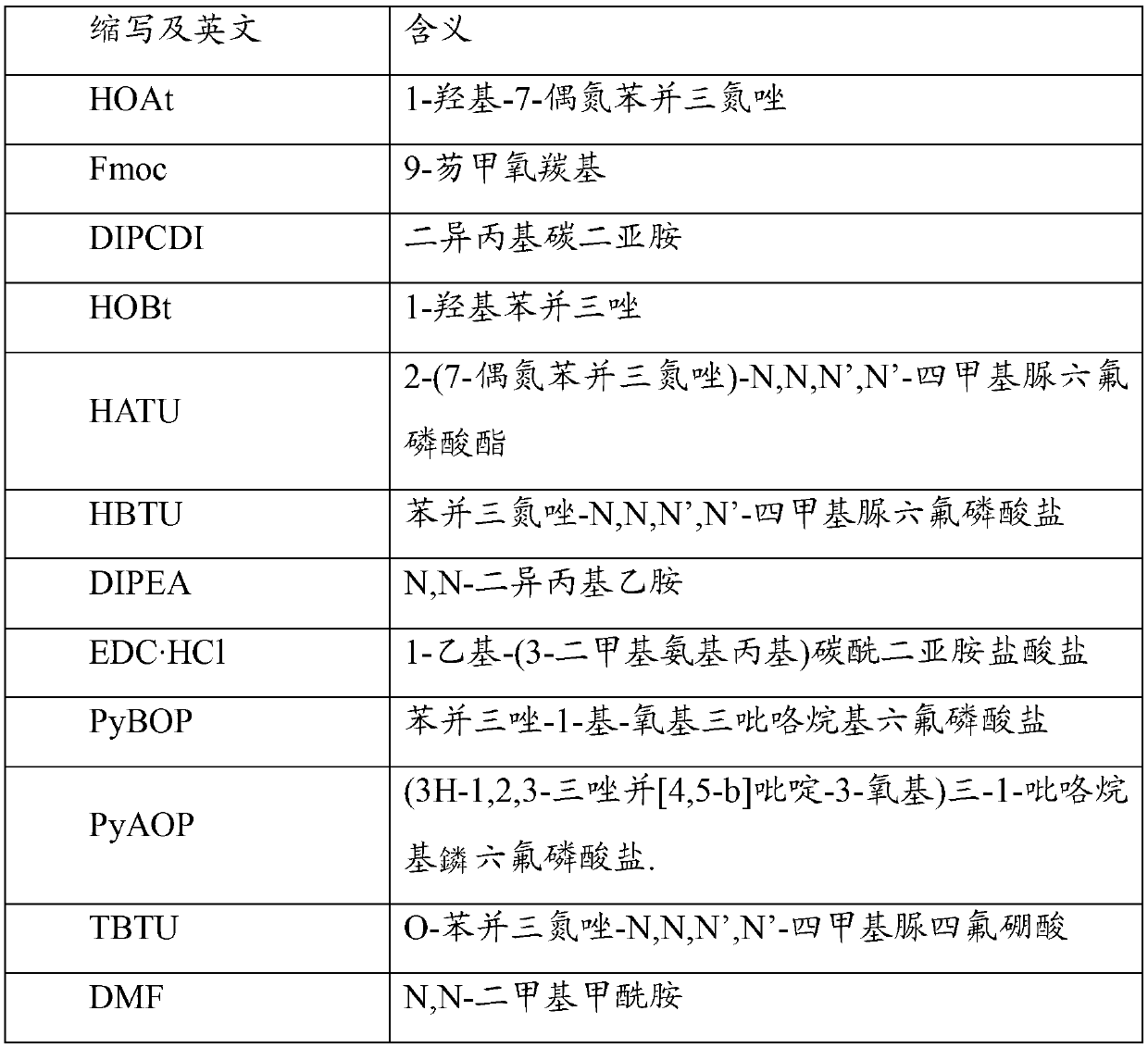
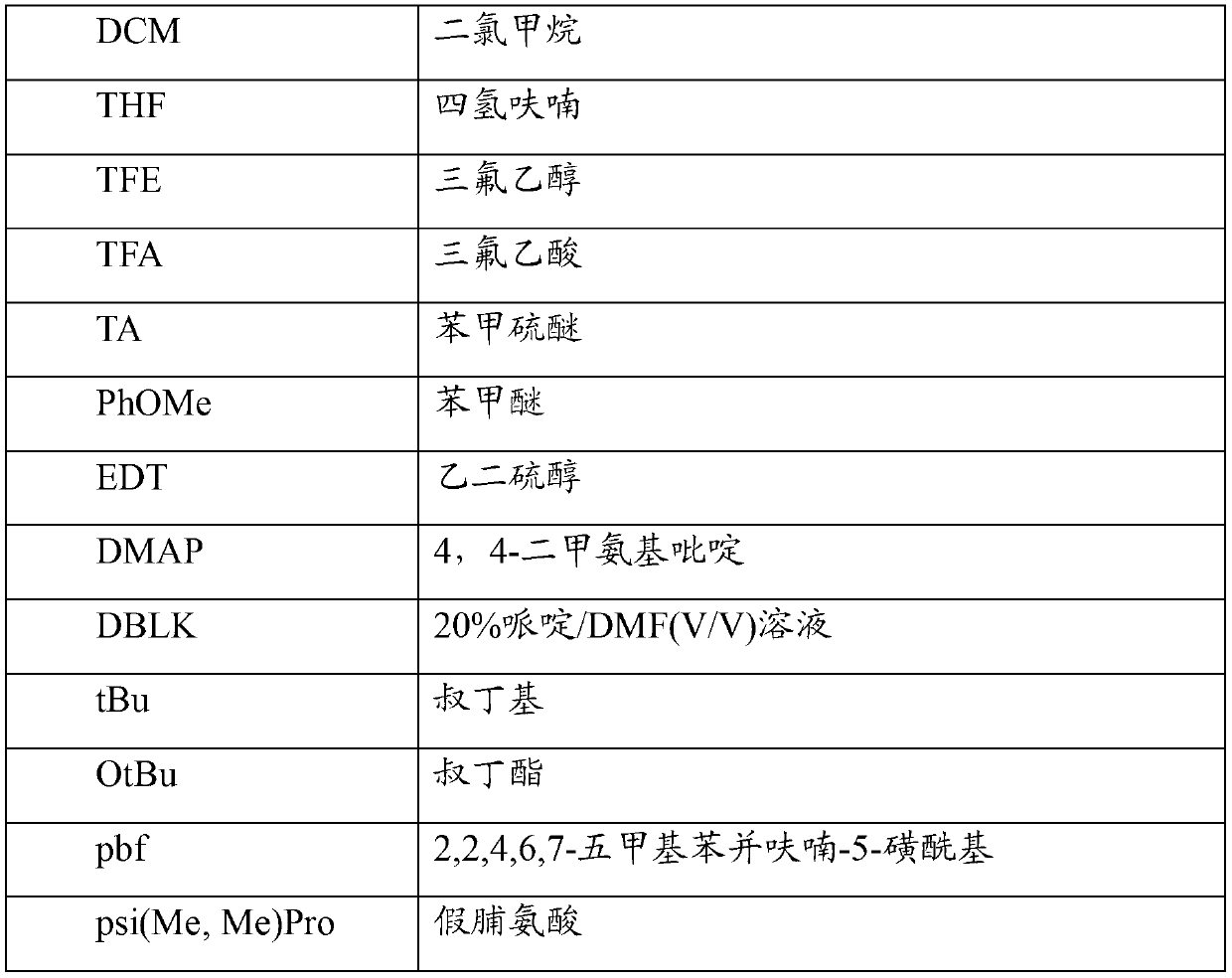
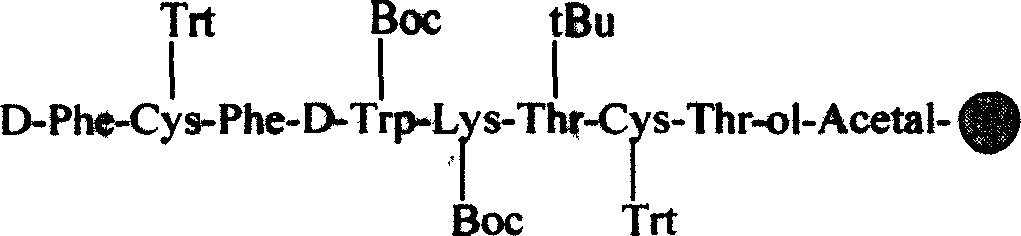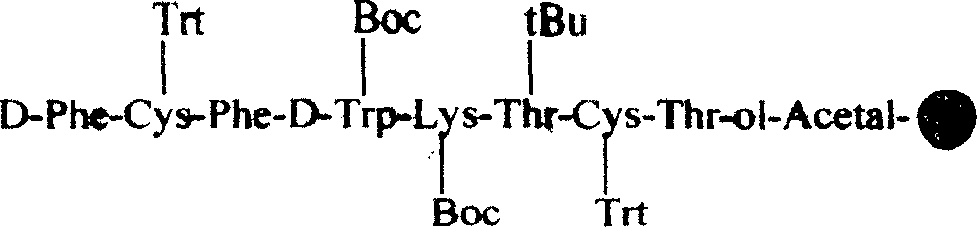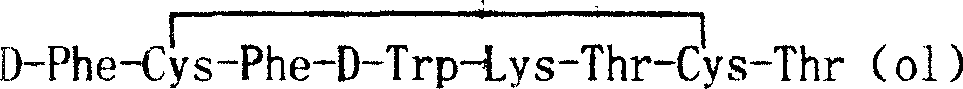Patents
Literature
38results about How to "Reduce racemization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
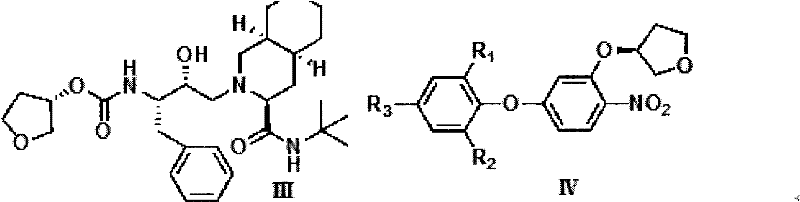
Process for the production of 2'-branched nucleosides
ActiveUS20050020825A1High purityHigh yieldOrganic active ingredientsSugar derivativesNucleoside XNucleoside
Owner:INDENIX PHARM LLC
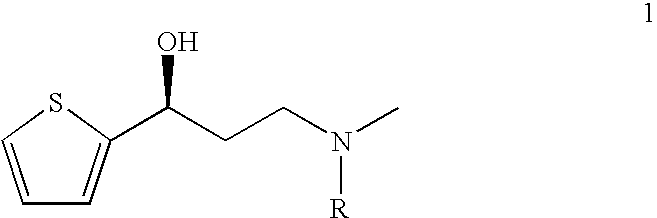
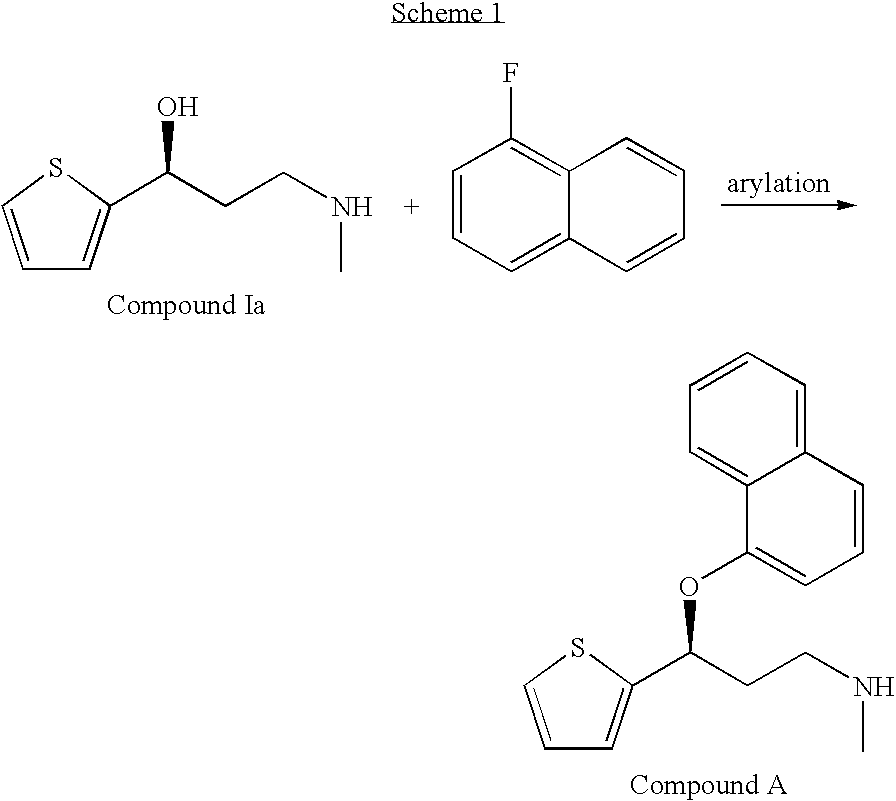
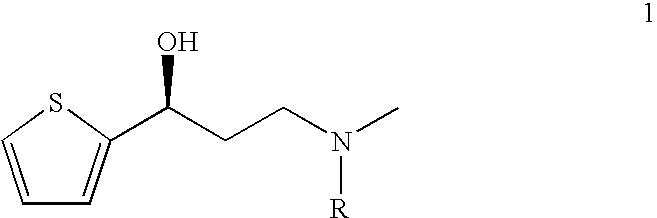
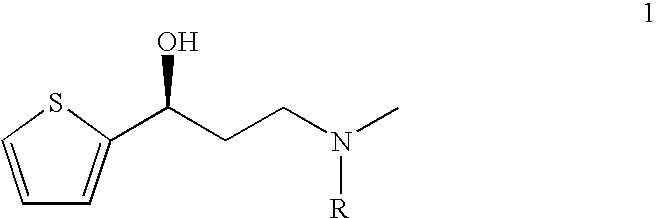
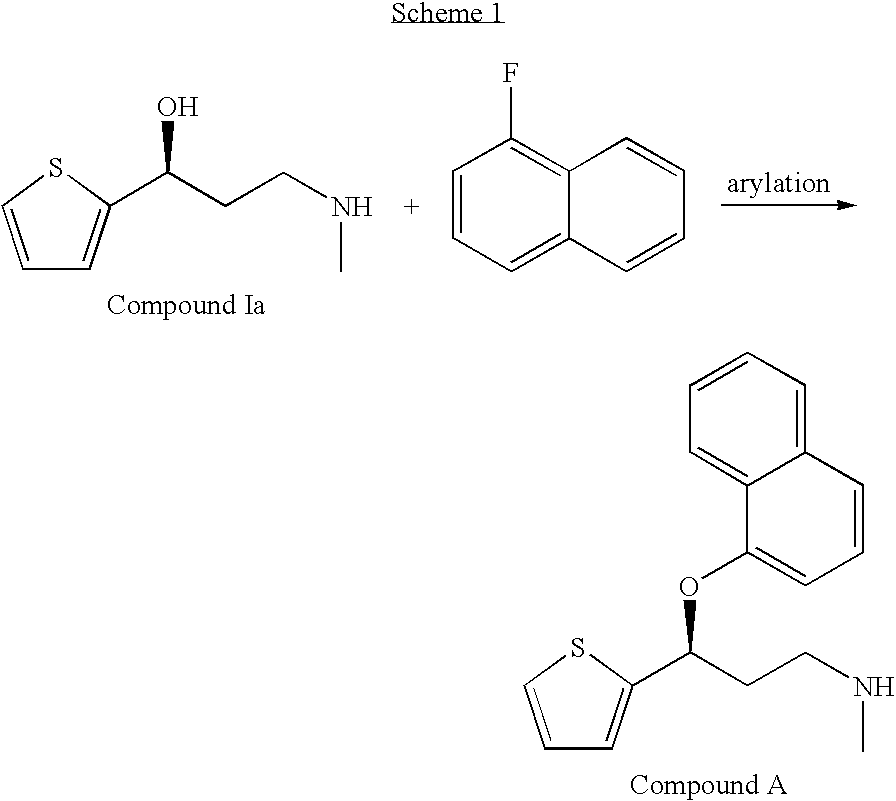
Improved process for the asymmetric synthesis of duloxetine
InactiveUS20070167636A1Reduce racemizationHigh amount of racemizationOrganic chemistryChemistryDuloxetine
This invention provides an improved asymmetric process for the synthesis of duloxetine involving arylation of Compounds of Formula I.
Owner:ELI LILLY & CO
Process for the asymmetric synthesis of duloxetine
InactiveUS7538232B2Reduce racemizationReduce the amount of solutionOrganic chemistryDuloxetineBiochemistry
This invention provides an improved asymmetric process for the synthesis of duloxetine involving arylation of Compounds of Formula I.
Owner:ELI LILLY & CO
Solid-phase synthesis process of octreotide acetate
ActiveCN1569890AIncrease reaction rateRapid responsePowder deliveryPeptide/protein ingredientsAutoxidationOctreotide acetate
The invention discloses the solid-phase synthesis process of octreotide acetate which consists of, bonding acetal product with macromolecular resin, merging the bonded products with protection amino acid residue in sequence to obtain octapeptide resin, cutting the octapeptide from the resin to obtain aqueous solution, carrying out naturally oxidation in air to obtain Octreotide, charging glacial acetic acid into Octreotide aqueous solution, and freeze-drying.
Owner:SINOPHARM A THINK PHARMA
Preparation method of teduglutide
InactiveCN104418949AEase of mass productionAvoid side effects such as degradationPeptide preparation methodsGlucagonsCombinatorial chemistrySide reaction
The invention belongs to the field of medicinal chemistry, and in particular relates to a preparation method of teduglutide. The preparation method of the invention is as below: respectively synthesizing a teduglutide sequence site No.4-33 peptide resin fragment and a teduglutide sequence No.1-3 peptide resin fragment through solid phase synthesis; then coupling the teduglutide sequence site No.4-33 peptide resin fragment and the teduglutide sequence No.1-3 peptide resin fragment by an appropriate resin vector to obtain a teduglutide resin; and finally cracking to obtain crude teduglutide, and purifying to obtain the pure teduglutide. The preparation method provided by the invention can effectively avoid side reaction such as degradation caused by an Asp-Gly structure of the No. 3-4 site, also can reduce the His1 racemization, thus effectively improving the purity of crude peptide and improve the purification yield. Compared with the prior art, the preparation method of the invention has the advanategs of high purity of the prepared teduglutide product, less by-product and high product yield, and is beneficial for mass production of teduglutide.
Owner:HYBIO PHARMA
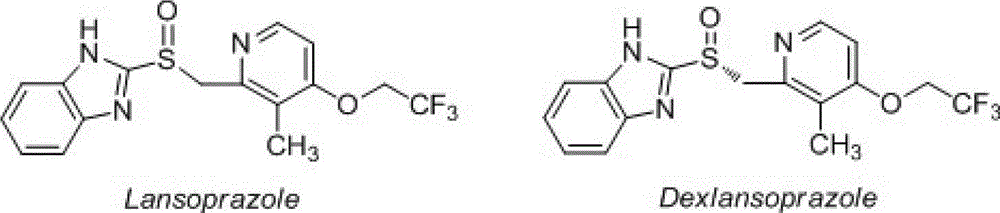
Dextral lansoprazole freeze-drying preparation and preparation method thereof
InactiveCN102908322AImprove convenienceImprove securityOrganic active ingredientsPowder deliveryFreeze-dryingDexlansoprazole
The invention relates to a dextral lansoprazole freeze-drying preparation, which comprises the following components: dextral lansoprazole, Hydroxypropyl-Beta-Cyclodextrin and PH modifier. The invention also provides a preparation method for the freeze-drying preparation. The freeze-drying preparation obtained according to the technical scheme of the invention can maintain better redissolving performance and solution stability, and enhance the convenience and security of clinical application; and furthermore, the Hydroxypropyl-Beta-Cyclodextrin used in the preparation method plays the function of stabilizer and excipient, that is, on one hand, the racemization of the dextral lansoprazole is effectively reduced, and on the other hand, the freeze-drying preparation obtained through the preparation method is loose, full and integral in appearance and is suitable for industrialized production.
Owner:NANJING YOKO PHARMA GRP CO LTD
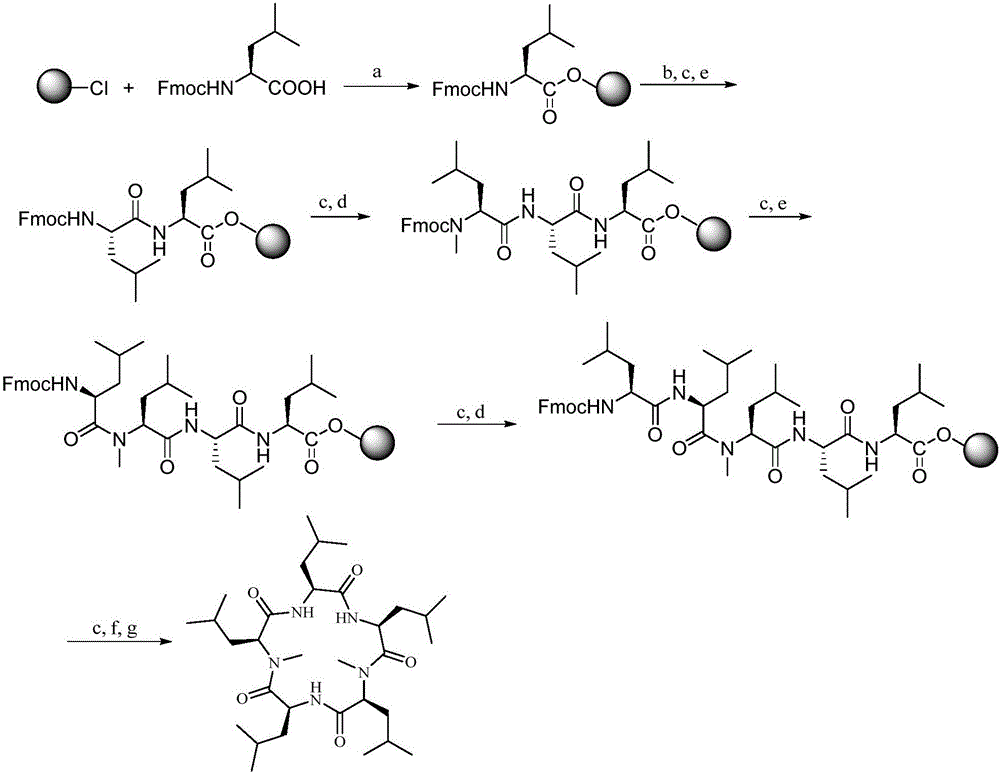
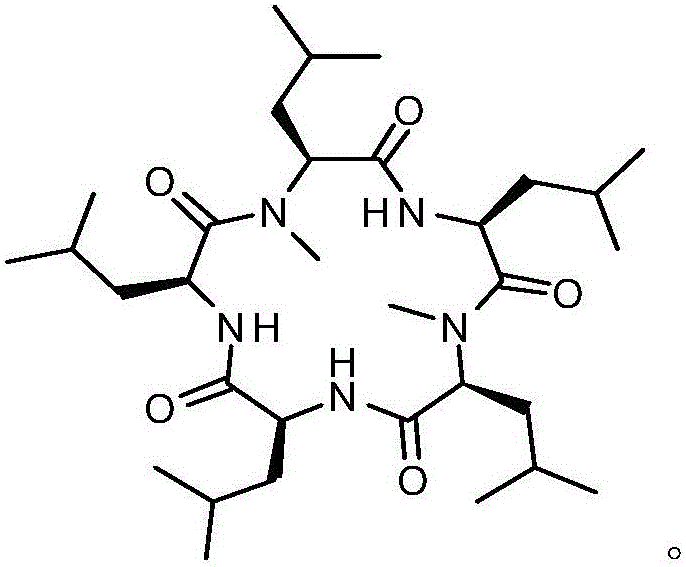
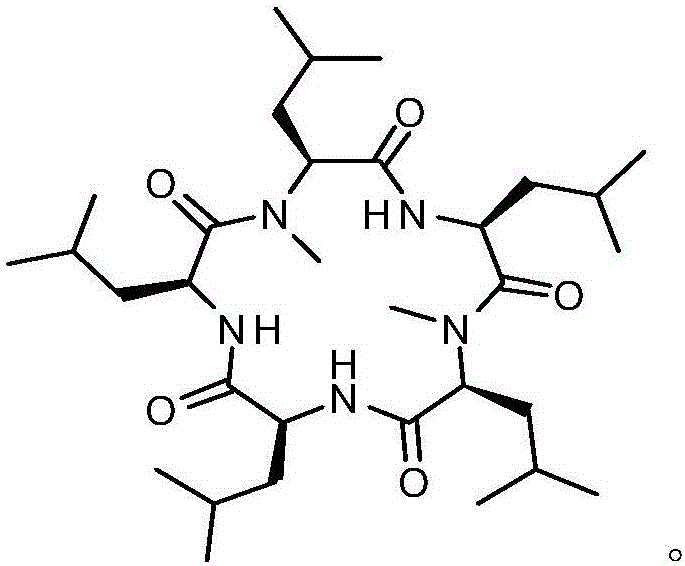
Synthesis method of cyclic pentapeptide
ActiveCN105777871AHigh purityReduce racemizationPeptide preparation methodsSynthesis methodsDrug biological activity
The invention discloses a synthesis method of cyclic pentapeptide. According to the synthesis method, the 'solid-liquid combined production' strategy is adopted, a proper condensation agent is used for a condensation reaction, linear pentapeptides are prepared respectively through a proper process, the cyclization site is located between N-methyl-leucine and leucine, and the efficiency of linear peptide preparation is effectively improved.The proper condensation agent is used for the condensation reaction, racemization in amido bond formation can be effectively reduced, therefore, the purity of a target product can be effectively improved, reaching 99% or above, the requirements of biological activity tests and clinical application can be fully met, and the synthesis method of cyclic pentapeptide has high medical value. The cyclization agent PyBOP is used for a cyclization reaction, the cyclization yield can be effectively increased and reach up to 84.2%.The synthesis method does not require complex purification by column chromatography, redundant amino acids can be simply washed away, the synthesis process is simple, the cost of raw materials is low, reaction conditions are mild, the purity is high, industrialization is easy, and the synthesis method has broad market prospects.
Owner:JINAN UNIVERSITY
Process for producing a stable low concentration, injectable solution of noradrenaline
ActiveUS20170049720A1Prevent oxidationReduce occurrenceOrganic active ingredientsNervous disorderAntioxidantPreservative
In a first aspect, the present invention relates to a process for producing a stable, injectable solution with low content of noradrenaline, which includes dissolving noradrenaline and optionally an excipient in deoxygenated or degassed water, filtrating the resulting noradrenaline solution in a nitrogen current, distributing the solution in a nitrogen current, and sterilization, preferably hot. The invention further provides a stable, injectable solution with low content of noradrenaline, substantially free of anti-oxidizing and preservative agents, as well as uses thereof in the medical and pharmaceutical fields.
Owner:SINTETICA SA
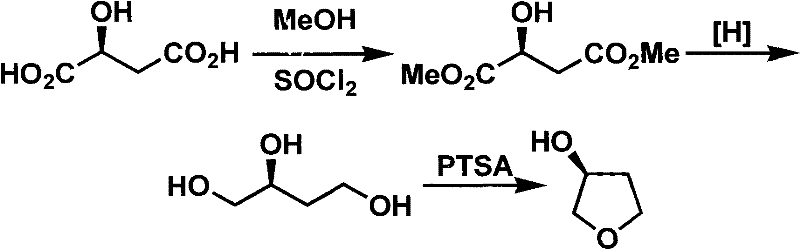
Novel method for preparing S-3-hydroxytetrahydrofuran
InactiveCN102477019AIncrease fat solubilityFacilitate product separationOrganic chemistry3-HydroxytetrahydrofuranEnvironmentally friendly
The invention discloses a novel method for preparing an important medical farm chemical intermediate, i.e., S-3-hydroxytetrahydrofuran. The method comprises the following steps of: performing hydroxy group protection on S-4-chloro-3-hydroxybutyrate serving as a raw material; and undergoing a reduction reaction, a cyclization reaction and a deprotection reaction to obtain a target product. A synthesis method disclosed by the invention has the advantages of high optical purity and high yield of the target product, easiness and convenience for reaction operation, environmentally-friendly process and suitability for industrial production.
Owner:苏州凯达生物医药技术有限公司
Process for producing a stable low concentration, injectable solution of noradrenaline
ActiveUS10251848B2Prevent oxidationReduce occurrenceOrganic active ingredientsNervous disorderAntioxidantNitrogen
In a first aspect, the present invention relates to a process for producing a stable, injectable solution with low content of noradrenaline, which includes dissolving noradrenaline and optionally an excipient in deoxygenated or degassed water, filtrating the resulting noradrenaline solution in a nitrogen current, distributing the solution in a nitrogen current, and sterilization, preferably hot. The invention further provides a stable, injectable solution with low content of noradrenaline, substantially free of anti-oxidizing and preservative agents, as well as uses thereof in the medical and pharmaceutical fields.
Owner:SINTETICA SA
Solid phase method of secretin
InactiveCN103214568AHigh yieldHigh puritySecretinsPeptide preparation methodsCombinatorial chemistrySecretin
The invention provides a solid phase method of secretin, which comprises the following steps: 1) selecting an appropriate solid phase carrier; 2) coupling amino acids one by one according to a solid phase synthetic method; 3) splitting for obtaining a crude peptide; 4) obtaining the secretin by purifying the crude peptide, wherein, the solid phase synthesis employs Fmoc-strategy, and false proline is used for replacing parts of serine in the peptide chain during the solid phase synthesis. The solid phase method of secretin provided by the invention has the advantages of simple operation, small impurity, easy purifying and high yield, and is beneficial to realization of industrialization.
Owner:HYBIO PHARMA
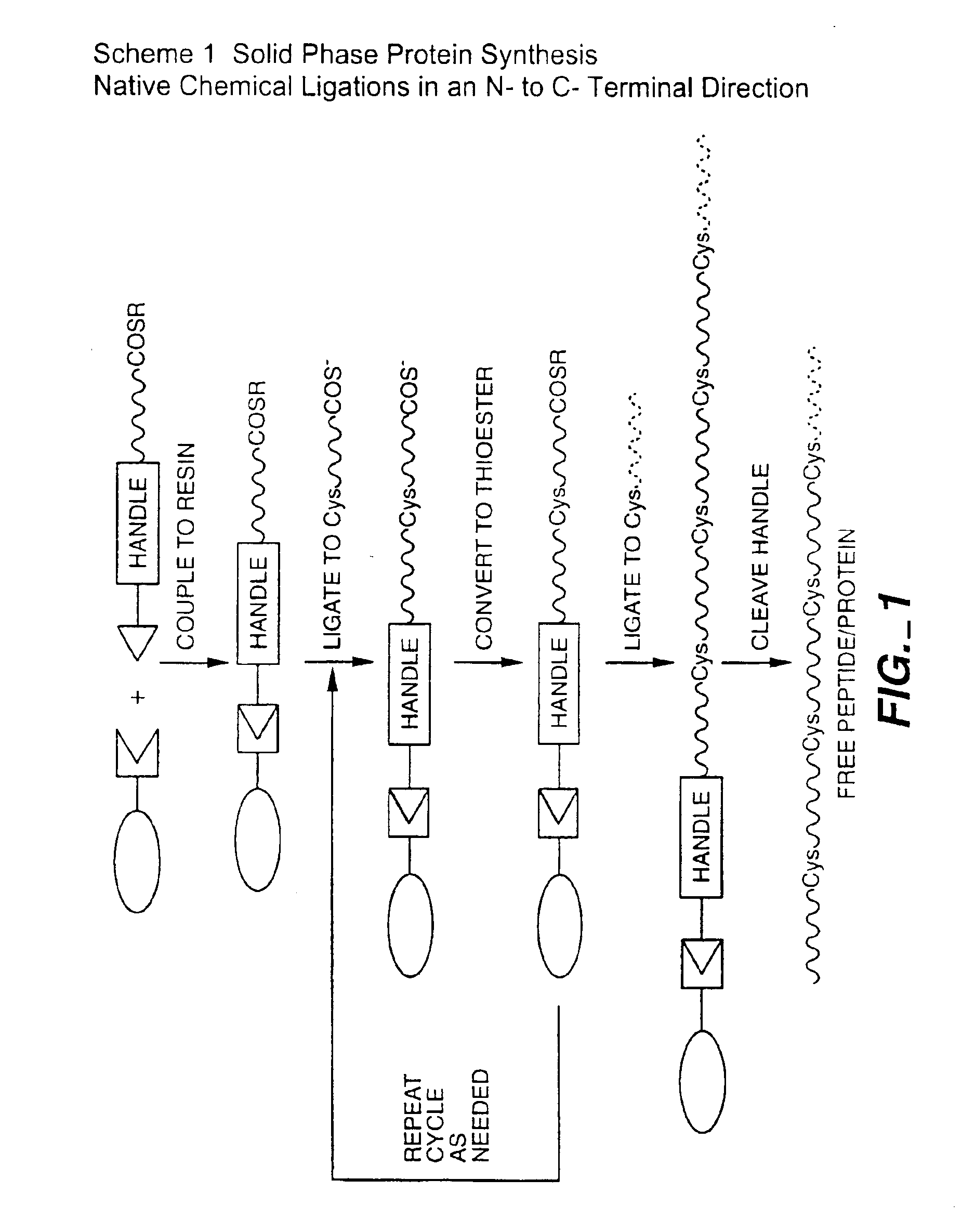
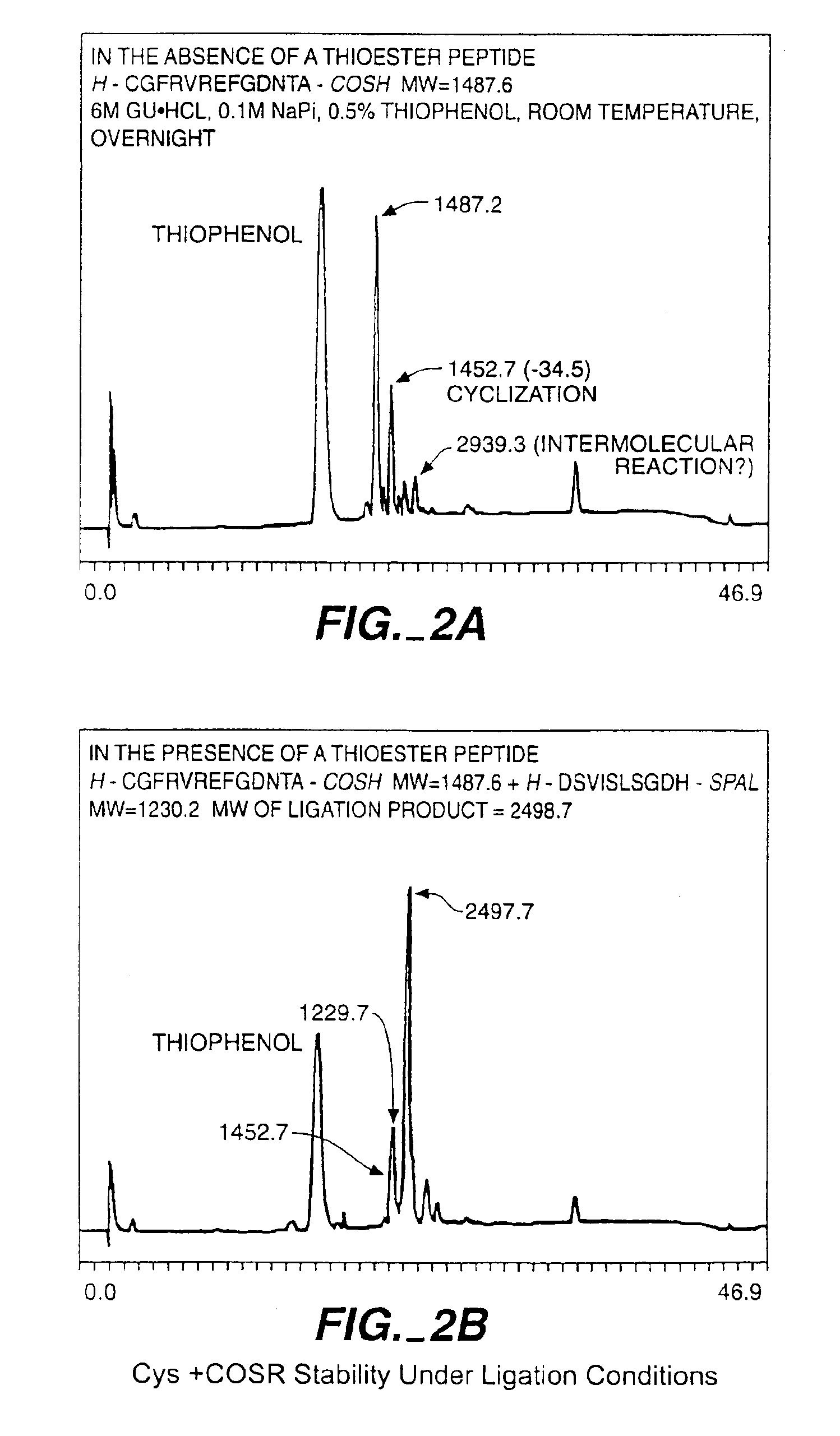
Solid phase native chemical ligation of unprotected or N-terminal cysteine protected peptides in aqueous solution
InactiveUS7030217B2Easy to purifySynthesis fastBioreactor/fermenter combinationsBiological substance pretreatmentsESI mass spectrometryChemical ligation
Owner:AMYLIN PHARMA INC
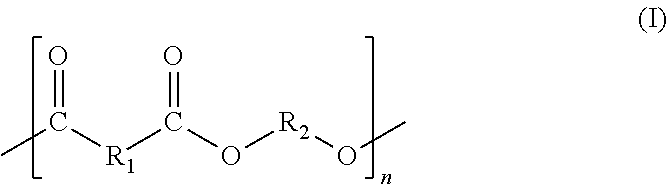
Polylactic acid block copolymers and preparation methods thereof
Disclosed are a polylactic acid block copolymer and a preparation method thereof. The polylactic acid block copolymer comprises block A and block B, and is presented as B-b-A-b-B triblock structure, wherein the block A is a cyclic aromatic polyester oligomer block, and the block B is a polylactic acid block. The polylactic acid block copolymer is obtained by ring-opening copolymerization of a cyclic aromatic polyester oligomer and a lactide. Disclosed are another polylactic acid block copolymer and a preparation method thereof. The polylactic acid block copolymer comprises block A and block B, and is presented as B-b-A-b-B triblock structure, wherein the block A is an aromatic polyester block with two hydroxyl end groups, and the block B is a polylactic acid block. The polylactic acid block copolymer is obtained by ring-opening copolymerization of an aromatic polyester with two hydroxyl end groups and a lactide.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Solid phase synthesis method for adrenomedullin (27-52)
InactiveCN101165067AMild reaction conditionsLess side effectsHormone peptidesPeptide preparation methodsReaction rateTarget peptide
The present invention discloses solid phase synthesis process of adrenal medullarin (27-52). The synthesis process includes the first solid phase Fmoc polypeptide synthesis to graft protective amino acid Fmoc-Ala-OH onto resin, connecting the rest protective amino acids successively, cutting down the synthesized coarse peptide with peptide cutting reagent, and final separating and purifying to obtain the adrenal medullarin (27-52) as the target peptide. The present invention has mild reaction condition, high reaction rate, less side reactions, easy purification, high yield, no corrosion to the equipment and no environmental pollution.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Method for production of peptide thioester compound
ActiveUS8058394B2Reduce racemizationPeptide-nucleic acidsPeptide/protein ingredientsSolventC-terminus
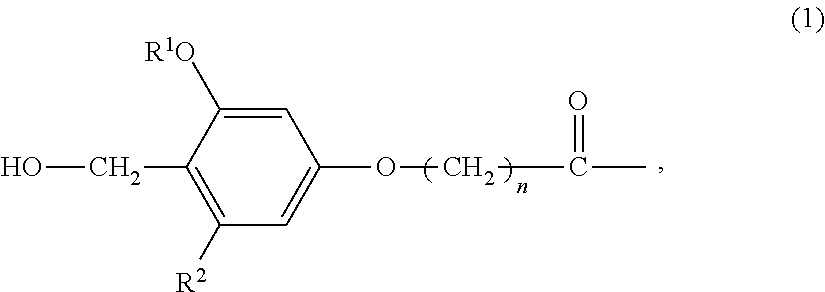
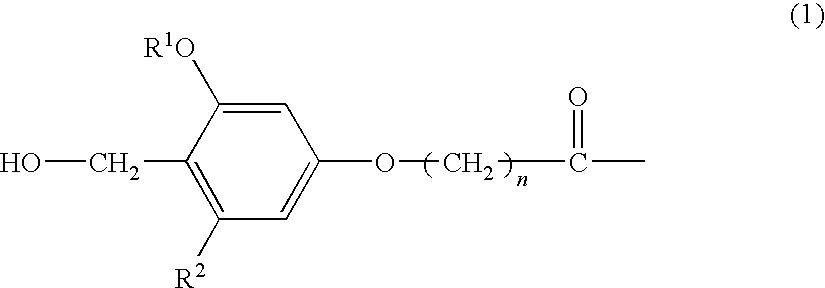
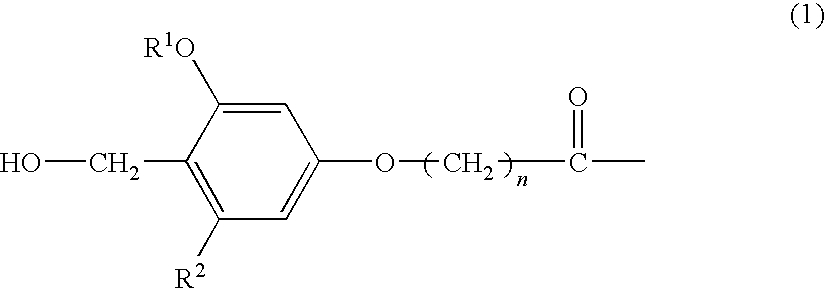
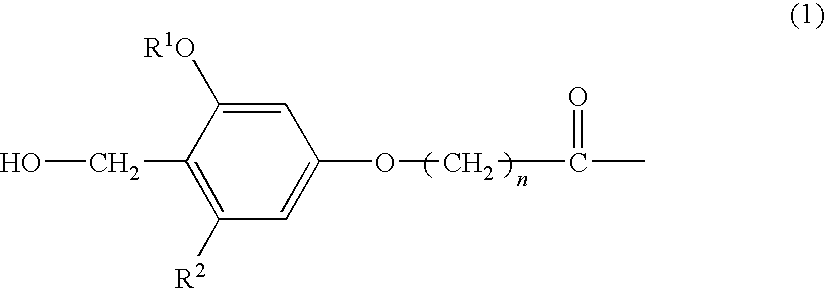
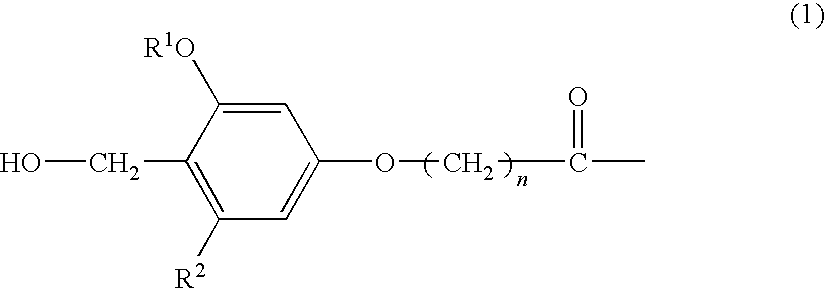
The present invention provides a process for producing a peptide thioester compound. The process involves: (A) forming a peptide by a solid-phase synthesis method using a resin modified with a linker represented by the formula (1) as a solid phase:wherein R1 represents C1-4 alkyl group, R2 represents hydrogen atom or C1-4 alkoxy group, and n represents an integer of 1 to 4; (B) cleaving a bond between the solid phase and the peptide with at least one acid selected from dilute hydrochloric acid, dilute sulfuric acid, formic acid, and acetic acid to produce a peptide having a carboxyl group at the C-terminus; and (C) reacting a thiol compound with the peptide at −100 to 0° C. in the presence of a condensing agent in a solvent.
Owner:GLYTECH
Solid-phase synthesis method of semaglutide
ActiveCN112679602AReduce generationLow costPeptide preparation methodsBulk chemical productionDipeptideCombinatorial chemistry
The invention discloses a solid-phase synthesis method of semaglutide. According to the solid-phase synthesis method of the semaglutide, the semaglutide is divided into three fragments to be synthesized simultaneously through a solid-phase fragment synthesis technology, and dipeptide or tripeptide is used for replacing original single amino acid at part of positions during fragment synthesis. According to the solid-phase synthesis method of the semaglutide, the synthesis period is effectively shortened by 50%; and the generation of racemization impurities is greatly reduced, especially formation of [D-His1]-semaglutide is greatly reduced, formation of deletion peptides or polyaminopeptides in conventional one-by-one coupling, especially generation of [+Ser11]-semaglutide, [+Ala19]-semaglutide and [+Gly29]-semaglutide impurities, is effectively avoided, the purity of crude peptides is improved, downstream purification is facilitated, the material cost is greatly reduced, and the solid-phase synthesis method of the semaglutide is suitable for industrial production, and the total yield reaches 35%.
Owner:苏州特瑞药业股份有限公司
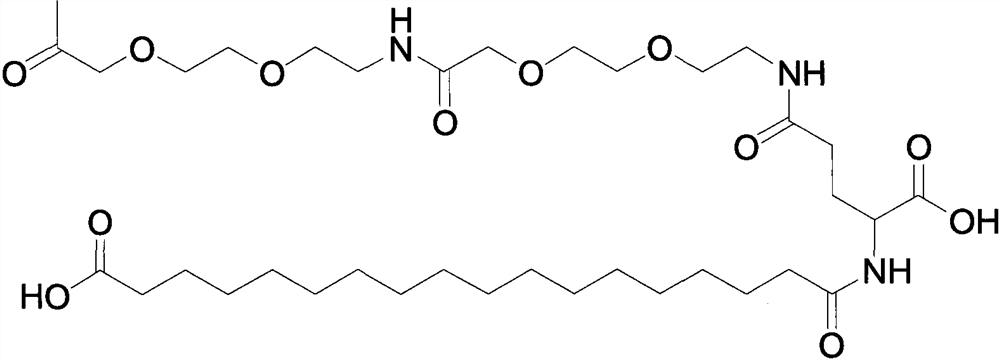
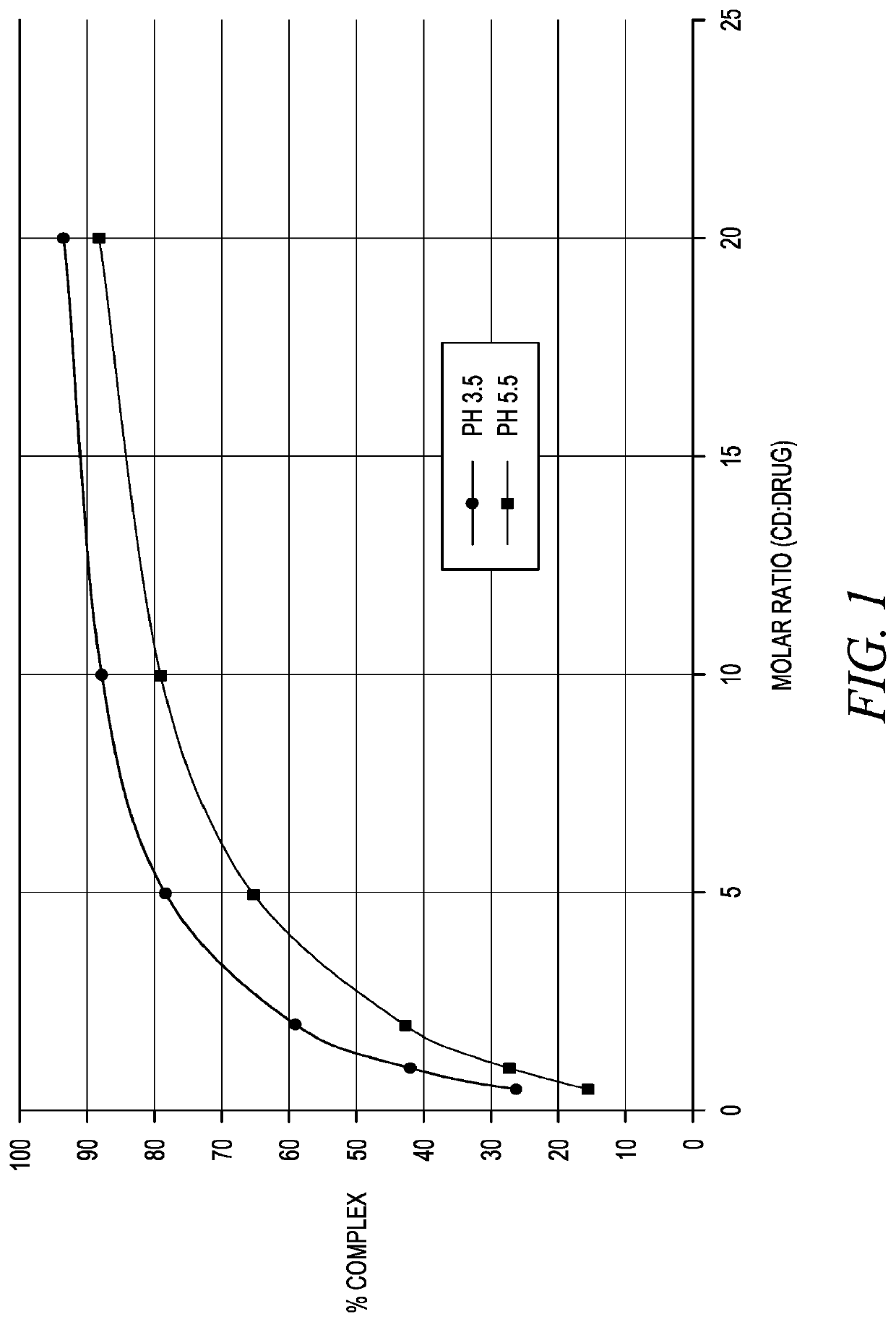
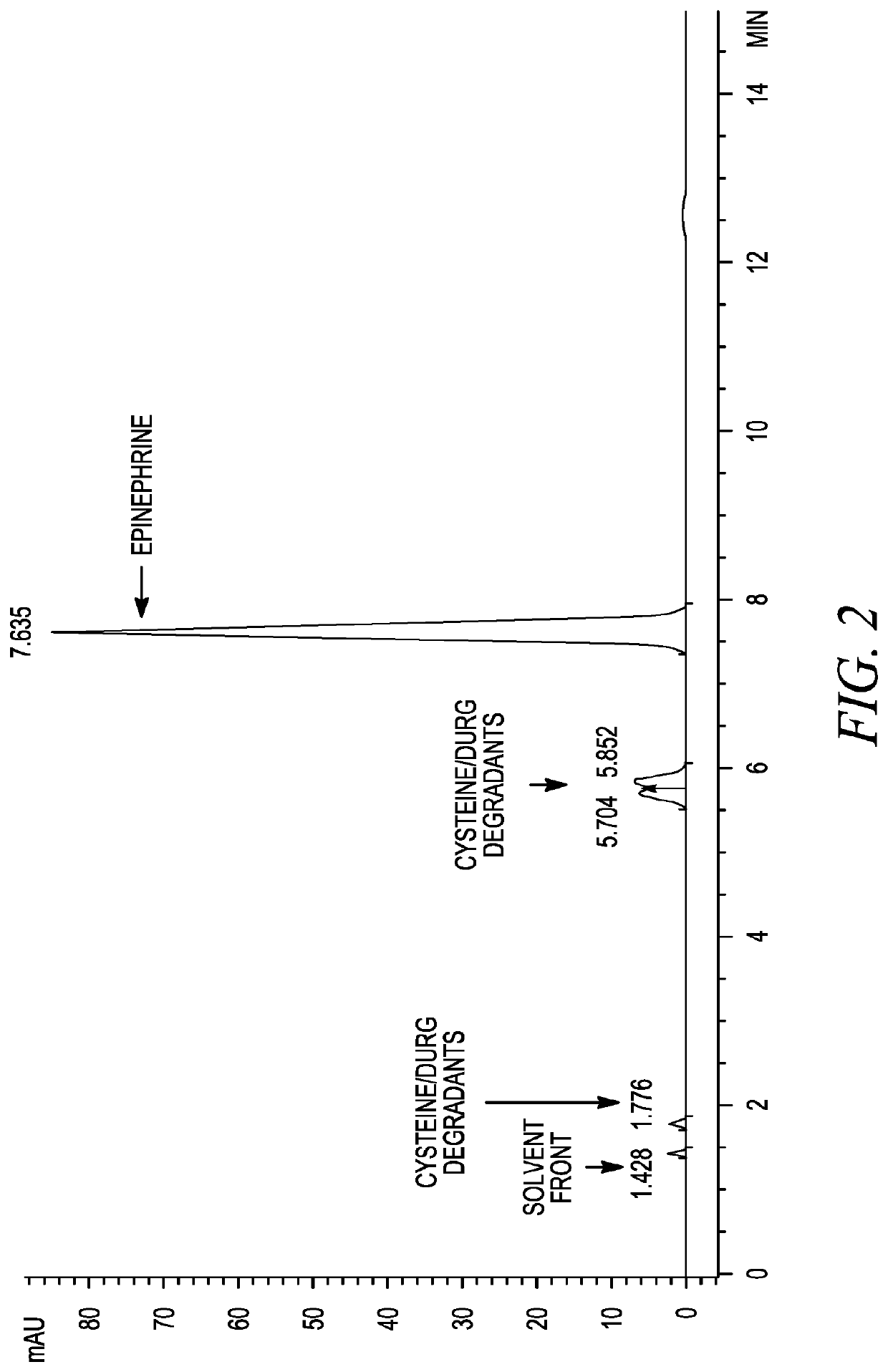
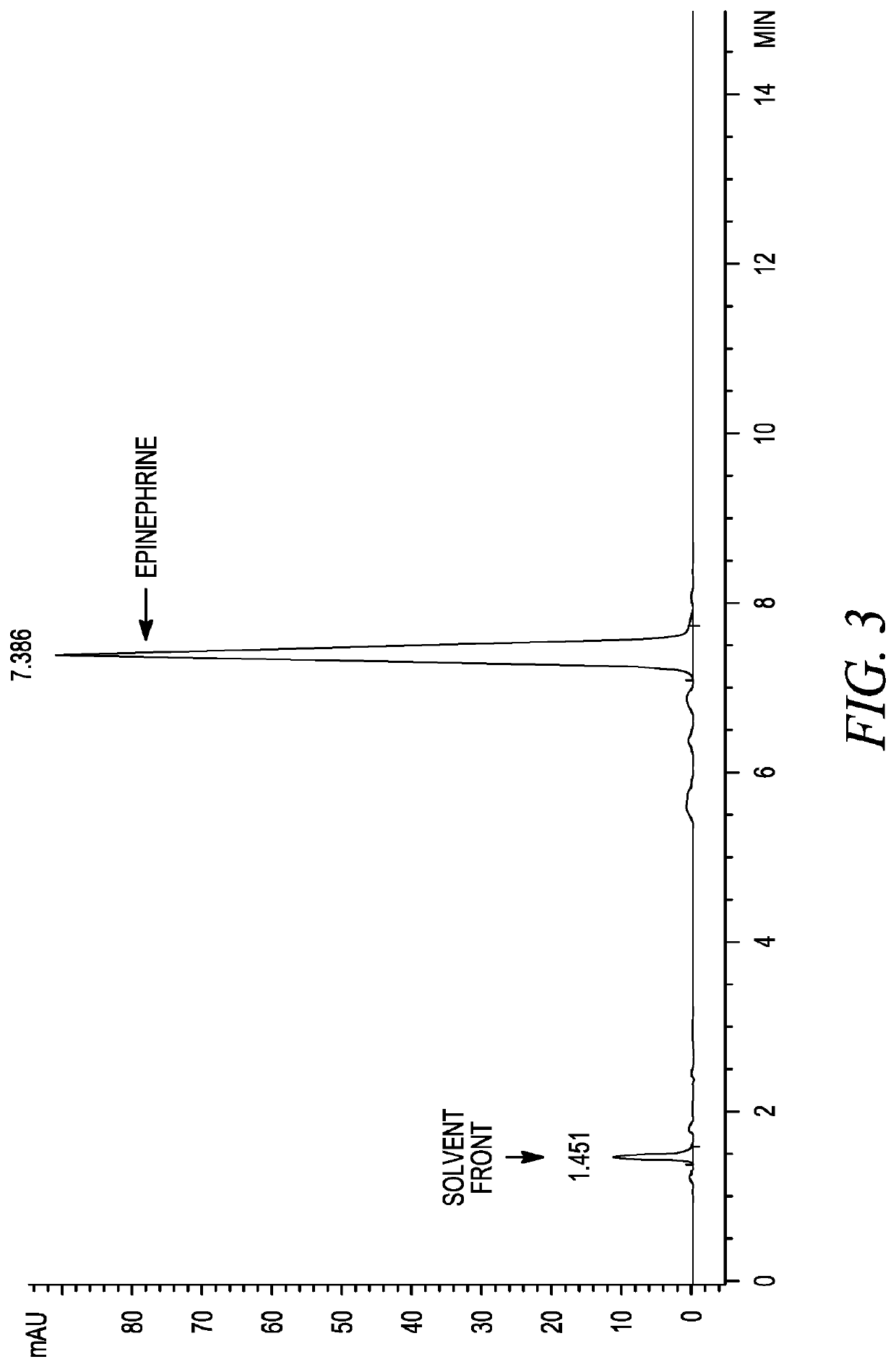
Stabilization of epinephrine formulations
PendingUS20200268689A1Reduce the amount requiredLow formationOrganic active ingredientsAmpoule syringesAntioxidantSulfite salt
The disclosure herein relates to the innovative epinephrine formulations in aqueous solution of medicinal products that enhance the physicochemical stabilities of epinephrine and extend the product shelf life. In some instances, the formulations comprise epinephrine or a salt thereof, a complexing agent, and a “non-sulfite” antioxidant. The epinephrine formulations substantially demonstrated the superior physicochemical stabilities to conventional sulfite formulation of commercial medications currently available. In some instances, sulfite-free formulations further provide further benefit (e.g., safety benefits) to sulfite-sensitive patients. The compositions, methods for preparing the formulations, and methods of using the same (e.g., in the treatment of anaphylaxis) are also provided.
Owner:YS PHARMTECH
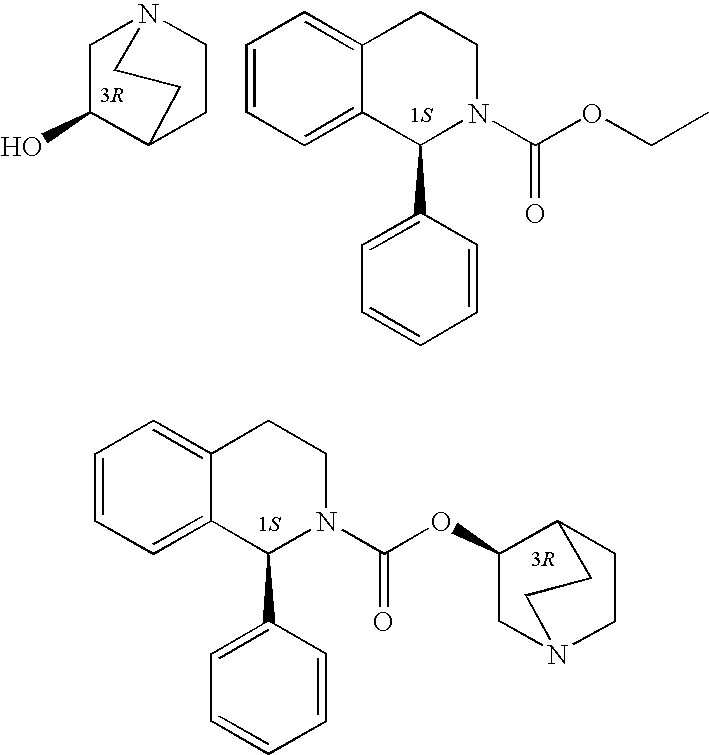
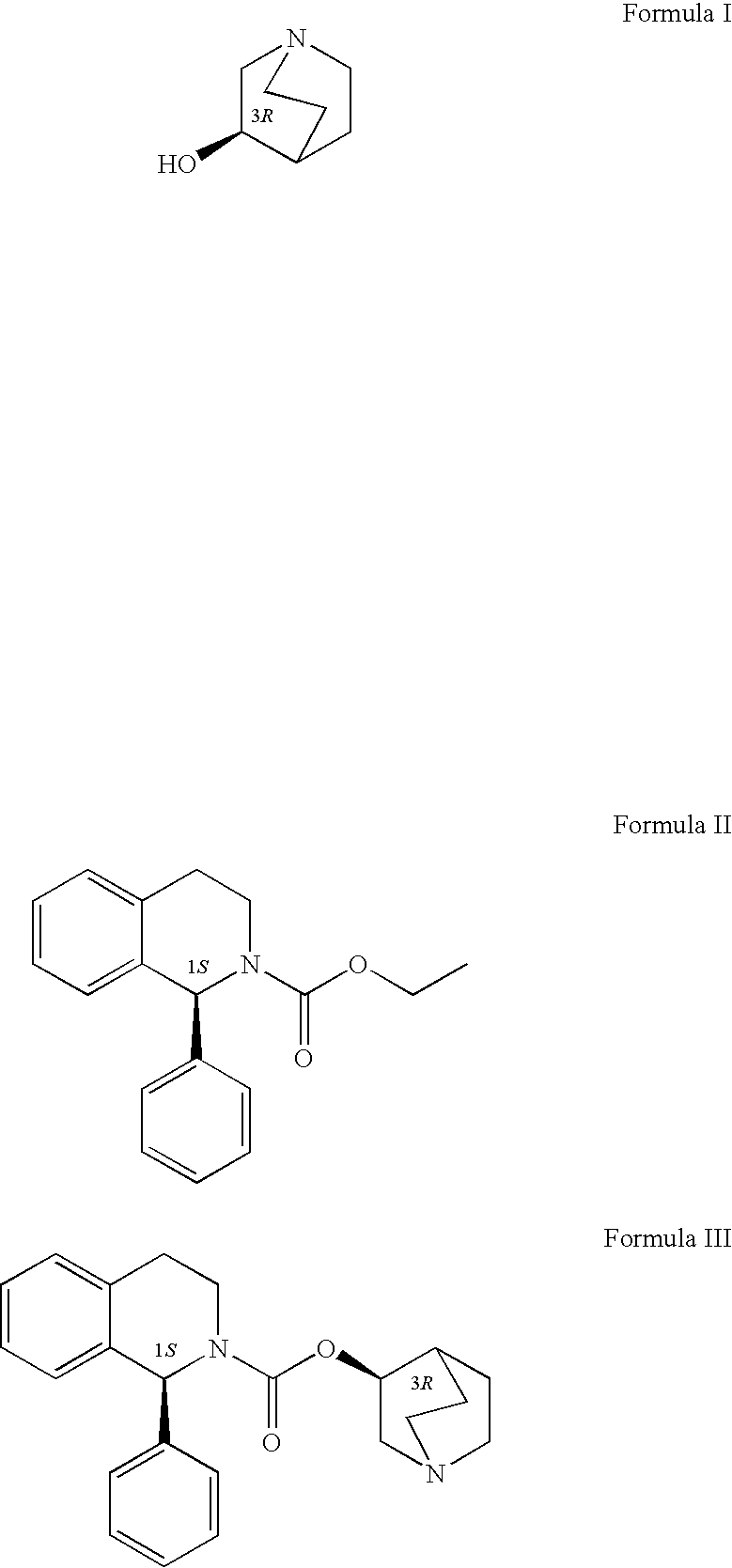
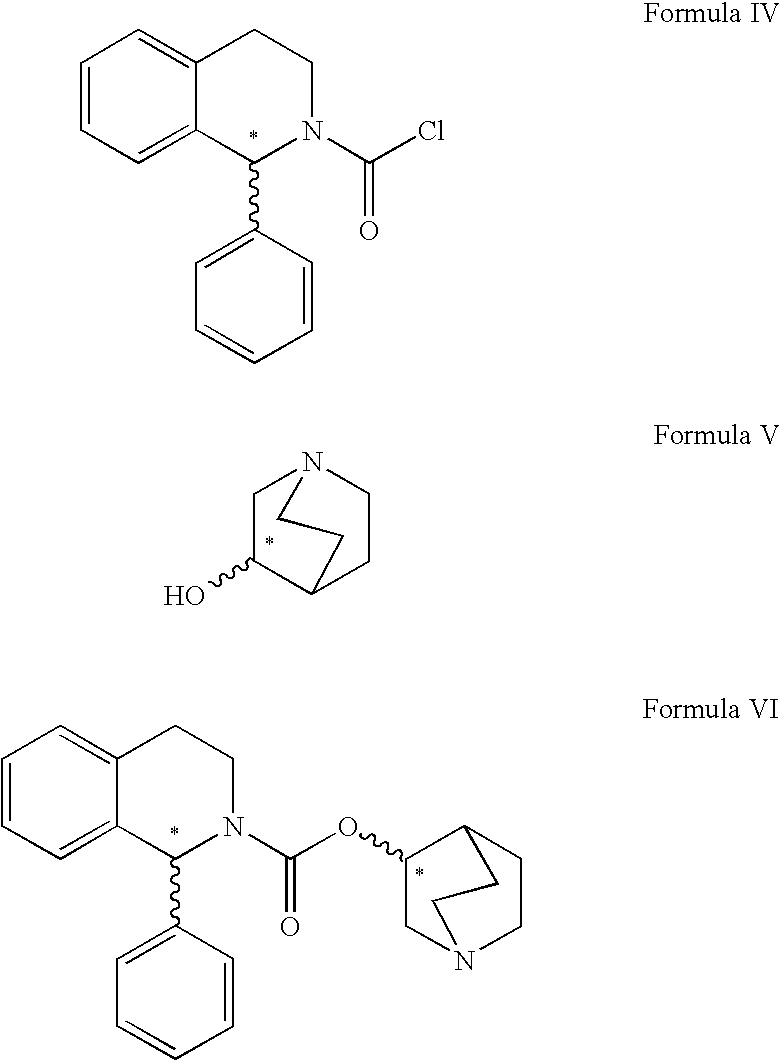
Process for the preparation of solifenacin
InactiveUS20090203914A1High degree of conversionReduce racemizationOrganic chemistryIsoquinolineHydrogen
A process for the preparation of (1S)-QR)-I -azabicyclo[2.2.2.]oct-3-yl 3,4-dihydro-1-phenyl- 2(1H)-isoquino-line carboxylate by reacting (1S)-alkyl 1-phenyl-1,2,3,4-tetrahydro-2-isoquinoline carboxylate with 3-(R)-quinuclidol in an inert solvent, where a primary alkyl ester of the carboxylate whose alkyl length is C1-C4 is used and the reaction is catalyzed by a non-nu-cleophilic base.
Owner:ZENTIVA AS
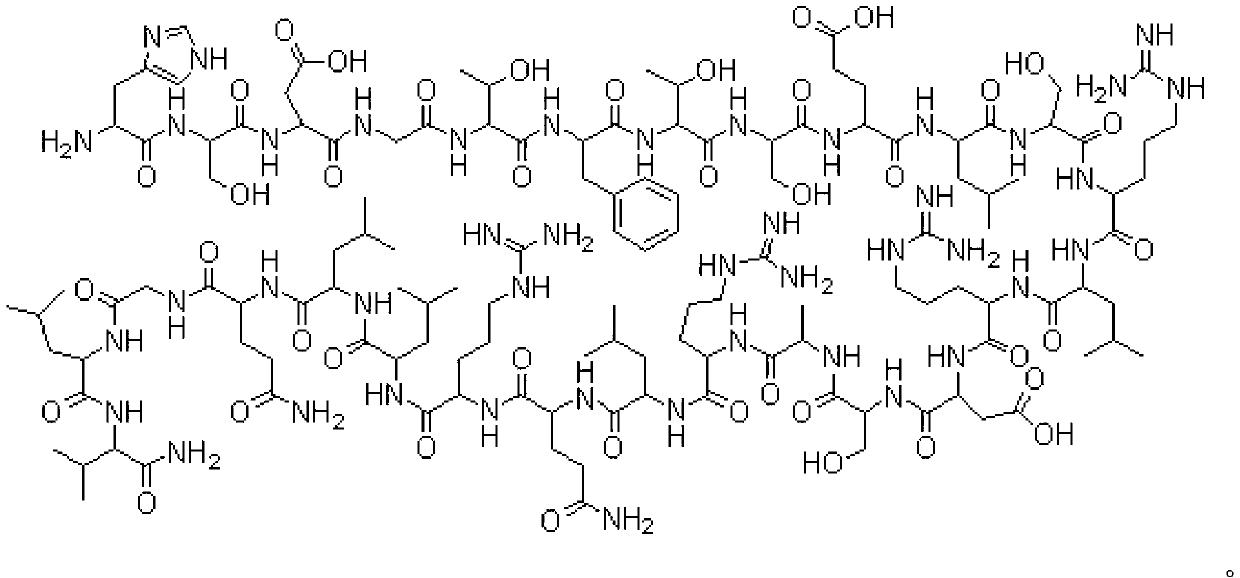
Preparation method of human vascular endothelial inhibitory peptide
InactiveCN103183733AReduce racemizationImprove product qualityPeptide preparation methodsAnimals/human peptidesVascular endotheliumWang resin
The invention provides a solid-phase synthesis process of a human vascular endothelial inhibitory peptide: H-His-Ser-His-Arg-Asp-Phe-Gln-Pro-Val-Leu-His-Leu-Val-Ala-Leu-Asn-Ser-Pro-Leu-Ser-Gly-Gly-Met-Arg-Gly-Asp-Arg-Gly-Arg. The process includes: taking Fmoc-Asp(OtBu)-Wang resin as a raw material and putting it in a polypeptide synthesis reactor, using N, N-dimethylformamide (DMF) as a solvent, taking 20% piperidine (PIP) as an alpha-amino deprotection agent, adopting N, N-diisopropyl carbodiimide (DIC) as a condensation agent, and introducing protected amino acid (Fmoc-AA) in order to perform a programmed reaction; then carrying out cracking, precipitation by anhydrous ether, and oxidation to obtain a crude product; and subjecting the crude product to purification, desalination and freeze-drying so as to obtain a refined end product.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG +1
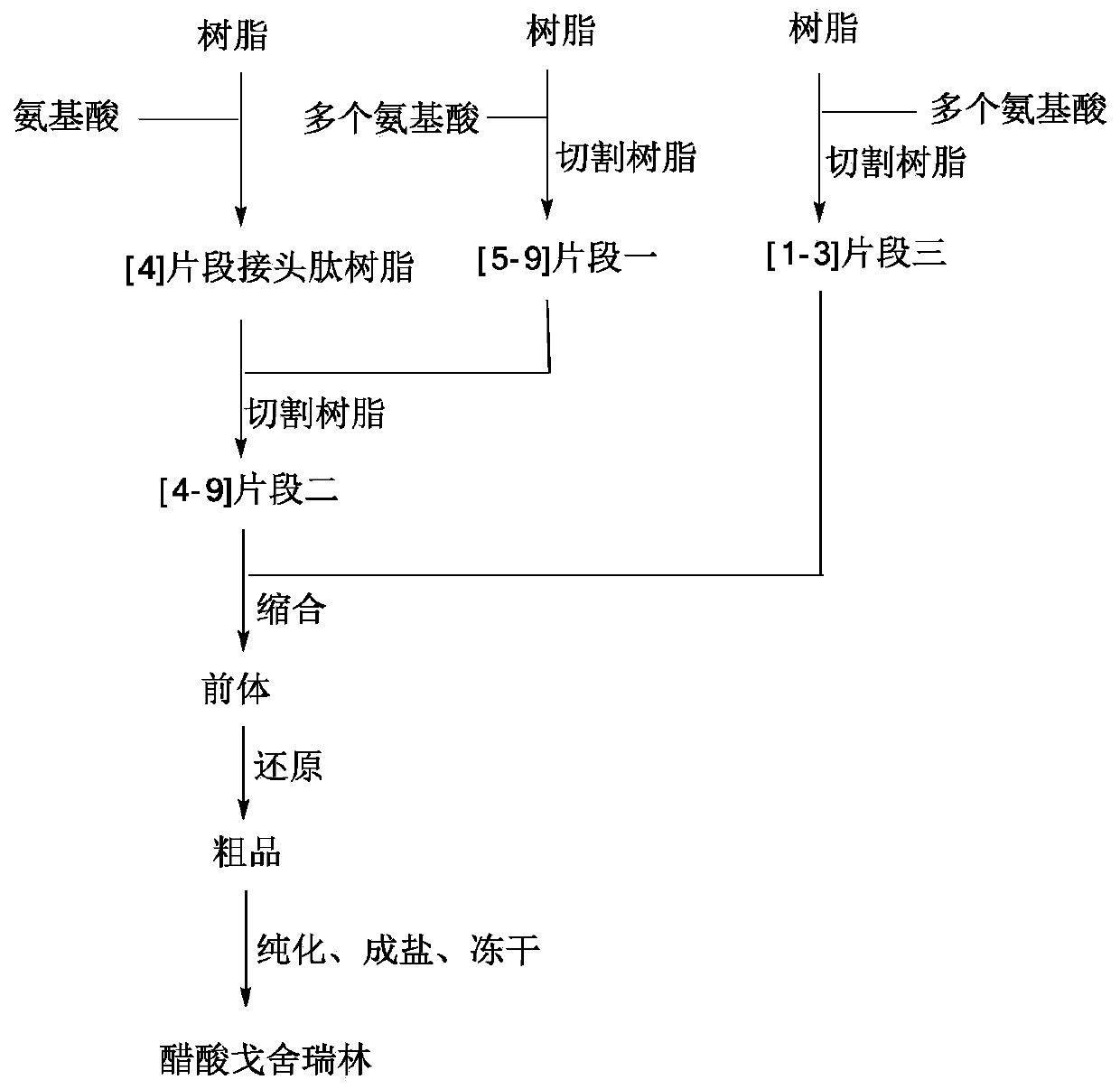
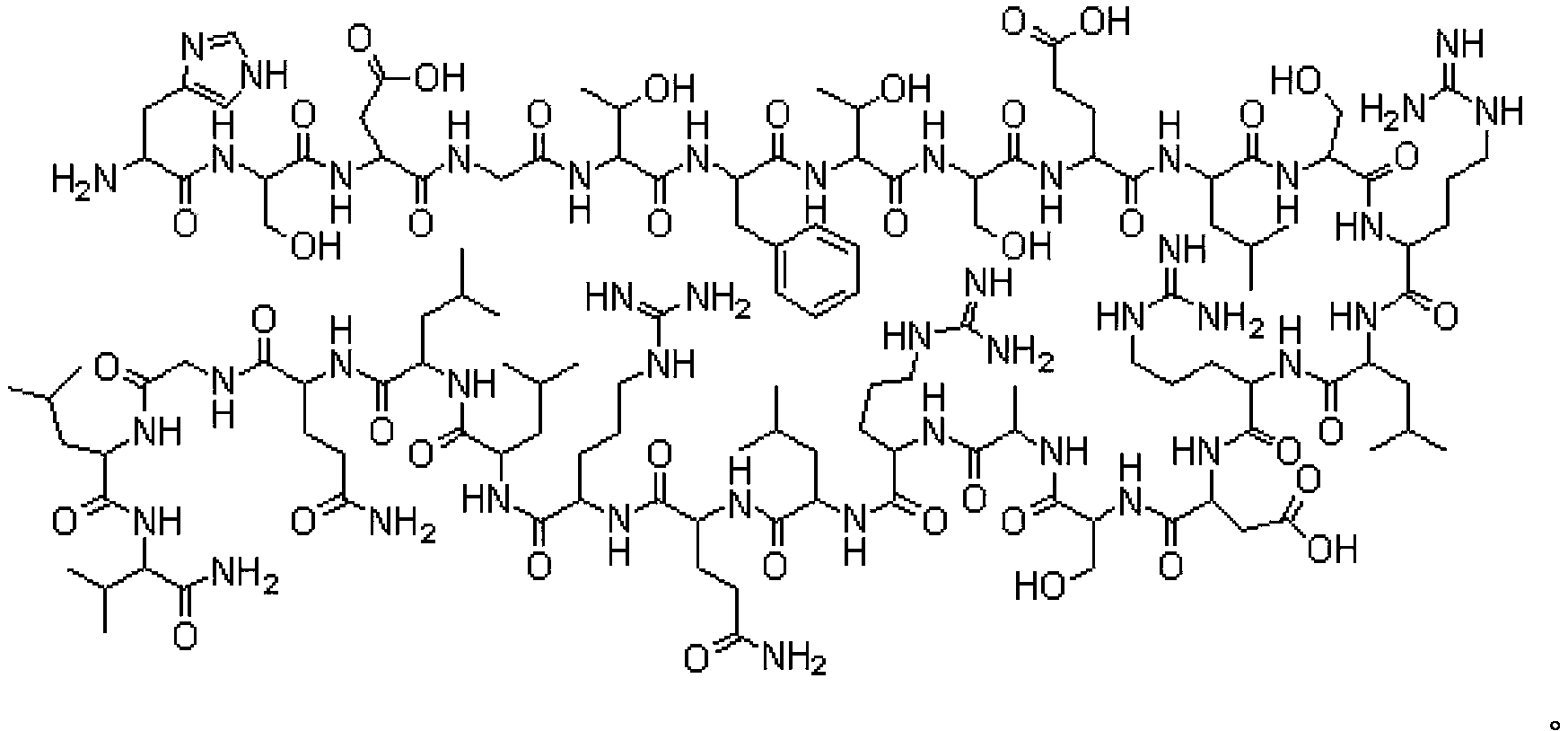
Fragment-process synthesis method of goserelin
ActiveCN111233980AShort synthesis timeHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseCombinatorial chemistry
The invention synthesizes goserelin by a synthesis method of combining a solid-phase method and a liquid-phase method. Fragments II and III are easy to synthesize and purify and high in purity; different fragments can be synthesized simultaneously, so that the synthesis time of the goserelin is effectively shortened, and the preparation efficiency is improved; and finally, the two fragments are butt-jointed by the liquid-phase method to obtain a goserelin precursor, low-cost coupling is realized, and industrial enlarged preparation is facilitated.
Owner:NANJING LEEWE BIOPHARMACEUTICAL CO LTD
Solid phase method of secretin
InactiveCN103214568BEasy for industrial synthesisEasy to removeSecretinsPeptide preparation methodsCombinatorial chemistrySecretin
The invention provides a solid phase method of secretin, which comprises the following steps: 1) selecting an appropriate solid phase carrier; 2) coupling amino acids one by one according to a solid phase synthetic method; 3) splitting for obtaining a crude peptide; 4) obtaining the secretin by purifying the crude peptide, wherein, the solid phase synthesis employs Fmoc-strategy, and false proline is used for replacing parts of serine in the peptide chain during the solid phase synthesis. The solid phase method of secretin provided by the invention has the advantages of simple operation, small impurity, easy purifying and high yield, and is beneficial to realization of industrialization.
Owner:HYBIO PHARMA
Method for production of peptide thioester compound
ActiveUS20090137780A1Reduce racemizationPeptide-nucleic acidsPeptide/protein ingredientsSolventC-terminus
The present invention provides a process for producing a peptide thioester compound, characterized by comprising: (A) forming a peptide by a solid-phase synthesis method using a resin modified with a linker represented by the formula (1) as a solid phase; (B) cleaving a bond between the solid phase and the peptide with at least one acid selected from dilute hydrochloric acid, dilute sulfuric acid, formic acid, and acetic acid to produce a peptide having a carboxyl group at the C-terminus; and (C) reacting a thiol compound with the peptide at −100 to 0° C. in the presence of a condensing agent in a solvent: (1) wherein R1 represents C1-4 alkyl group, R2 represents hydrogen atom or C1-4 alkoxy group, and n represents an integer of 1 to 4.
Owner:GLYTECH
Solid-phase synthesis process of octreotide acetate
ActiveCN1254484CIncrease reaction rateRapid responsePowder deliveryPeptide/protein ingredientsSide reactionSolid-phase synthesis
Owner:SINOPHARM A THINK PHARMA
Amrubicin hydrochloride intermediate compound I
InactiveCN111646915AIncrease spatial selectivityImprove conversion rateSugar derivativesCarboxylic acid nitrile preparationCombinatorial chemistryAmrubicin
The invention belongs to the technical field of medicines, and particularly relates to an amrubicin hydrochloride intermediate compound and a preparation method thereof. A novel intermediate is provided, and is used for synthesizing the amrubicin hydrochloride important intermediate. The preparation method solves the problem of low yield caused by chiral resolution of the amrubicin intermediate inthe prior art, and the new intermediate compound synthesized through chirality has the advantages of high product yield, simple operation, substantial reduction of the production cost, and suitableness for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
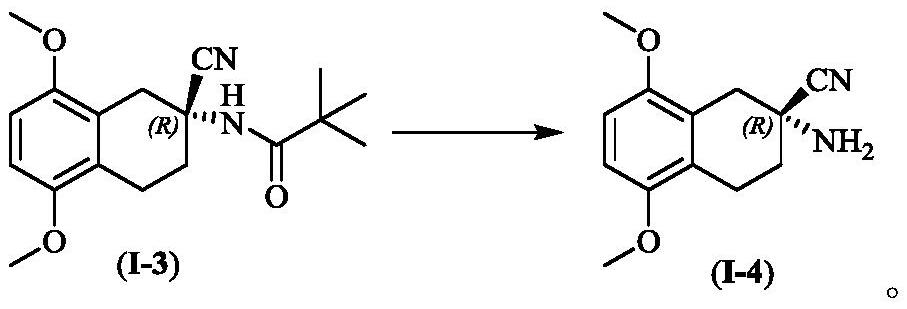
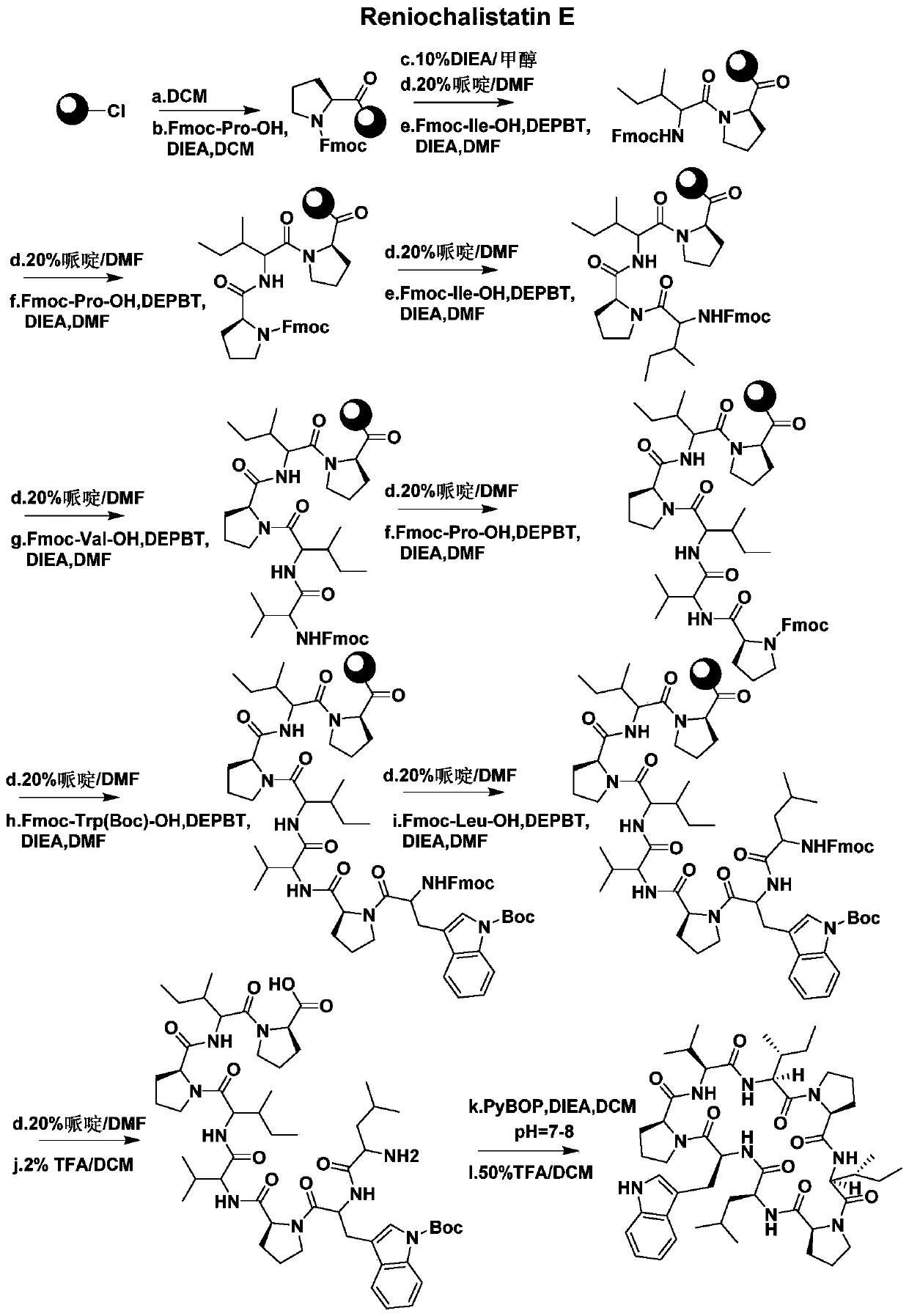
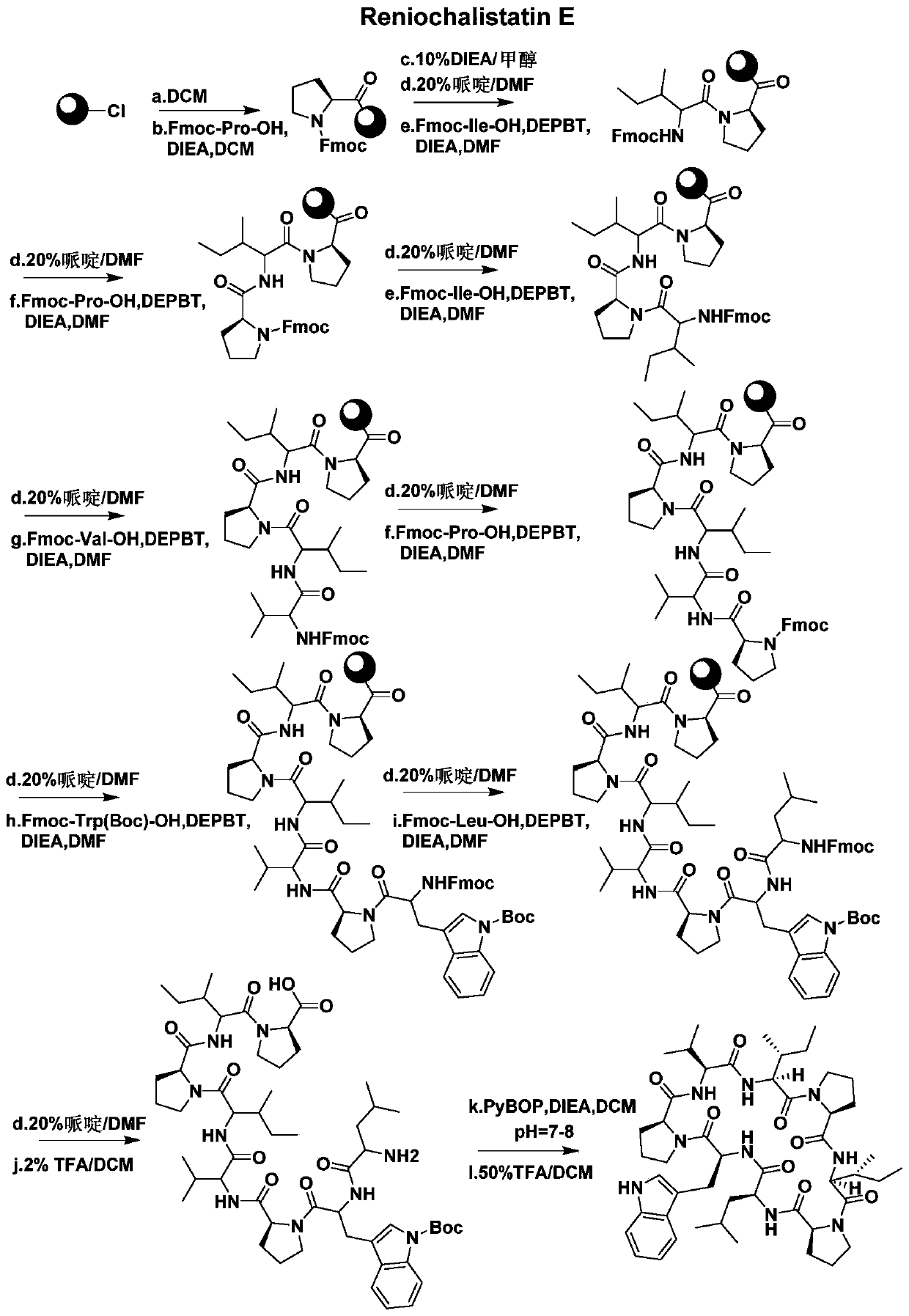
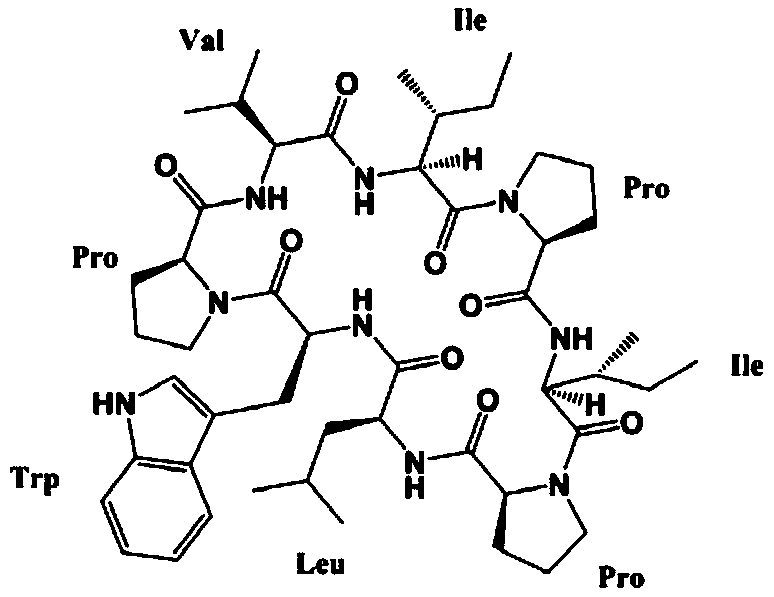
A kind of synthetic method of active cyclic octapeptide reniochalistatin E and its analogs
InactiveCN107827956BLow costShorten the compositing timePeptide preparation methodsBulk chemical productionTryptophanReniochalistatin E
The invention discloses a synthesis method of active cyclic octapeptide Reniochalistatin E and an analogue thereof. A complete synthesis method of the cyclic octapeptide disclosed by the invention adopts a polypeptide solid phase synthesis method, and the synthesis time and purification processes are greatly shortened; in order to prevent the condition that hydrogen on tryptophan indole nitrogen possibly causes decrease of a connection rate and increase of by-products, tryptophan with protection of carbonyl tert-butyl (Boc) on indole is adopted as a raw material, and finally, after cyclizationis finished, a TFA / DCM solution with a high volume ratio is adopted for removing Boc protection to obtain a final product. Compared with traditional condensation reagents and cyclization reagents, condensation reagents DEPBT and DIEA and a cyclization reagent PyBOP adopted by the invention have the advantages of greatly reducing racemization caused by reaction processes and reducing toxicity hazards to the body of a user. The product obtained by the synthesis method provided by the invention is simple in synthesis process, low in raw material cost, mild in reaction condition, high in purity and easy to industrialize, and has broad market prospects.
Owner:JINAN UNIVERSITY
Solid phase synthesis method for adrenomedullin (27-52)
InactiveCN101165067BMild reaction conditionsLess side effectsHormone peptidesPeptide preparation methodsReaction rateTarget peptide
The present invention discloses solid phase synthesis process of adrenal medullarin (27-52). The synthesis process includes the first solid phase Fmoc polypeptide synthesis to graft protective amino acid Fmoc-Ala-OH onto resin, connecting the rest protective amino acids successively, cutting down the synthesized coarse peptide with peptide cutting reagent, and final separating and purifying to obtain the adrenal medullarin (27-52) as the target peptide. The present invention has mild reaction condition, high reaction rate, less side reactions, easy purification, high yield, no corrosion to the equipment and no environmental pollution.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
A method for separating L-glutamic acid and L-pyroglutamic acid from L-glutamic acid refining mother liquor
ActiveCN104177269BAchieve separationSpeed up the coking reaction rateOrganic compound preparationAmino-carboxyl compound preparationL-Pyroglutamic AcidGlutamic acid
The invention relates to a method for separating L-glutamic acid and L-pyroglutamic acid from a glutamic acid refinement mother solution. The method comprises the following steps: 1) carrying out vacuum concentration on the glutamic acid refinement mother solution, cooling to crystallize, and centrifugating, wherein the solid is the L-glutamic acid crude product, and the liquid is for later use; 2) washing the L-glutamic acid crude product with water to obtain pure L-glutamic acid; and 3) heating the liquid obtained in the step 1), carrying out pressurized pyrogenic reaction, cooling, concentrating, cooling to crystallize, centrifugating, collecting the precipitate, adding water to a saturated state, recrystallizing, cooling, and carrying out centrifugal separation to obtain the L-pyroglutamic acid. The method is simple in technical process, and can well solve the problem of the separation technique for glutamic acid and pyroglutamic acid, thereby achieving the goals of enhancing the L-glutamic acid refinement yield and coproducing the L-pyroglutamic acid.
Owner:BENGBU BBCA MEDICINE SCI DEV
Method for improving substitution rate and/or substitution efficiency of hyaluronan-drug conjugates
ActiveUS11458204B2Increase ratingsImprove substitutionPharmaceutical non-active ingredientsDiimineIsopropyl
Disclosed herein is a method for preparing a hyaluronan-drug conjugate. The method uses the ethyl cyano(hydroxyimino)acetate / diisopropylcarbodiimide coupling system in a homogeneous reaction phase, which unexpectedly improves the substitution rate and substitution efficiency of hyaluronan-drug conjugates for various drugs.
Owner:AIHOL CORP
Method for improving substitution rate and/or substitution efficiency of hyaluronan-drug conjugates
ActiveUS20210369852A1Improves degree of substitutionImprove substitution efficiencyPharmaceutical non-active ingredientsDiimineIsopropyl
Disclosed herein is a method for preparing a hyaluronan-drug conjugate. The method uses the ethyl cyano(hydroxyimino)acetate / diisopropylcarbodiimide coupling system in a homogeneous reaction phase, which unexpectedly improves the substitution rate and substitution efficiency of hyaluronan-drug conjugates for various drugs.
Owner:AIHOL CORP
Method for preparing plecanatide
InactiveCN112851761AEffectively stretchExposed reaction sitePeptide preparation methodsBulk chemical productionPlecanatideBiochemical engineering
An embodiment of the invention discloses a method for preparing plecanatide. The method is a novel process route for preparing plecanatide. According to the method, cysteine at a key position in a sequence adopts a special fragment and structure, so the polycondensation of a polypeptide sequence can be broken in a long distance, and the all-solid-phase large-scale production of the linear structure is realized. The plecanatid linear peptide obtained by the method has extremely high purity, and a crude product obtained by primary cyclization and secondary cyclization also has extremely high purity, is convenient to purify and is beneficial to the development of commercial large-scale production.
Owner:杭州多普源生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com