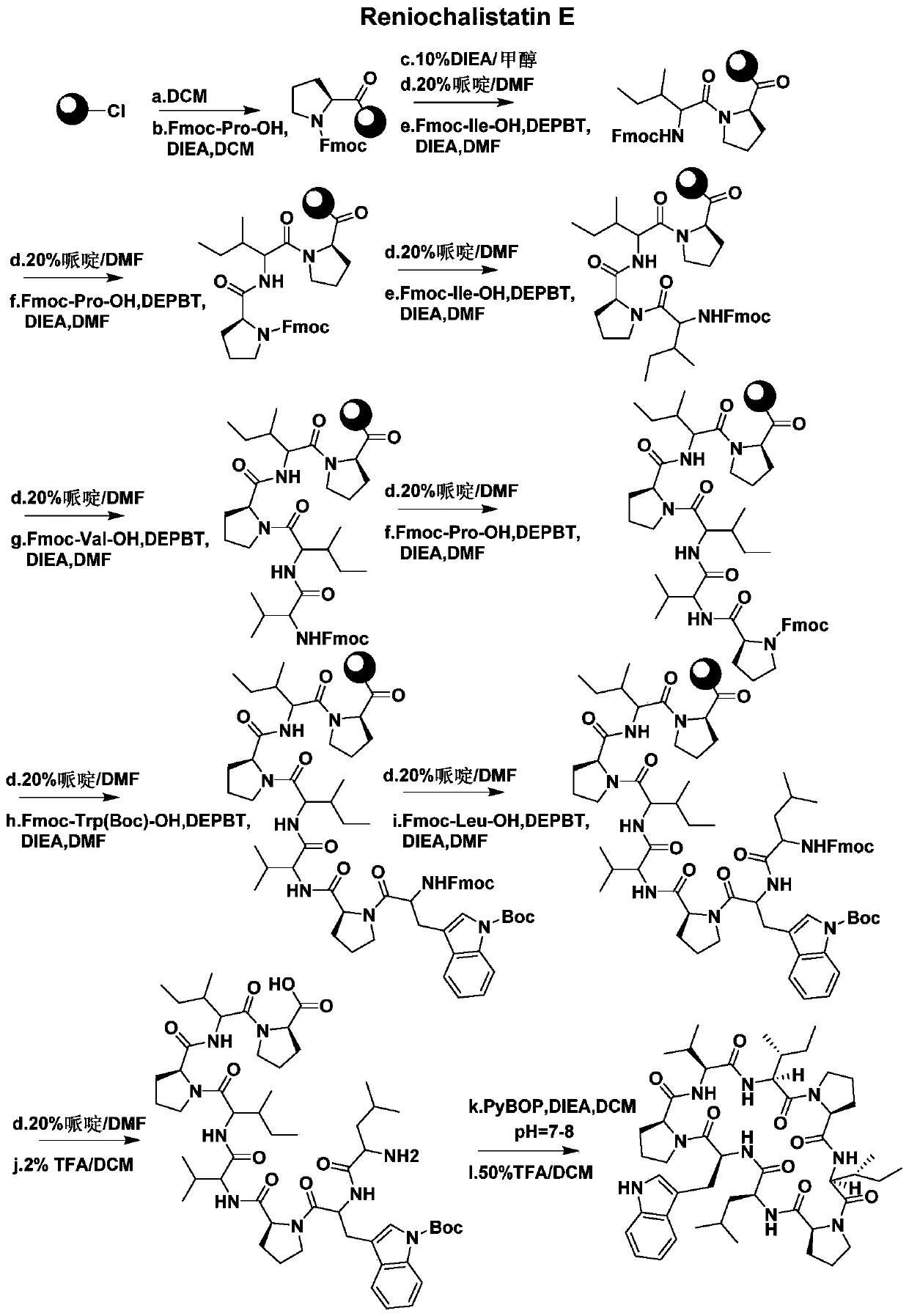
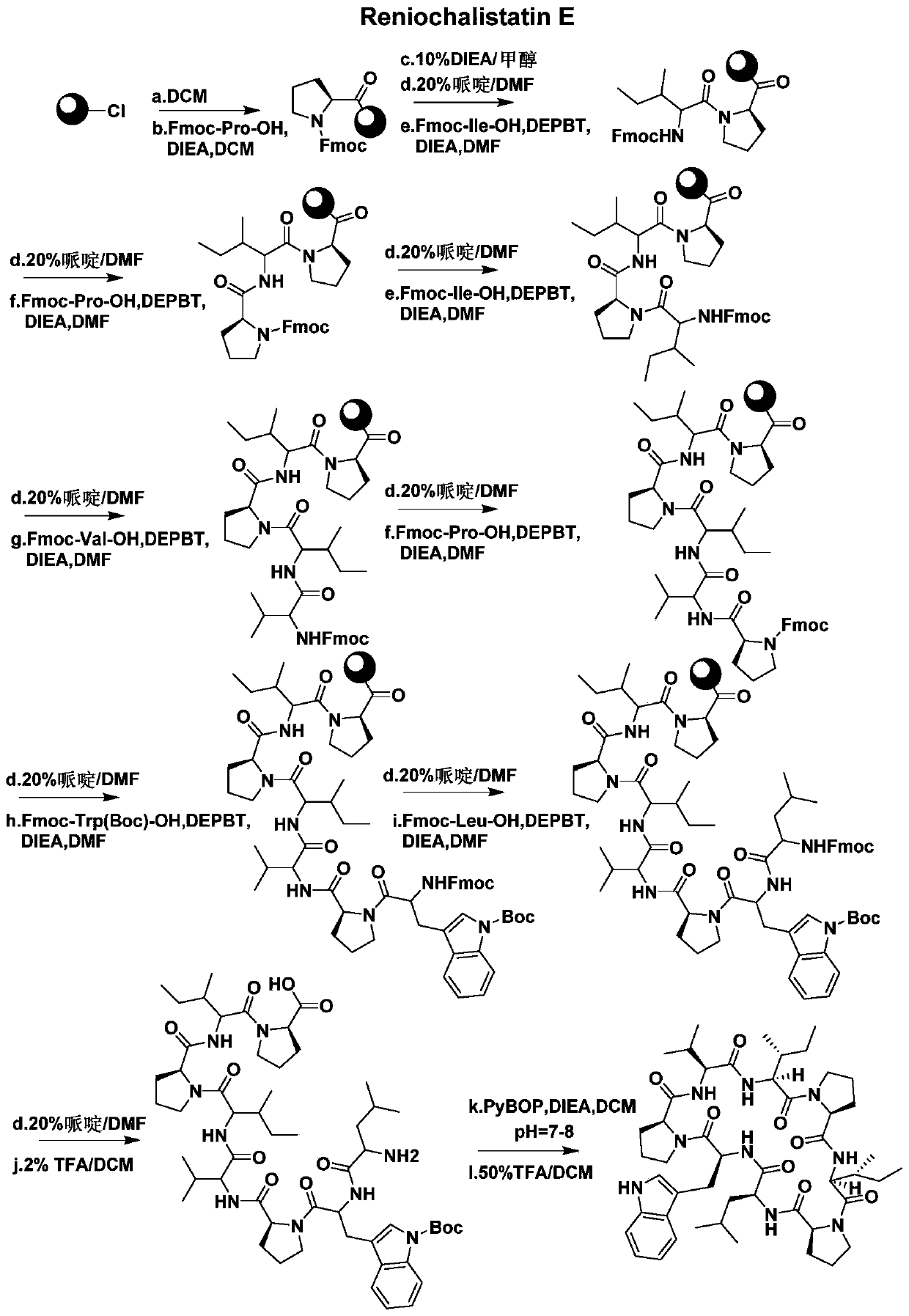
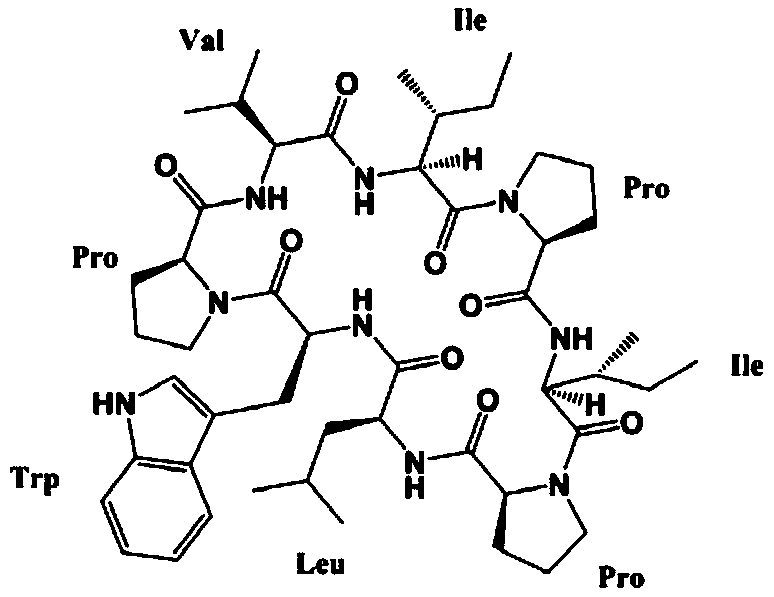
A kind of synthetic method of active cyclic octapeptide reniochalistatin E and its analogs
A synthesis method and technology of cyclic octapeptide, which is applied in the field of synthesis of active cyclic octapeptide Reniochalistatin E and its analogues, can solve the problems of unsatisfactory practical application and small amount of cyclic peptide, and achieve reduction of racemization, reduction of synthesis time, The effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the resin complex (Fmoc-Pro-resin) of fluorenyl methaneoxycarbonyl proline
[0046] Take 1.00 g of 2-chlorotrityl chloride resin (loading capacity 0.985 mmol / g) in a 100 mL peptide synthesis tube, add 20 mL of DCM to swell for 30 minutes. Drain the filtrate, add 0.84g (3mmol) of Fmoc-Pro-OH to it and dissolve it in 20mL of DCM, adjust the pH to 7-8 with nitrogen, react on a shaker for 4 hours, drain the filtrate, and use 10mL of DCM and DMF were washed alternately for 3 times, and the filtrate was drained to obtain Fmoc-Pro-resin.
Embodiment 2
[0047] Example 2: Residual site blocking on resin
[0048] Add 20 mL of DIEA / MeOH solution with a volume ratio of 10% to Example 1, react on a shaker for 1 hour, drain the filtrate, wash with 10 mL of DCM and DMF alternately for 3 times, and drain the filtrate.
Embodiment 3
[0049] Example 3: Protected linear dipeptide-resin complex (Fmoc-Ile-Pro-resin)
[0050] 1. Add 10 mL of 20% piperidine / DMF solution to Example 2, react for 10 to 15 minutes, wash with 10 mL of DCM and DMF alternately for 3 times, and drain the filtrate.
[0051] Repeat step I once.
[0052] Ⅱ. Weigh 1.06g (3mmol) of Fmoc-Ile-OH and 0.90g (3mmol) of DEPBT in a 50mL conical flask, add 15mL of DMF to dissolve, then add 0.52mL (3mmol) of DIEA, and keep at room temperature After activating for 3 to 5 minutes, add it to Example 2 treated in step I, pass nitrogen gas, react on a shaking table for 3 hours, drain the filtrate, wash it with 10 mL of DCM and DMF alternately for 3 times, and drain the filtrate to obtain Fmoc-Ile-Pro-resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com