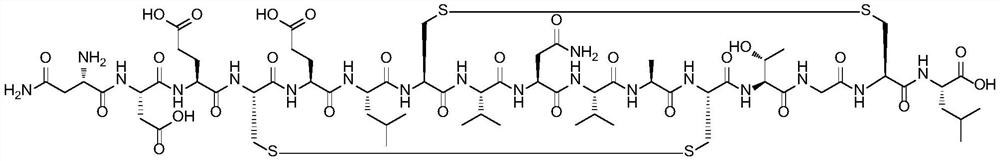
Method for preparing plecanatide
A technology of canatide and puna, applied in the field of polypeptide drug preparation, can solve the problems of long cycle, high cost, complicated process route, etc., and achieve the effect of solving polycondensation and increasing reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of (2R / S,4R)-2-(2,4-dimethoxyphenyl)-thiazole-4-carboxylic acid
[0039] Add 12g of cysteine and 16.6g of 2,4-dimethoxybenzaldehyde into 100mL of water and 100mL of mixed solvent, add 13.6g of water and sodium acetate, stir to form a homogeneous reaction solution, After stirring at room temperature for two hours, a large amount of white solid gradually formed. After 12 hours, the reaction was stopped, filtered, and the obtained paste solid was washed three times with water, washed three times with ethanol, dried to constant weight, and pulverized to obtain 22.5g of (2R / S,4R)-2-(2, 4-Dimethoxyphenyl)-thiazole-4-carboxylic acid, white solid, yield 75%.
Embodiment 2
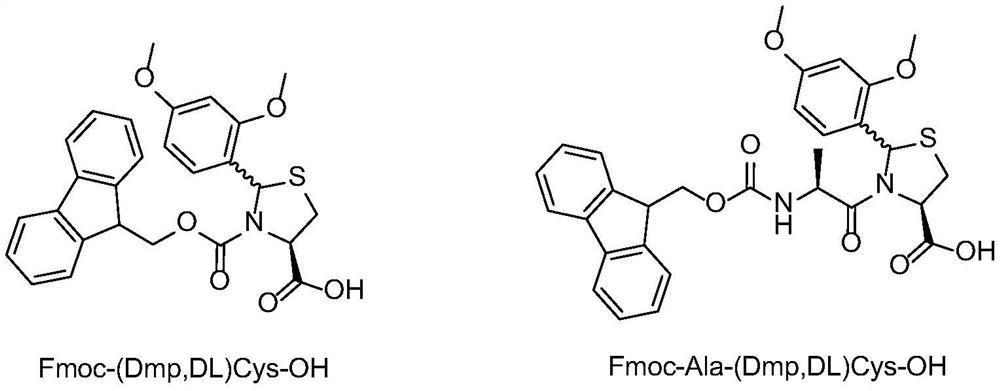
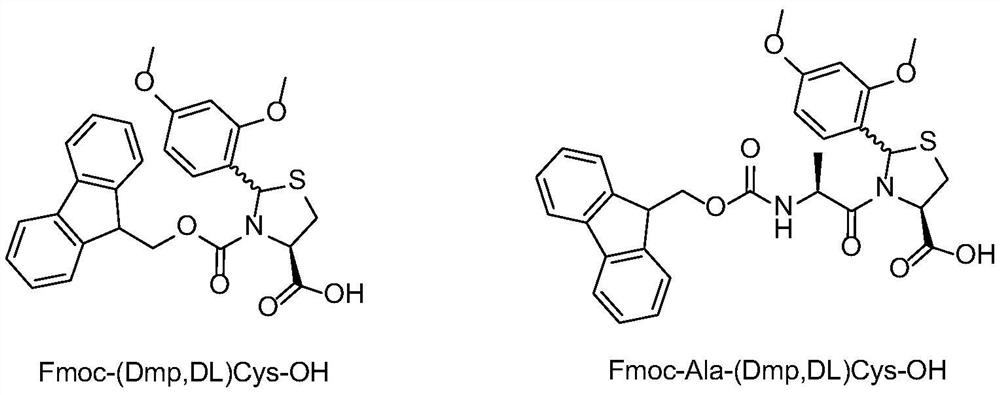
[0041] Synthesis of Fmoc-(Dmp,DL)Cys-OH
[0042] Dissolve 22.5 g of (2R / S, 4R)-2-(2,4-dimethoxyphenyl)-thiazole-4-carboxylic acid obtained in Example 1 in 400 mL of DMF, add 42.5 g of Fmoc -OSu, cooled to 0-5 degrees, 40mL of DIEA is slowly added dropwise to the reaction solution, after the dropwise addition is completed, the entire reaction solution is warmed up to 25 degrees, and after 5 hours of reaction, the HPLC detection reaction has been completed. Add 400mL of dichloromethane to the mixture, wash with water three times, dry the organic phase and spin dry, add 250mL MTBE to the solid, filter after beating for 3 times, and dry the filter cake to constant weight to get the product Fmoc-(2R / S, 4R)-2-(2,4-dimethoxyphenyl)-thiazole-4-carboxylic acid, namely Fmoc-(Dmp,DL)Cys-OH, the yield is 83%.
Embodiment 3
[0044] Synthesis of Fmoc-Ala-(Dmp,DL)Cys-OH
[0045] Dissolve 10 g of (2R / S, 4R)-2-(2,4-dimethoxyphenyl)-thiazole-4-carboxylic acid obtained in Example 1 in 150 mL of DMF, add 15 g of Fmoc-Ala -OSu, cooled to 0-5 degrees, slowly dripping 18mL of DIEA into the reaction solution, after the dropwise addition, the whole reaction solution was warmed up to 25 degrees, and after 5 hours of reaction, the HPLC detection reaction was completed. Add 400mL of dichloromethane to the mixture, wash with water three times, dry the organic phase and spin dry, add 250mL MTBE to the solid, filter after beating for 3 times, and dry the filter cake to constant weight to obtain the product Fmoc-Ala-(Dmp, DL) Cys-OH, yield 77%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com