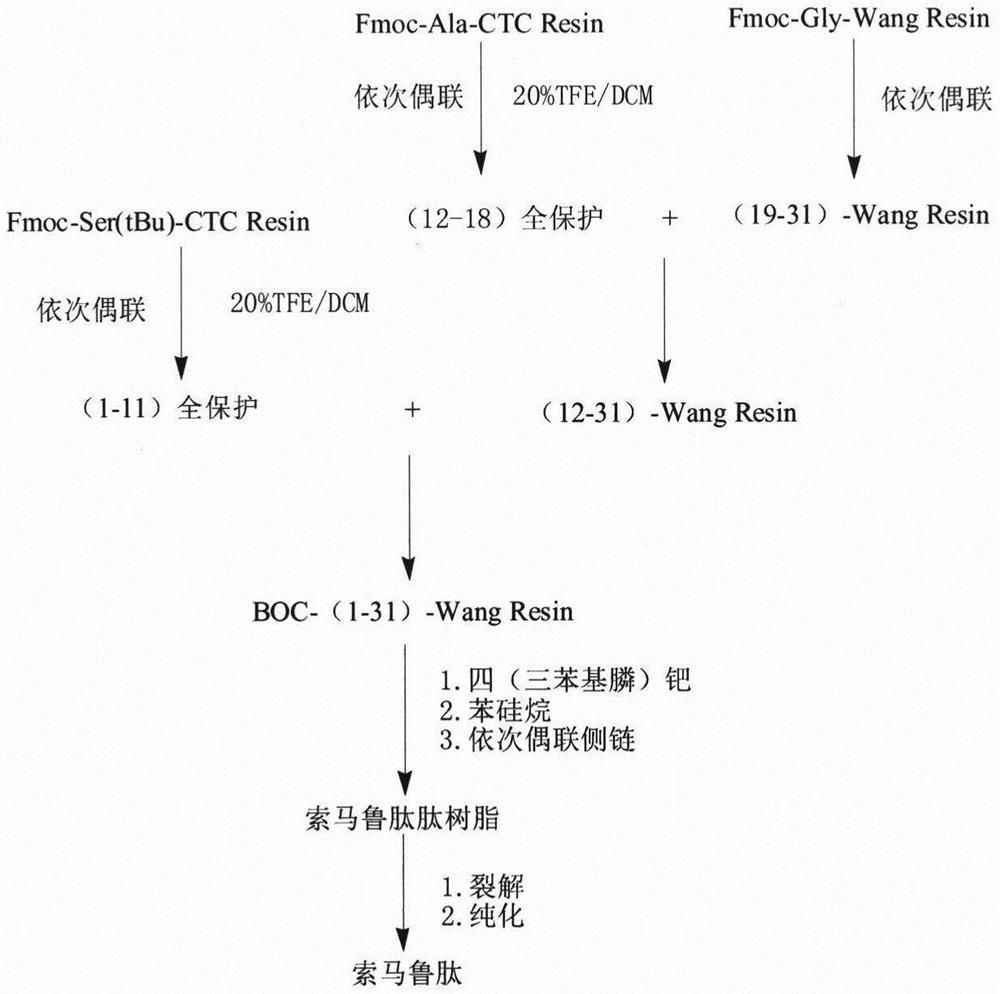
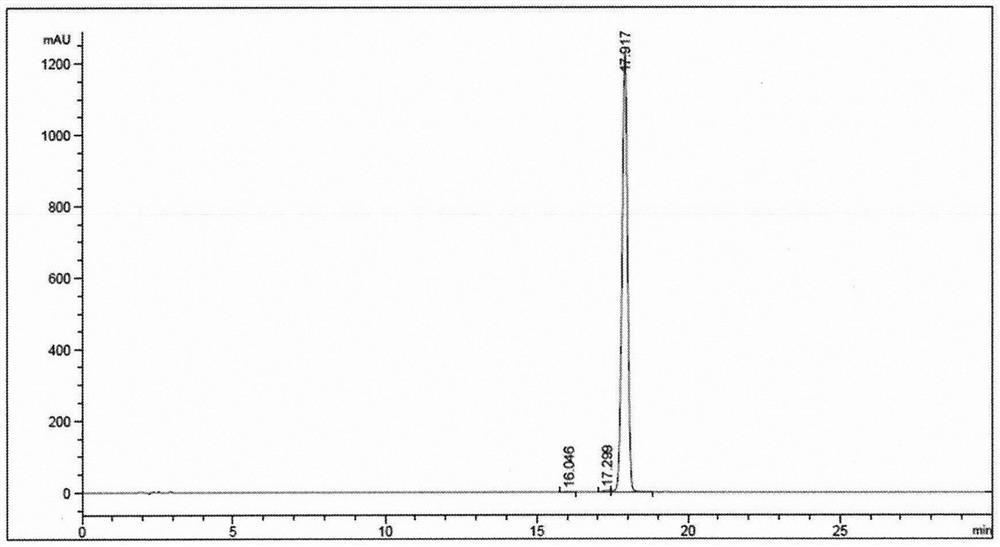
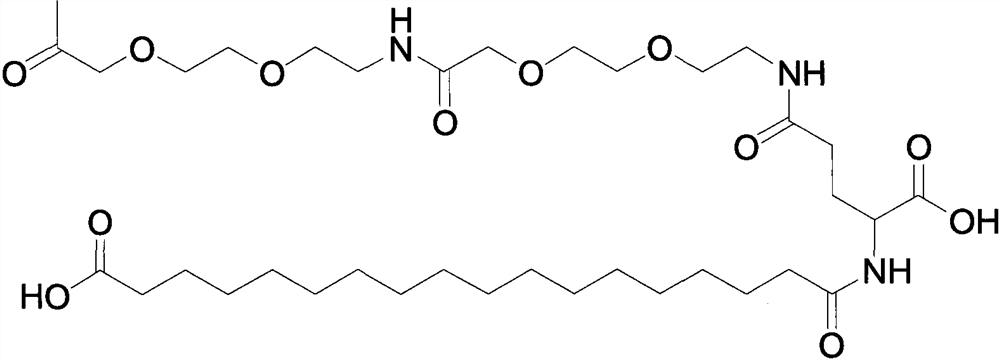
Solid-phase synthesis method of semaglutide
A technology of solid-phase synthesis and condensing agent, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., and can solve the problems of missing peptides, low efficiency, and reduced product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of fully protected peptide of the first fragment of semaglutide:
[0057] 1) Resin swelling: Weigh 100g of 2-Cl-CTC Resin with a substitution degree of 1.0mmol / g, add 800ml DCM to swell the resin for 0.5h, drain the solvent, add 500ml DMF to wash the resin twice, and drain the solvent.
[0058] 2) Preparation of Fmoc-Ser(tBu)-CTC Resin: according to the resin, Fmoc-Ser(tBu)-OH, DIEA molar mass ratio of 1:2:6, weigh 78.7g Fmoc-Ser(tBu)-OH and dissolve Add 105ml DIEA to 500ml DMF, mix well, add the solution to the reaction column for 2 hours, and drain the solvent; add 600ml DMF to wash the resin three times, and drain the solvent; according to the volume ratio of DCM, methanol, and DIEA is 17:2: 1, prepare 1200ml blocking solution, seal the resin twice, each 10min; add 600ml DMF to wash the resin three times, drain the washing solvent; wash with methanol three times, each 10min, drain the solvent, and after vacuum drying, obtain Fmoc-Ser(tBu )-CTC Resin 135....
Embodiment 2
[0062] Preparation of fully protected peptide of the second fragment of semaglutide:
[0063] 1) Resin swelling: Weigh 100g of 2-Cl-CTC Resin with a substitution degree of 1.0mmol / g, add 800ml DCM to swell the resin for 0.5h, drain the solvent, add 500ml DMF to wash the resin twice, and drain the solvent.
[0064] 2) Preparation of Fmoc-Ala-CTC Resin: according to the resin, Fmoc-Ala-OH, DIEA molar mass ratio of 1:2:6, weigh 62.2g Fmoc-Ala-OH and dissolve in 500ml DMF, add 105ml DIEA, After mixing evenly, put the solution into the reaction column to react for 2 hours, and drain the solvent; add 600ml DMF to wash the resin three times, and drain the washing solvent; Twice, 10min each time; add 600ml DMF to wash the resin three times, and drain the washing solvent; wash with methanol three times, each time for 10min, drain the solvent, and vacuum dry to obtain Fmoc-Ser(tBu)-CTC Resin 135.2g, measured The degree of substitution is 0.65mmol / g.
[0065] 3) Preparation of semaglut...
Embodiment 3
[0068] Preparation of semaglutide third fragment peptide resin:
[0069] 1) Resin swelling: Weigh 100g of Wang Resin with a substitution degree of 1.0mmol / g, add 800ml of DCM to swell the resin for 0.5h, drain the solvent, add 500ml of DMF to wash the resin twice, and drain the solvent.
[0070] 2) Preparation of Fmoc-Gly-Wang Resin: According to the molar mass ratio of resin, Fmoc-Gly-OH, HoBt, DIC, DMAP is 1:1:1.2:1.2:0.1, weigh 29.7g Fmoc-Ala-OH, 16.2 Dissolve g HoBt in 450ml DMF, add 18.6ml DIC to activate for 5min, add the activation solution to the resin and react at 30°C for 10min, weigh 1.2g DMAP and dissolve it in 50ml DMF, slowly drop into the reaction column at 30°C and continue to react for 1.5h, pump Dry the solvent; add 600ml DMF to wash the resin three times, and drain the washing solvent; according to the resin, acetic anhydride, and pyridine molar ratio of 1:20:5, add blocking solution, and react for 5 hours; add 600ml DMF to wash the resin three times, and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com