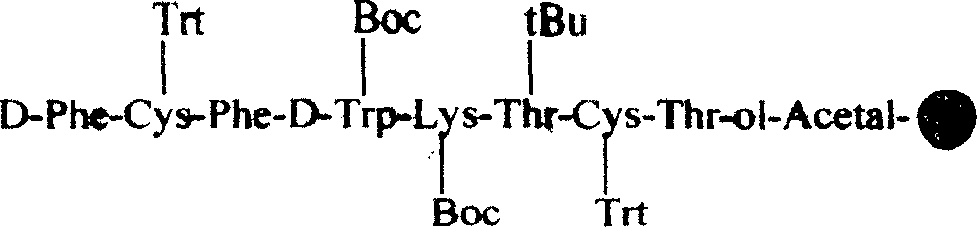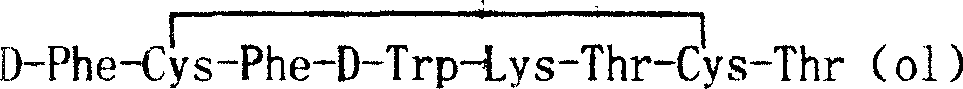Solid-phase synthesis process of octreotide acetate
A technology for solid-phase synthesis of octreotide acetate, which is applied in the field of solid-phase synthesis of octreotide acetate, can solve the problems of long liquid-phase synthesis preparation time, strong corrosive environmental pollution, waste of organic solvents, etc., and achieve high yield and few side reactions , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063]Example 1: Synthesis of Fmoc-Thr-ol-Acetal
[0064] Add Fmoc-Thr-ol (3.28g) into a four-necked flask, dissolve it with anhydrous chloroform (200ml), add 1.90g p-carboxybenzaldehyde, and add 100mg p-toluenesulfonic acid, and then heat and reflux in a water bath at 60°C for reaction. TLC monitoring, after the completion of the reaction, concentrated under reduced pressure on a rotary evaporator to remove the solvent to obtain a light yellow viscous substance. After diluting the viscous substance, it was chromatographed on silica gel, using the developing solution dichloromethane: ethyl acetate (3:1), collecting the solution, and distilling to obtain 3.0 g of the product.
[0065] Among them, Fmoc-Thr-ol can be used directly, or can be prepared by the following example method:
[0066] Fmoc-Thr-OH (7.5g) was added to 100ml THF to dissolve, then 3.1mL ethyl chloroformate and 3.5mL NMM were added, and reacted at 0℃ for 10min, then NaBH was added 1 1.50g, reacted for 10min, stirre...
Embodiment 2
[0067] Example 2 Synthesis of the first peptide
[0068] 1) Add NMP (30ml) to the acetalized product (3.4g), DCC (1.2g) and HOBt (1.6g) in an ice bath, stir electromagnetically, and react at room temperature for 1 hour.
[0069] 2) Put the resin (5.00g) in a solid phase reactor, add dichloromethane / dimethylformamide=1 / 1 30ml solvent, and pass high-purity nitrogen through it and stir for 60 minutes.
[0070] 3) Add the reaction solution of the acetalization product to the solid phase reactor, ventilate and stir, and react at room temperature for 16 hours.
Embodiment 3
[0071] Example 3: Synthesis of the second peptide
[0072] 1) The reaction solution was forced out through the waste liquid outlet with gas, washed with dichloromethane, and then 30 mL of dichloromethane and 1.5 mL of acetic anhydride and 1.5 mL of pyridine were added to the resin in the solid phase reactor, and the reaction was stirred at room temperature for 2 hours.
[0073] 2) The reaction solution was forced out through the waste liquid outlet with gas, washed with dichloromethane, and then 15 mL each of hexahydropyridine and dimethylformamide were added, and reacted at room temperature for 1 hour.
[0074] 3) The reaction solution was forced out through the waste liquid outlet with gas, washed with dichloromethane, and then Fmoc-Cys(Trt)-OH (4.34g), HOBt (1.10g), HBTU (3.10g) were added to the container In the solid phase reactor where the resin was reacted in the previous step, NMP (30 ml) was added to it, while nitrogen gas was introduced, and the reaction was carried out ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com