Preparation method of human vascular endothelial inhibitory peptide
A vascular endothelial and inhibitory peptide technology, applied in the field of anti-tumor applications, can solve problems such as residues, drug resistance, insensitivity, etc., and achieve the effects of low racemization, good product quality and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
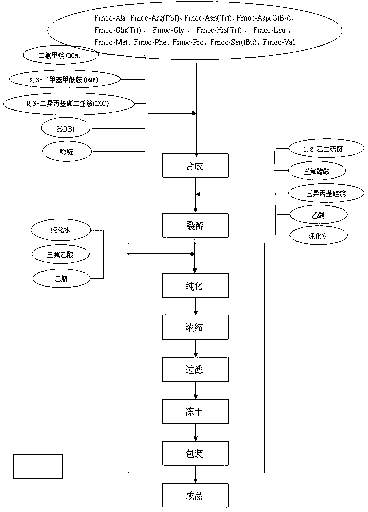
[0015] (1) Swell Fmoc-Asp(OtBu)-Wang with DMF for 30 minutes, remove DMF, add 20% PIP / DMF solution, stir for 5 minutes, wash with DMF, and wash twice.
[0016] (2) Then add 20% PIP / DMF solution, stir and react for 25 minutes, wash with DMF twice, and the ninhydrin test should be positive.
[0017] (3) Weigh Fmoc-Gly-OH (3-5 times the number of moles of amino groups contained in the resin) and equimolar 1-hydroxy-benzo-triazole (HOBt) to dissolve in DMF, and cool in an ice bath for 30 minutes; Take an equimolar amount of DIC, dilute it with DCM, and pre-cool it in a water bath at 0°C to -10°C, add the cooled DIC solution to the DMF solution of Fmoc-Gly-OH, continue to cool and stir for more than 60 minutes, and then add it to The condensation reaction was carried out in the reaction column, and after the reaction (no color detected by ninhydrin chromogenic method) was washed with DMF for 3 times to complete a peptide grafting reaction.
[0018] (4) Repeat the steps of removing...
Embodiment 2
[0023] (1) Preparative HPLC was used for separation and purification, and the mobile phase system was 0.1% TFA-acetonitrile; the preparative liquid chromatography system used 10μm reversed-phase C18 (77mm*250mm) packing, the UV detector was set at 280nm, and the flow rate was 90ml / min .
[0024] (2) Using gradient elution, take the crude product solution and put it on the chromatographic column, start the mobile phase elution, collect the spectrum, observe the change of the absorbance, collect the main peak and use the analytical liquid phase to test the purity, combine the main peak solution, at 30-40 Concentrate under reduced pressure at ℃ to obtain a concentrated solution after purification of the anti-cancer polypeptide. The purity is about 90%, and the refined yield is 60%. The next step of purification is carried out.
[0025] (3) High performance liquid chromatography was used for desalting, and the mobile phase system was 0.2% acetic acid-acetonitrile. Take the concen...
Embodiment 3
[0028] Take the CS8023 acetate aqueous solution, filter it with a 0.45 μm filter membrane, transfer it to a freeze-drying tray, and freeze-dry it. The freeze-drying conditions (which can be adjusted according to different specifications of the freeze-drying machine) are shown in Table 1.
[0029] temperature time (hours) Remark -40℃ 4 pre-freeze room temperature 72 Vacuum room temperature out of the box
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com