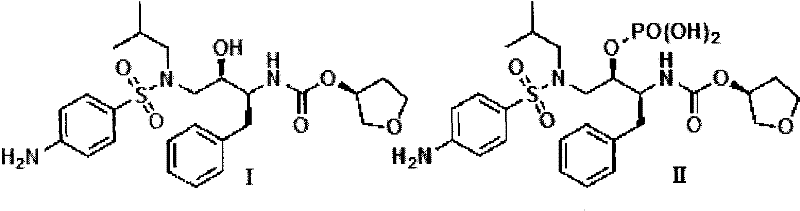
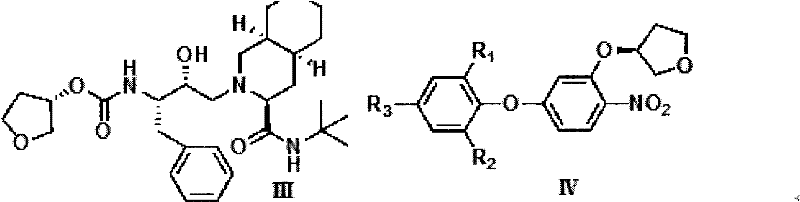
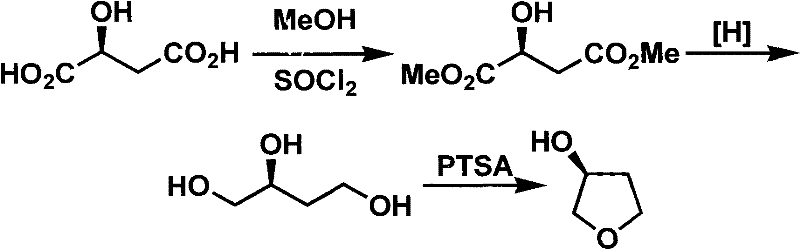
Novel method for preparing S-3-hydroxytetrahydrofuran
A technology of hydroxytetrahydrofuran and S-3-, which is applied in the direction of organic chemistry, can solve the problems of using too much solvent, unfavorable industrial production, and low yield, and achieves the effects of low cost, favorable product separation, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1, the preparation of S-4-chloro-3-methoxybutyric acid ethyl ester.
[0033] Dissolve 100 grams of S-4-chloro-3-hydroxybutyric acid ethyl ester in 500 milliliters of toluene, cool with ice water, add 190 grams of sodium carbonate, and slowly add 91 grams of dimethyl sulfate dropwise under vigorous stirring; the addition is complete , gradually rise to room temperature, after GC detects that the reaction of the raw materials is completed, the solid is removed by filtration, the organic layer is washed with saline, dried over anhydrous sodium sulfate, and the solvent is evaporated under reduced pressure to obtain 93.4 grams of S-4-chloro-3-methoxybutyl Acetate ethyl ester, yield 86%.
[0034] Step 2, the preparation of S-4-chloro-3-methoxy-1-butanol.
[0035] Dissolve 90 grams of S-4-chloro-3-methoxy ethyl butyrate in 150 milliliters of ethanol, cool with ice water, add 23 grams of sodium borohydride, and slowly add 66 grams of calcium chloride dropwise under vigoro...
Embodiment 2
[0042] Step 1: Preparation of ethyl S-4-chloro-3-tert-butoxybutyrate:
[0043] Dissolve 100 g of ethyl S-4-chloro-3-hydroxybutyrate in 400 ml of dichloromethane, add 2 ml of concentrated sulfuric acid, cool to -20°C, and slowly add isobutene until the volume doubles under vigorous stirring ; Gradually rise to room temperature, after GC detects that the raw material has reacted, after washing with ice water, washing with sodium carbonate solution, the organic layer is dried with anhydrous sodium sulfate, and the solvent is evaporated under reduced pressure to obtain 131 grams of S-4-chloro-3-tert-butyl Ethyl oxybutyrate, yield 98%.
[0044] Step 2: Preparation of S-4-chloro-3-tert-butoxy-1-butanol:
[0045] Suspend 18.8 grams of sodium borohydride in 200 milliliters of tetrahydrofuran, control the temperature at 40°C, slowly add 100 grams of tetrahydrofuran solution of S-4-chloro-3-tert-butoxybutyric acid ethyl ester dropwise, dropwise, at 40°C The reaction was complete (GC d...
Embodiment 3
[0053] Step 1: Preparation of ethyl S-4-chloro-3-benzyloxybutyrate
[0054] Dissolve 100 grams of S-4-chloro-3-hydroxybutyric acid ethyl ester in 500 milliliters of tetrahydrofuran, cool with ice water, add 127 grams of sodium carbonate, and slowly add 83.6 grams of benzyl chloride dropwise under vigorous stirring; Gradually rise to room temperature, after the disappearance of raw materials detected by GC, remove the solid by filtration, wash the organic layer with brine, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain 120 g of S-4-chloro-3-benzyloxybutanoic acid Ethyl ester, yield 78%.
[0055] Step 2: Preparation of S-4-chloro-3-benzyloxy-1-butanol
[0056] Under nitrogen protection, suspend 19 grams of sodium borohydride and 21 grams of lithium chloride in 100 milliliters of tetrahydrofuran, stir vigorously for ten minutes, dissolve 128 grams of S-4-chloro-3-benzyloxy ethyl butyrate in 500 milliliters of ethanol The solution was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com