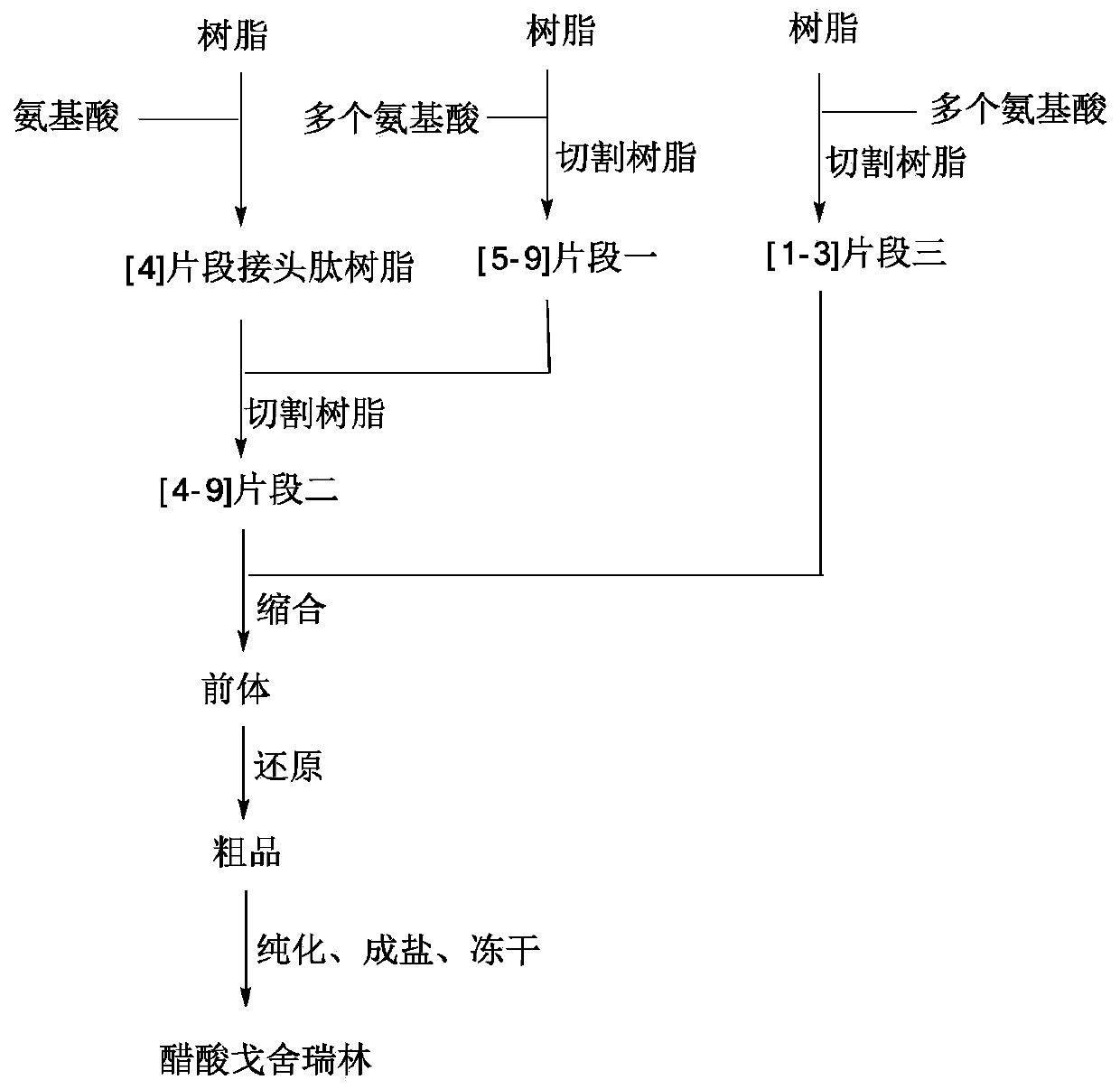
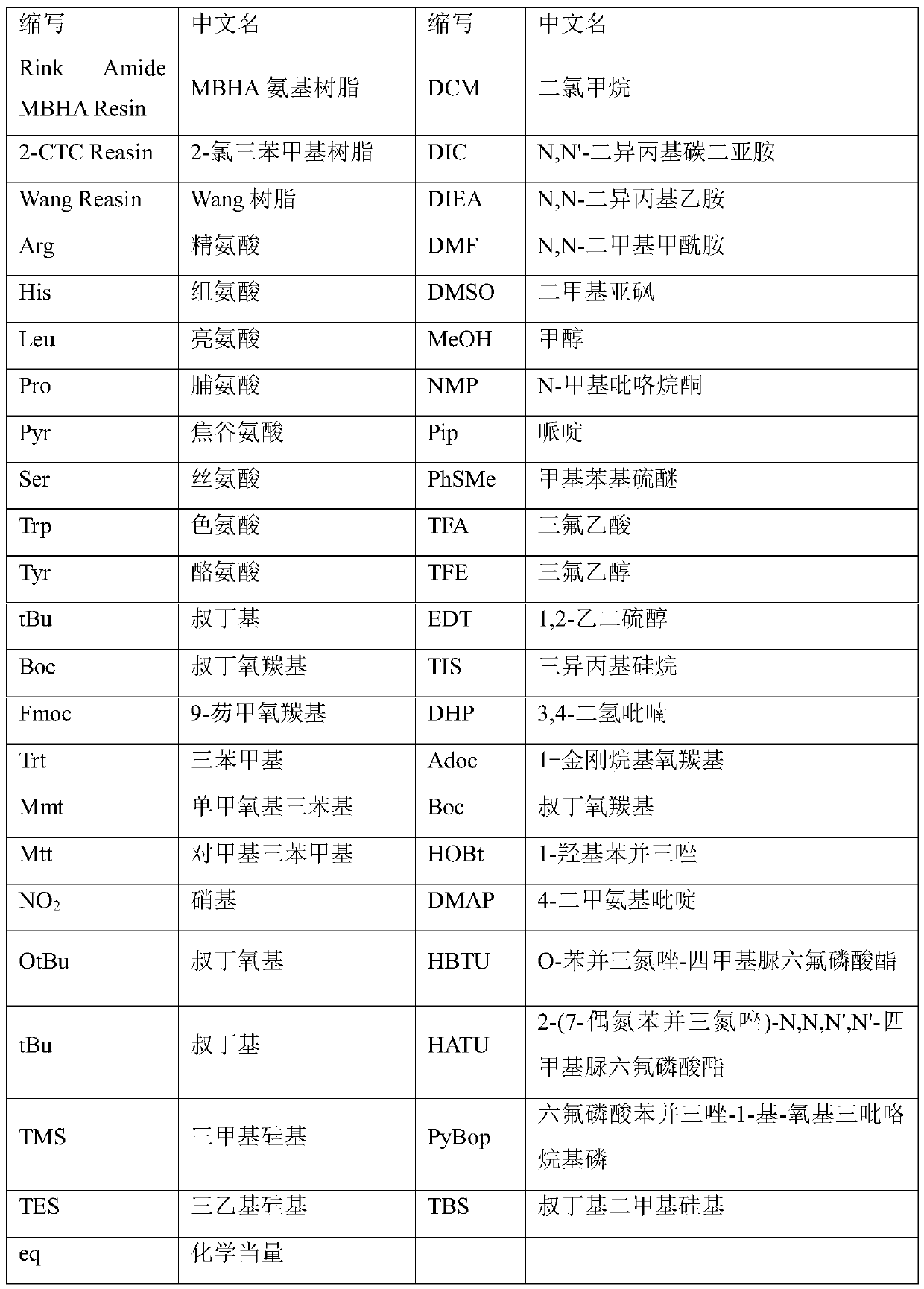
Fragment-process synthesis method of goserelin
A synthesis method and fragment technology, applied in the field of polypeptide drug preparation, can solve the problems of unfavorable industrial production, complicated operation steps, low purity and yield, etc., and achieve the effects of easy synthesis and purification, improved preparation efficiency and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Step (1) synthesis of fragment one
[0054] Add 60.00 g of Wang Reasin and 600 mL of DCM to the solid-phase reaction bottle in turn, stir for 15 minutes, and filter with suction after swelling to obtain a filter cake. Add DMF120mL, Fmoc-Tyr(tBu)-OH (3eq, 41.40g), catalyst DMAP (0.6eq, 2.19g), DIC (3.0eq, 11.37g), HOBt (3.0eq, 12.17g) to the above bottle in turn , stirred and reacted under nitrogen for 2 hours, and filtered with suction to obtain a filter cake. The filter cake was washed with an appropriate amount of DMF, and the filter cake was obtained by suction filtration. The Fmoc deprotection group reaction adopts Pip / DMF mixed solution: the filter cake is mixed with 300mL20% Pip / DMF solution (the volume ratio of DMF and Pip is 80:20, the same below), stirred for 5 minutes, and suction filtered to obtain the filter cake; 300mL of 20% Pip / DMF solution, stirred for 15 minutes, and filtered with suction. The filter cake was washed with an appropriate amount of DMF,...
Embodiment 2
[0067] The difference from Example 1 is the synthesis of Fragment One
[0068] 60.00 g of Wang resin and 600 mL of DCM were sequentially added into the solid-phase reaction bottle for the synthesis of Fragment 1, stirred for 15 minutes, and filtered with suction to obtain a filter cake. Add DMF120mL, Fmoc-Tyr(tBu)-OH (3eq, 41.40g), catalyst DMAP (0.6eq, 2.19g), HBTU (3.0eq, 34.14g), HOBt (3.0eq, 12.17g) to the above bottle in turn , DIEA (3.0 equivalents, 11.67g) was stirred and reacted under nitrogen for 2 hours, and the filter cake was obtained by suction filtration. The filter cake was washed with an appropriate amount of DMF, and the filter cake was obtained by suction filtration. The filter cake was mixed with 300 mL of 20% Pip / DMF solution, stirred for 5 minutes, and suction filtered to obtain the filter cake; then 300 mL of 20% Pip / DMF solution was added, stirred for 15 minutes, and suction filtered. The filter cake is washed with an appropriate amount of DMF, and the...
Embodiment 3
[0071] The difference from Example 1 is the synthesis of Fragment One
[0072] Synthesis of Fragment One
[0073] Add 60.00 g of Wang resin and 600 mL of DCM in sequence to the solid-phase reaction flask, stir for 15 minutes, and filter with suction to obtain a filter cake. Add DMF120mL, Fmoc-Tyr(tBu)-OH (3 equivalents, 41.40g), catalyst DMAP (0.6 equivalents, 2.19g), PyBop (3.0eq, 46.87g), HOBt (3.0eq, 12.17g) to the above bottle in sequence ), DIEA (3.0eq, 11.67g) was stirred and reacted under nitrogen for 2 hours, and the filter cake was obtained by suction filtration. The filter cake was washed with an appropriate amount of DMF, and the filter cake was obtained by suction filtration. The filter cake was mixed with 300mL 20% Pip / DMF solution (DMF:Pip; 80:20, volume ratio), stirred for 5 minutes, and suction filtered to obtain the filter cake; then 300mL 20% Pip / DMF solution was added, stirred for 15 minutes, and suction filtered. The filter cake was washed with an approp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com