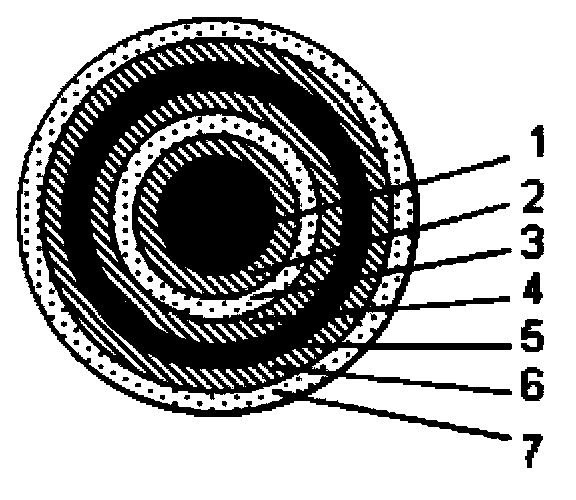
Multilayer coating system enteric preparation for dexlansoprazole
A technology of dexlansoprazole and multi-layer coating, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, digestive system, etc., and can solve problems such as insufficient time for acid suppression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Drug layer Ⅰ:
[0046] The ratio of each substance is as follows:
[0047] components
%(w / w)
Weight (g)
50.00
300.0
30.00
180.0
5.00
30.0
10.00
60.0
5.00
30.0
1200
[0048] The preparation steps are as follows:
[0049] 1) Dissolve hypromellose, meglumine and sodium lauryl sulfate in 1200g of purified water;
[0050] 2) Add dexlansoprazole to the above solution, stir and disperse evenly, and set aside;
[0051] 3) The mixed solution in step 2) is coated on the surface of the sucrose core with a GPCG2.0 fluidized bed to prepare the drug layer I. The temperature of the product during the preparation process is 35-37°C, and the ventilation volume is 1.2-1.4m 3 / min / kg, the spray speed is 10-12g / min / kg. After the preparation is completed, use a fluidized bed to...
Embodiment 2
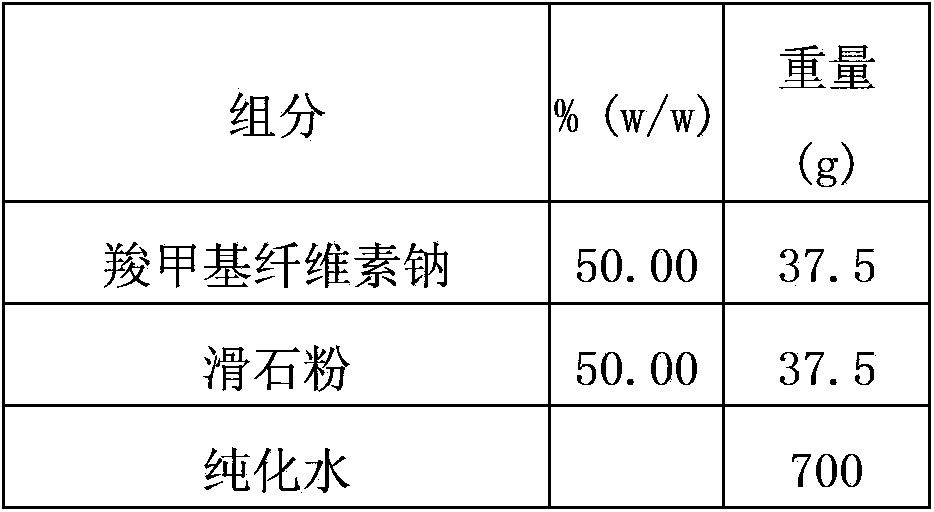
[0102] Different isolation layer formulations have different water vapor permeability, and the isolation layer is prepared according to the following isolation layer formulation, and the water vapor permeability is tested with a W3 / 230 water vapor transmission rate tester.
[0103] The ratio of each material in the isolation layer is as follows:
[0104]
[0105] The preparation steps are as follows:
[0106] 1) Add sodium carboxymethyl cellulose to 3g of purified water and stir to dissolve;
[0107] 2) Add talcum powder to the remaining water and disperse evenly;
[0108] 3) Add the dispersion in step 2) to the solution in step 1), and stir to form a uniform suspension;
[0109] 4) Cut a 11cm square air-permeable membrane, fix the four corners, apply the suspension in step 3) on the air-permeable membrane, and dry while coating, and prepare a 50 mg / cm2 isolation layer after coating membrane.
[0110] 5) Use the W3 / 230 water vapor transmission rate tester to test the wa...
Embodiment 3
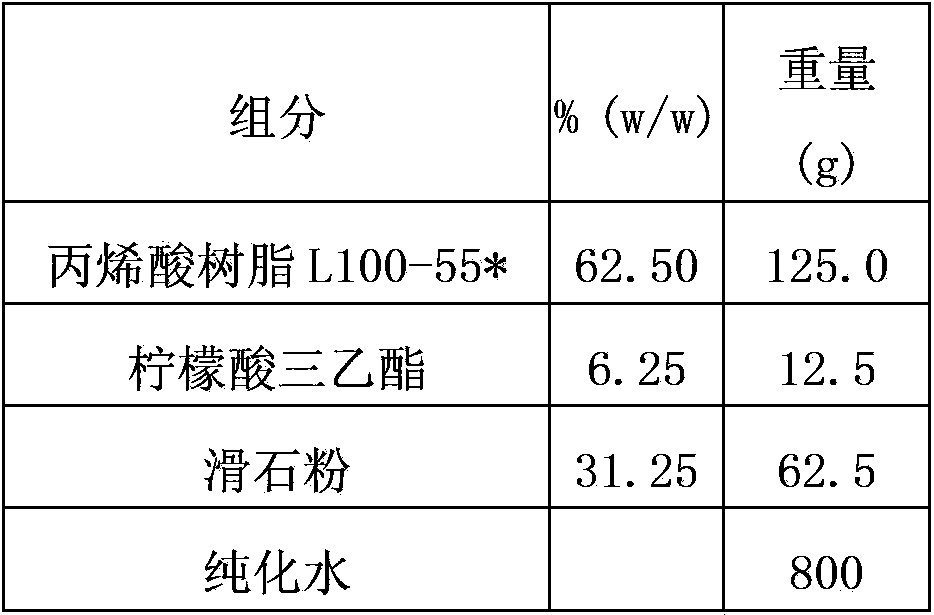
[0112] Prepare the isolation layer according to the following isolation layer prescription, and use the W3 / 230 water vapor transmission rate tester to test the water vapor permeability.
[0113] The ratio of each material in the isolation layer is as follows:
[0114]
[0115] The preparation steps are as follows:
[0116] 1) Add sodium carboxymethyl cellulose to 30g of purified water and stir to dissolve;
[0117] 2) Add talcum powder to the remaining water and disperse evenly;
[0118] 3) Add the dispersion in step 2) to the solution in step 1), and stir to form a uniform suspension;
[0119] 4) Cut a 11cm square air-permeable membrane, fix the four corners, apply the suspension in step 3) on the air-permeable membrane, and dry while coating, and prepare a 50 mg / cm2 isolation layer after coating membrane.
[0120] 5) Use the W3 / 230 water vapor transmission rate tester to test the water vapor transmission rate, and the measured result is 198 g / m2 / day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com