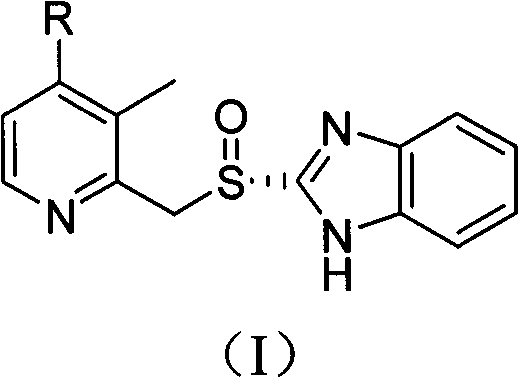
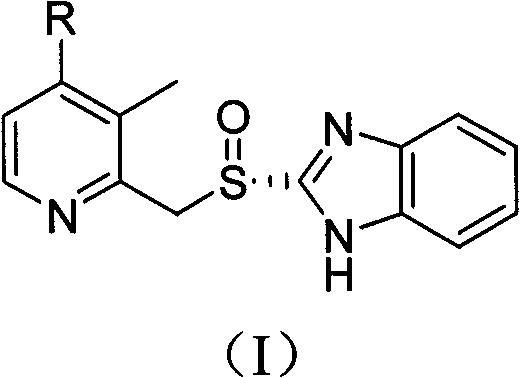
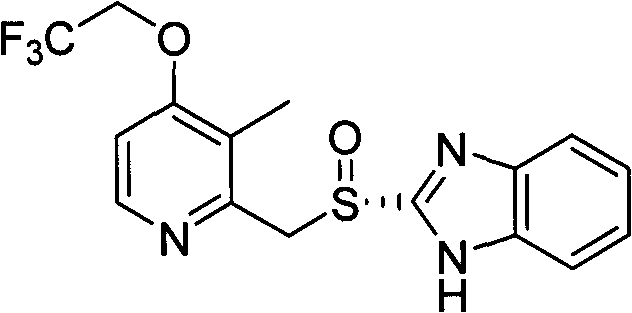
Method for preparing dexlansoprazole
A technology for dexlansoprazole and a compound, which is applied in the field of preparing dexlansoprazole, can solve the problems of high cost, toxicity of active components, prolonging the technological process, etc., and achieves improved yield, good stability, and simplified technological process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Synthesis of R-2-[[(4-chloro-3-methyl-2-pyridyl)methyl]sulfinyl]benzimidazole
[0026]
[0027] Raw material 2-[[(4-chloro-3-methyl-2-pyridyl)methyl]sulfanyl]benzimidazole (645.1g), toluene (3600ml) and L-(+) diethyl tartrate ( 168ml) were mixed, heated to 50-60°C for 0.5h, added titanium tetraisopropoxide (131ml), and continued to react at this temperature for 1h. The reaction solution was cooled to 20°C, diisopropylethylamine (135ml) was added, the temperature was lowered to -10°C, the temperature was controlled from -10°C to 0°C, and 80% cumene hydroperoxide (1203ml) was added to control the temperature at -5°C. ℃ to 0 ℃ reaction 4h. Thin-layer chromatography analysis basically completed the reaction, added 30% sodium thiosulfate solution (1600ml), stirred for 10min, and added dropwise n-hexane (1550ml), tert-butyl methyl ether (1550ml), n-hexane (13000ml) in sequence from 0°C to 10°C ), a white solid precipitated, filtered, and washed once with tert-b...
Embodiment 2
[0030] The synthesis of embodiment two dexlansoprazole
[0031]
[0032] Add the intermediate R-2-[[(4-chloro-3-methyl-2-pyridyl)methyl]sulfinyl]benzimidazole (322.2g) and dimethyl Sulfoxide (2100ml), trifluoroethanol (727.8g) and sodium hydroxide (235.6g) were heated up to 60-70°C and reacted for about 4 hours, and the reaction was basically completed by TLC analysis. Cool the reaction solution to room temperature, add water (20 L) dropwise, adjust the pH to about 7 with 330 ml of glacial acetic acid, precipitate a solid, stir for 5 min, and filter to obtain an off-white solid. This solid was dissolved in ethyl acetate (5 L), dried over anhydrous magnesium sulfate (1000.0 g). After filtration, the filtrate was concentrated under reduced pressure to obtain a brown oil. The oil was chromatographed on a short column of silica gel, the eluent was ethyl acetate-n-hexane-methanol (10:10:1), the qualified components were collected, concentrated, and dried with n-hexane (2 L) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com