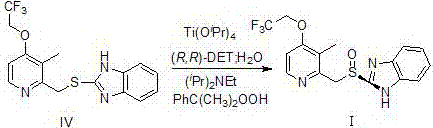Preparation method of (R)-lansoprazole
A technology of dexlansoprazole and dexlansoprazole, which is applied in the field of preparation of dexlansoprazole, can solve problems such as high price of resolving agent, unfavorable large-scale production, serious environmental pollution, etc., and achieve material cost The effect of reducing energy consumption, convenient post-processing, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
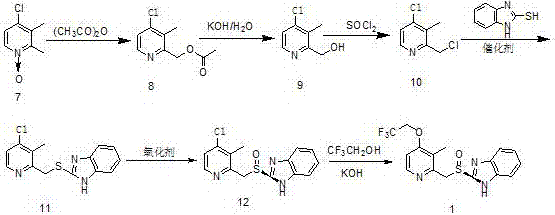
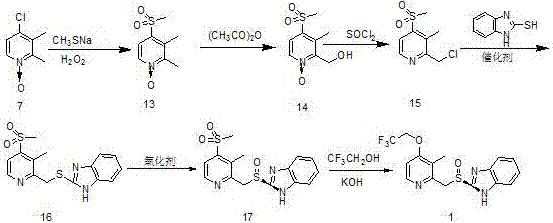
Image
Examples
Embodiment 1
[0035] (1) Preparation of (IV)
[0036]
[0037]Add sodium hydroxide (20.0 g, 0.5 mol) into ethanol (95%, 0.5 L ), stir and dissolve, then add compound Ⅱ (34.1 g, 0.23 mol) and compound Ⅲ (62.7 g, 0.23 mol) in turn, at room temperature The reaction was stirred for 2 h. About 400mL of ethanol was distilled off, water (0.46 L ) was added to the residue, stirred at room temperature for 1 hour, then suction filtered, and the filter cake was washed with water (300mL×3). The obtained solid was beaten and washed with ethyl acetate (300ml) for 1 h, then suction filtered, and the filter cake was dried at 45°C to obtain white powdery solid IV (76.32g), with a yield of 95%.
[0038] (2) Preparation of (I)
[0039]
[0040] Compound IV (60 g, 169.8 mmol), toluene (700 ml), D-(-)-C)-diethyl tartrate (70.1 g, 340.4 mmol), titanium tetraisopropoxide (48.4 g, 170.4mmol) and water (1.53 g, 84.8mmol) were sequentially added to the three-necked flask, stirred and heated to reflux for 1h...
Embodiment 2
[0043] Embodiment 2: the difference with embodiment 1 is that the preparation of (I) adopts sodium methylate and methyl alcohol.
[0044] Add sodium methoxide (27.0g, 0.5mol) into methanol (95%, 0.5L), stir to dissolve, add compound II (34.1g, 0.23mol) and compound III (62.7 g, 0.23mol) in turn, and stir at room temperature React for 2 h. About 400mL of methanol was distilled off, water (0.46 L ) was added to the residue, stirred at room temperature for 1 hour, then suction filtered, and the filter cake was washed with water (300mL×3). The obtained solid was beaten and washed with ethyl acetate (300ml) for 1 h, then suction filtered, and the filter cake was dried at 45°C to obtain white powdery solid IV (74.23g), with a yield of 92.4%.
Embodiment 3
[0045] Embodiment 3: the difference with embodiment 1 is that (I) refining used solvent is different.
[0046] Under nitrogen protection, compound IV (25g, 70.8mmol) was thrown into toluene (100ml), and L-diethyl tartrate (12.1ml, 70.8mmol) and vanadyl acetylacetonate (9.42g, 35.4mmol) were added successively under stirring. ), and stirred for 1 h at 50-55 °C. After cooling, add triethylamine (7.3mL, 53mmol), slowly add cumene hydroperoxide (85%, 18.4ml, 106.2mmol) under stirring, react at room temperature for 8 h, evaporate the solvent under reduced pressure, and obtain an off-white solid Throw it into acetonitrile (180ml), stir for 15min, then filter with suction, and repeat the above operation once for the filtrate (the volume of acetonitrile used is 90ml). The solvent is evaporated under reduced pressure to obtain compound I1 (51.2g, 81.5%) as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com