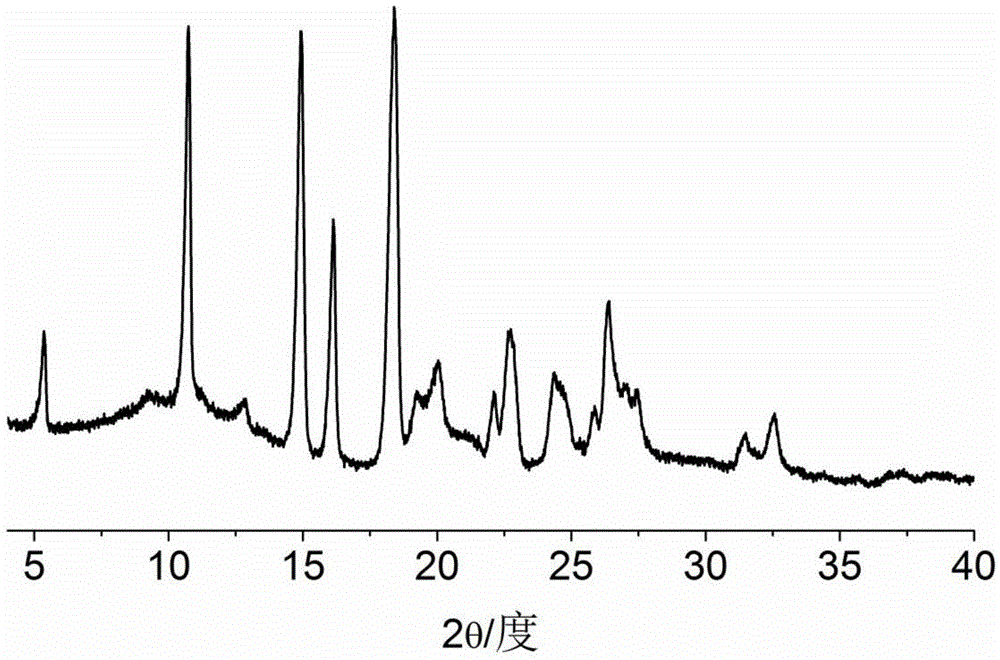
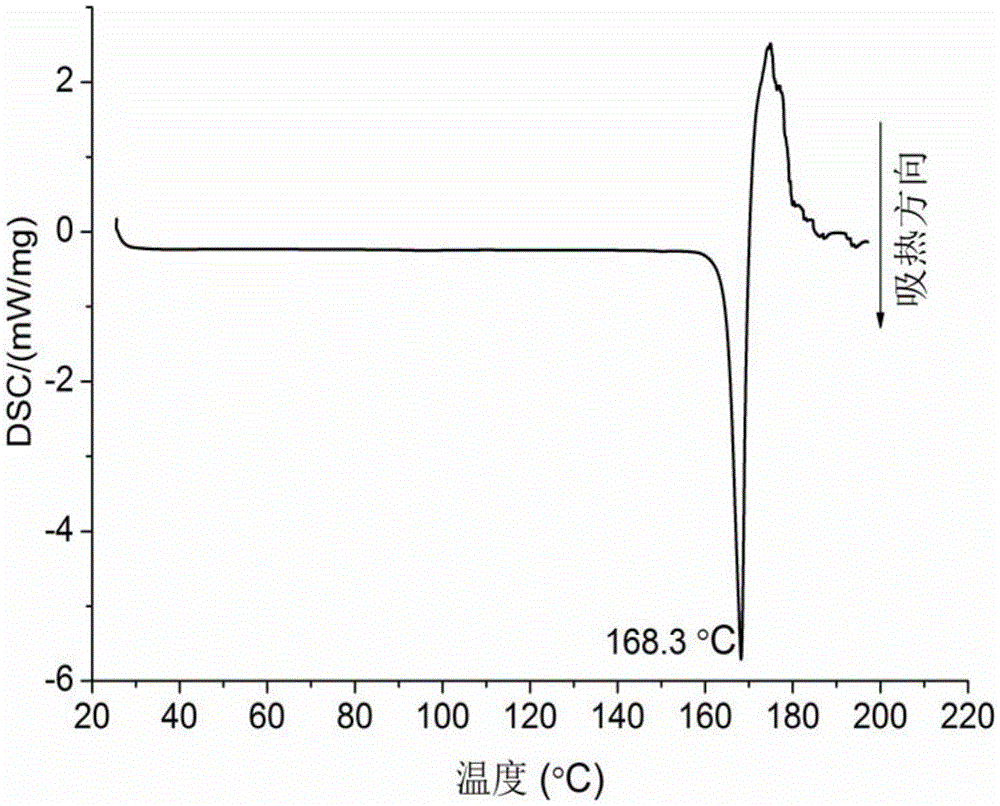
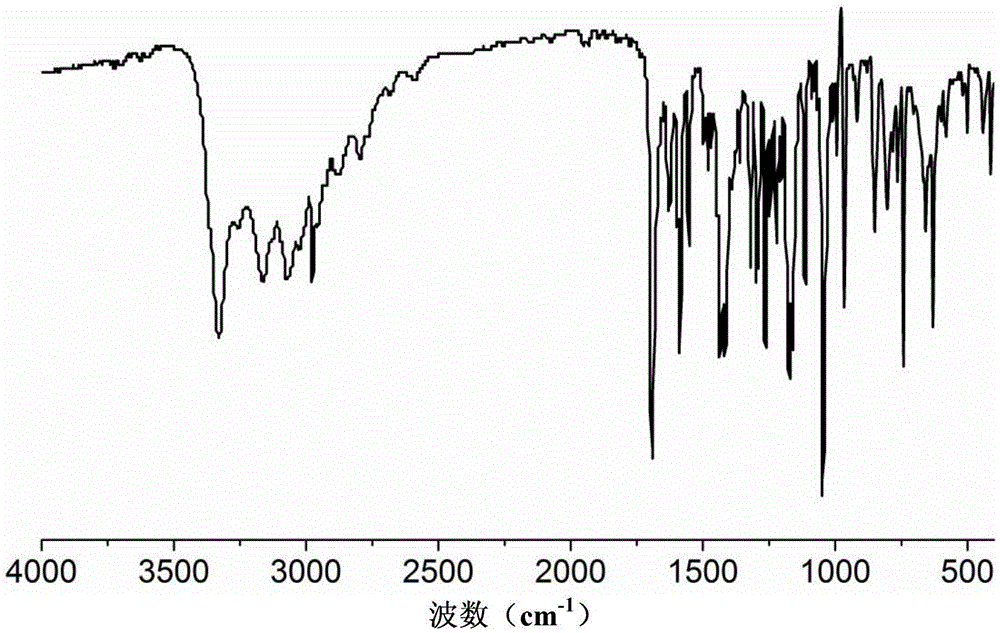
Dexlansoprazole-isonicotine eutectic and application of eutectic
A technology of dexlansoprazole and isonicotine, applied in the direction of medical preparations containing active ingredients, digestive system, organic active ingredients, etc. Poor stability and other problems, to achieve the effect of high crystal purity, excellent crystal stability and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Dissolve 144 mg of dexlansoprazole and about 50 mg of isonicotine (molar ratio 1:1) in 2 to 3 mL of acetonitrile solvent, heat to 40°C, and use an organic nylon head with a filter pore size of about 0.22 μm After filtration, the two solutions were mixed, left to stand at room temperature for 24 hours to evaporate, then filtered, and vacuum-dried at room temperature for 24 hours to obtain 134 mg of a white blocky solid with a yield of about 69%.
Embodiment 2
[0035] Example 2 Dissolve 295 mg of dexlansoprazole and about 50 mg of isonicotine (molar ratio: 2:1) in 2 to 3 mL of acetonitrile solvent, heat to 40°C, and use an organic nickel with a filter pore size of about 0.22 μm. After the faucet was filtered, the two solutions were mixed, left to stand at room temperature for 24 hours to evaporate, then filtered, and vacuum-dried at room temperature for 24 hours to obtain 180 mg of a white blocky solid with a yield of about 52%.
Embodiment 3
[0036] Example 3 Dissolve 142mg of dexlansoprazole and about 98mg of isonicotine (molar ratio: 1:2) in 2-3mL of acetonitrile solvent respectively, heat to 40°C, and use organic nickel with a filter pore diameter of about 0.22μm After the faucet was filtered, the two solutions were mixed, left to evaporate at room temperature for 24 hours, then filtered, and vacuum-dried at room temperature for 24 hours to obtain 159 mg of white blocky solid, with a yield of about 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com