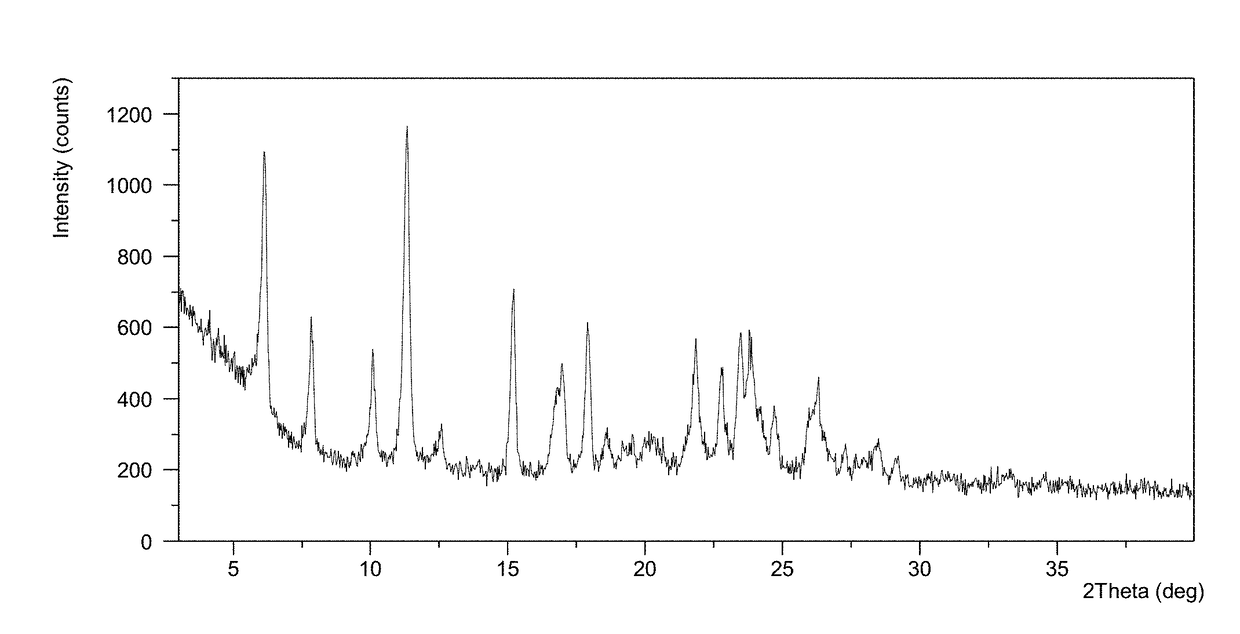
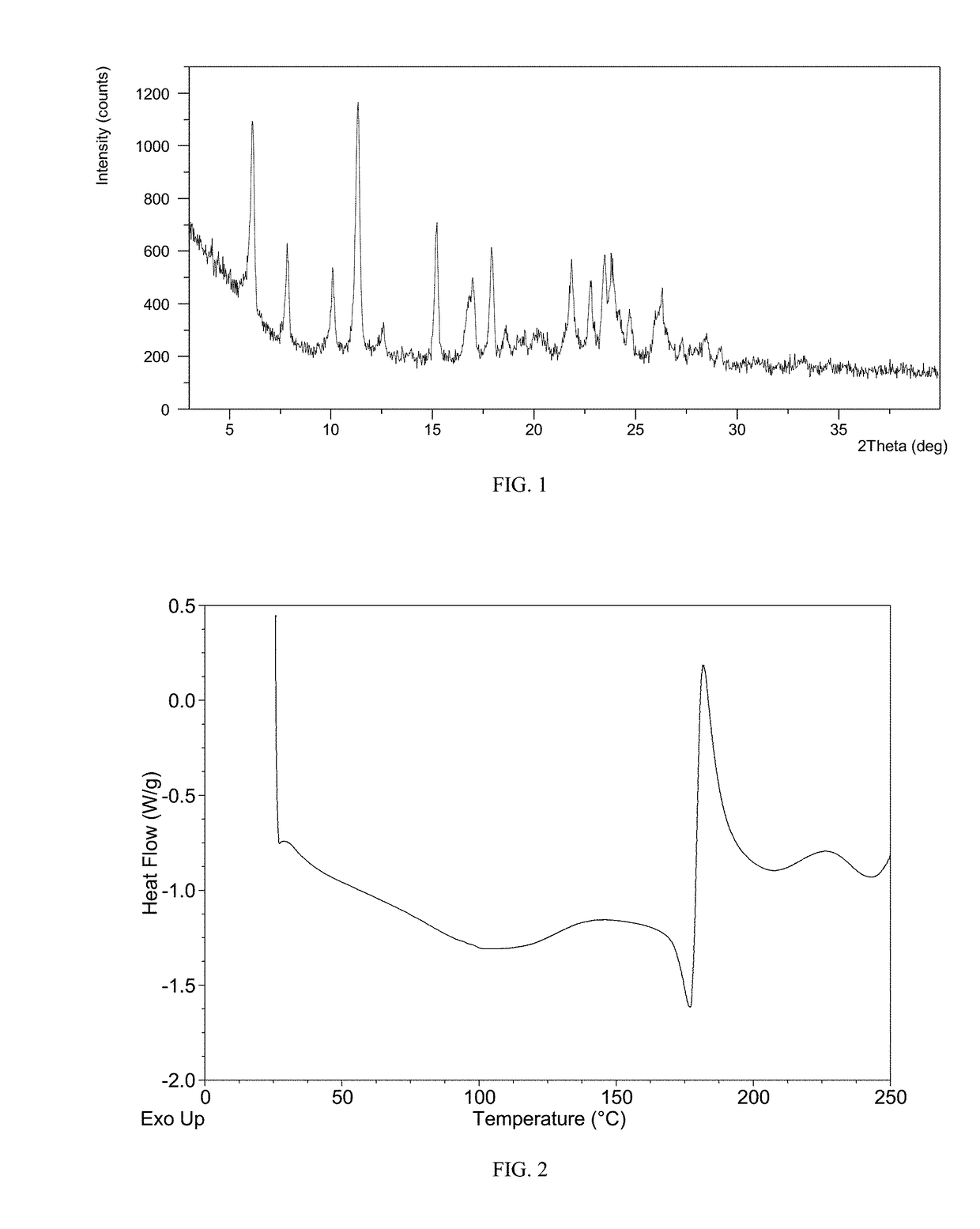
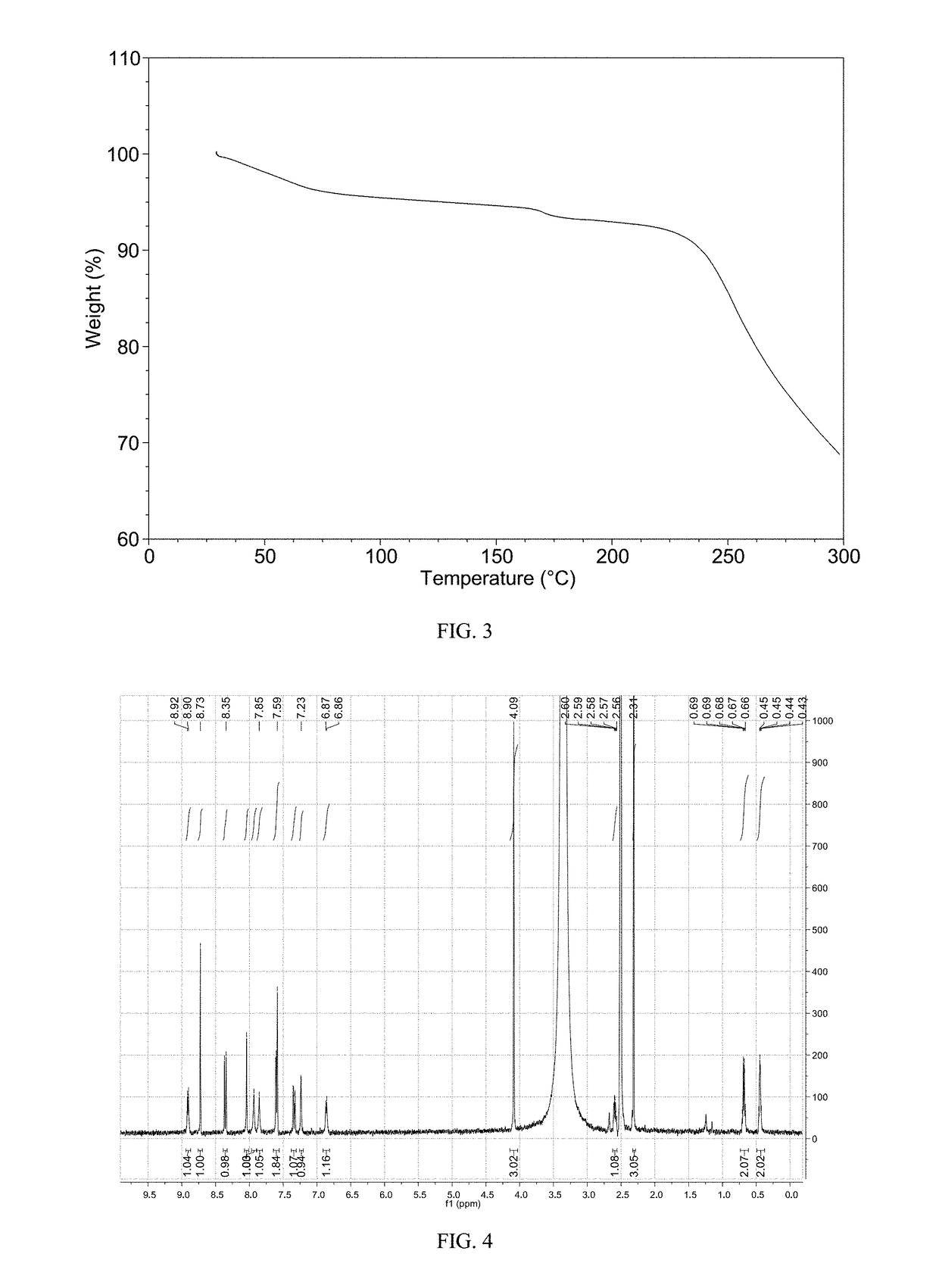
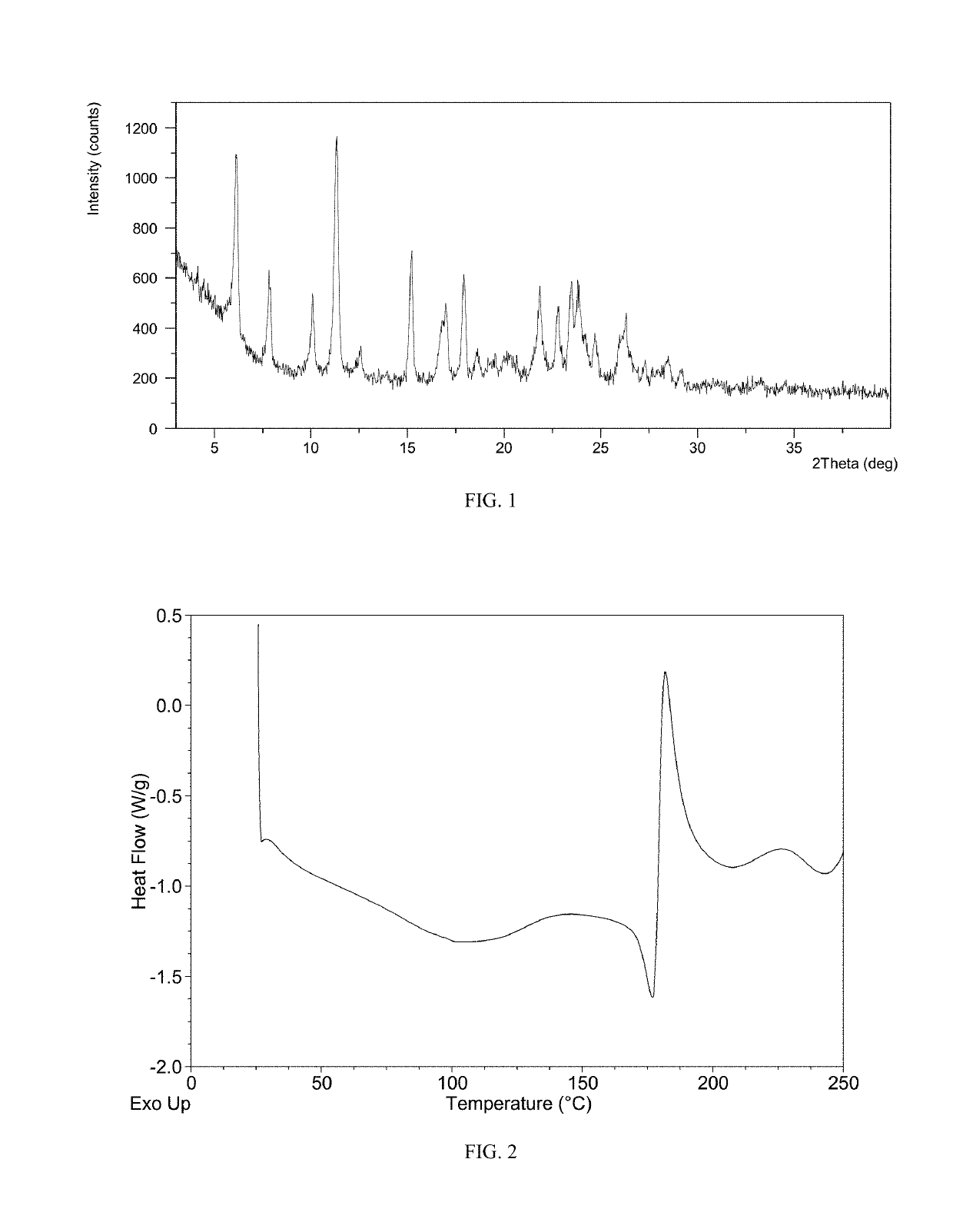
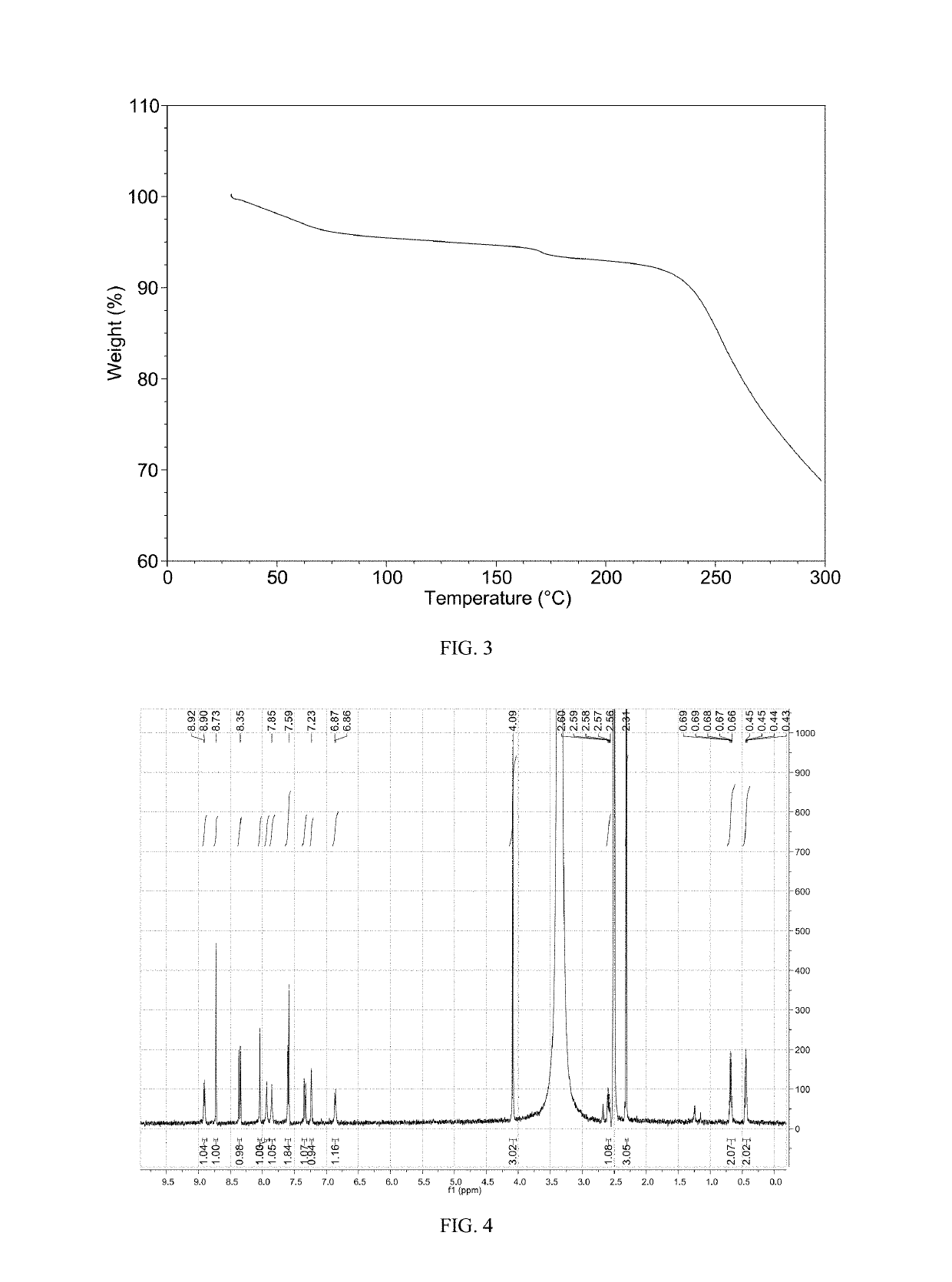
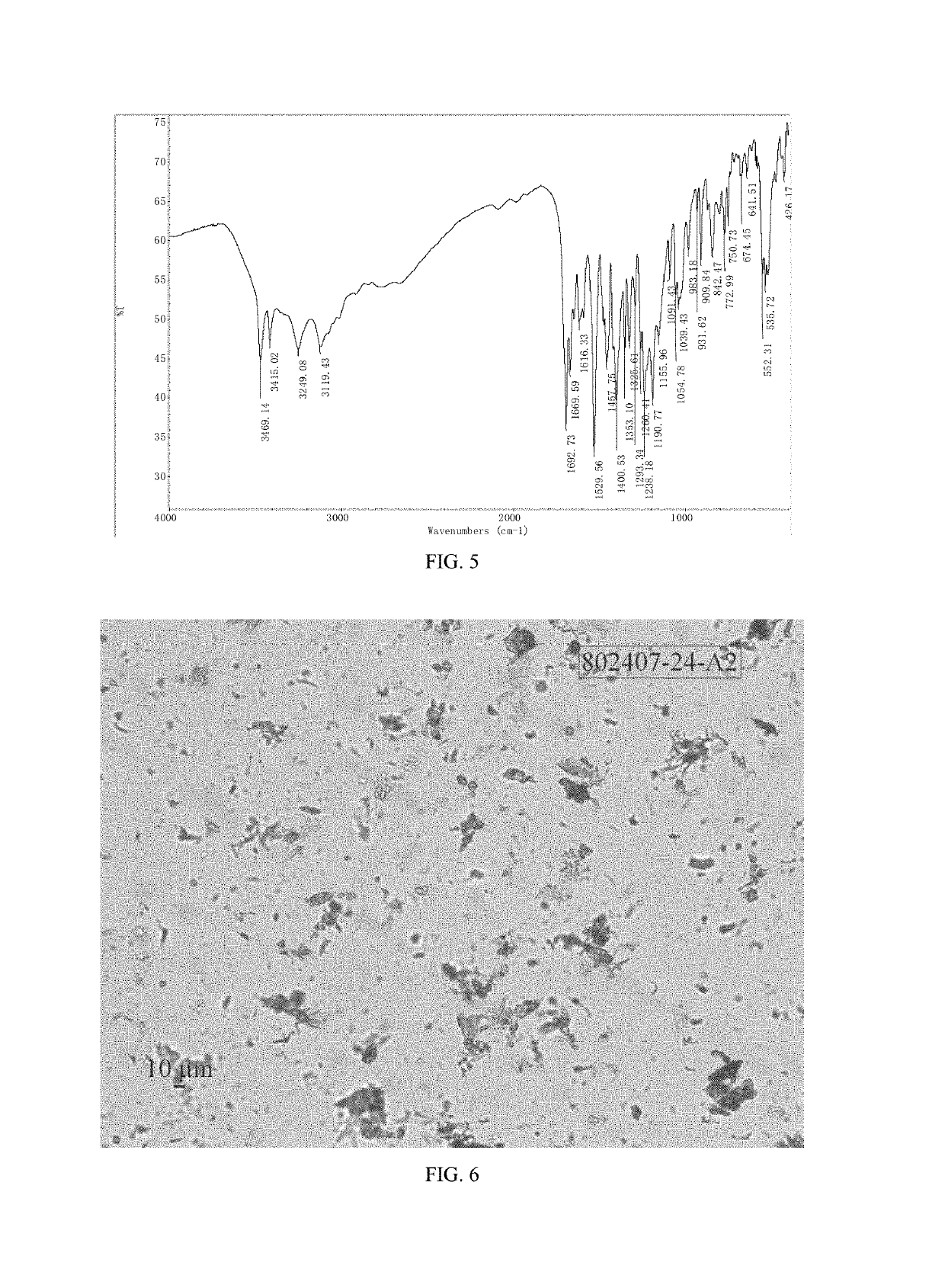
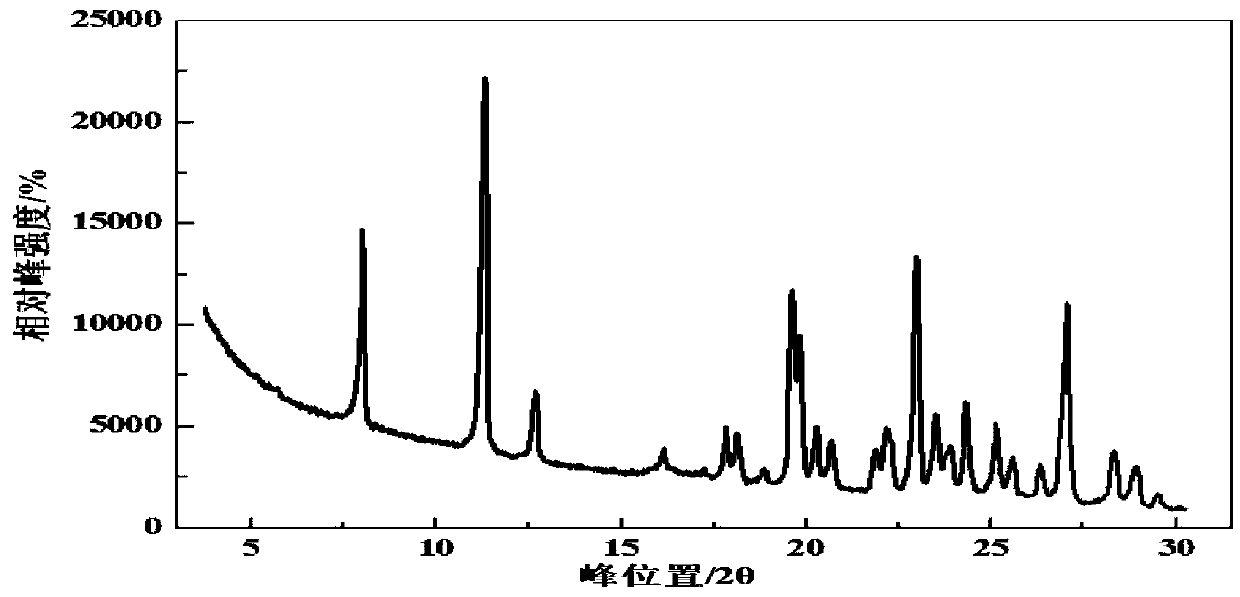
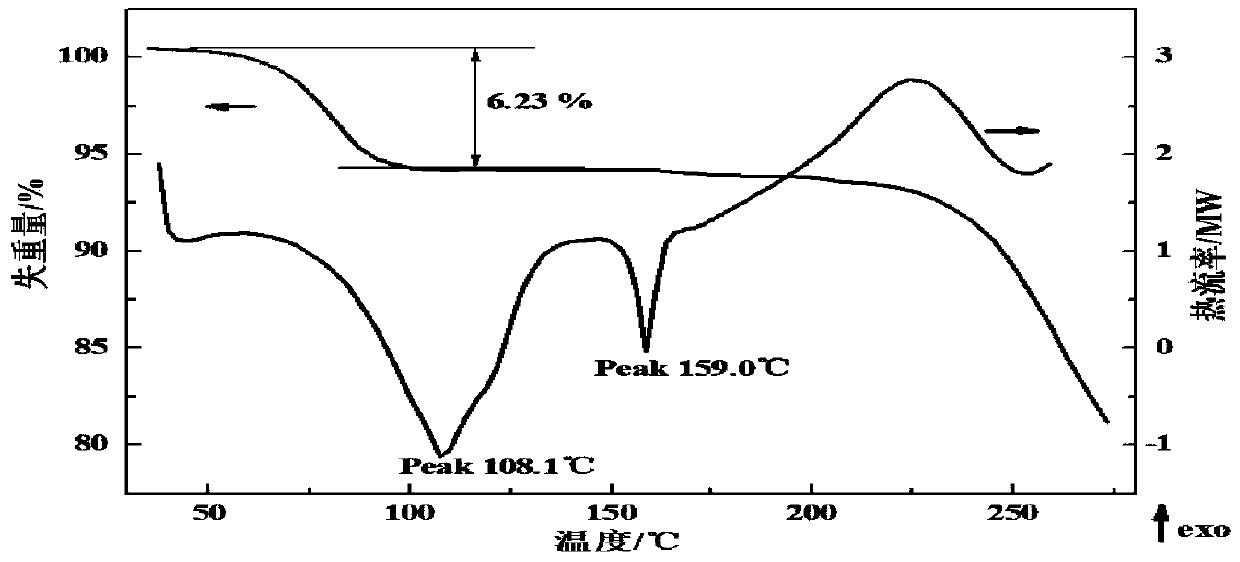
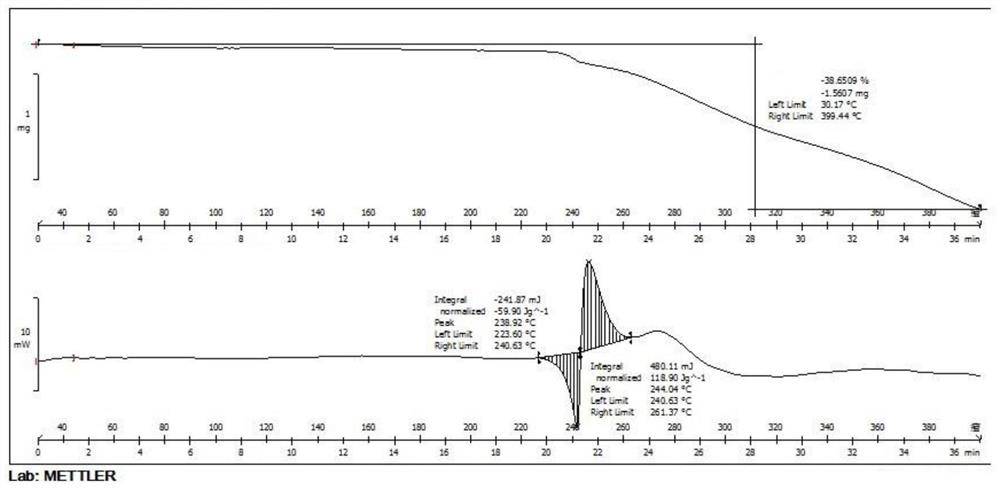
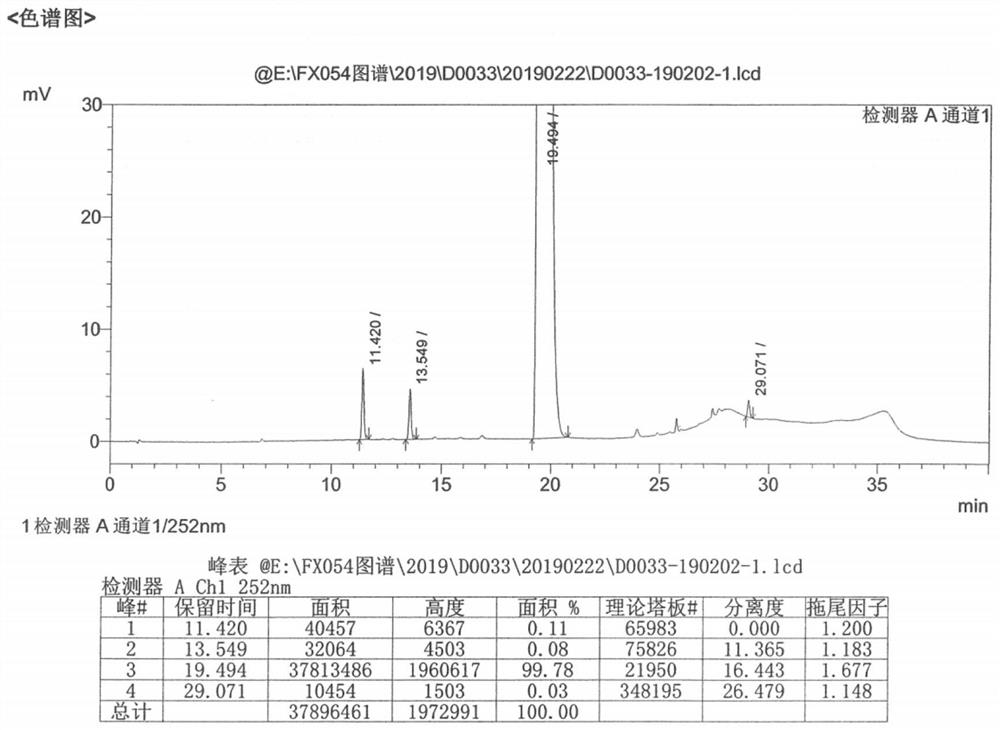
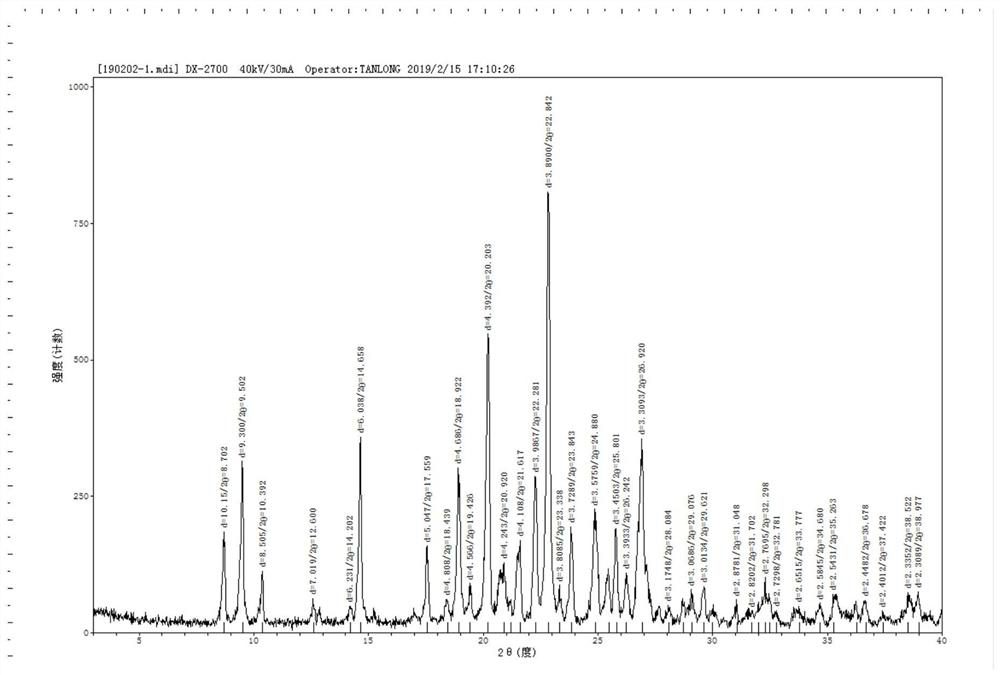
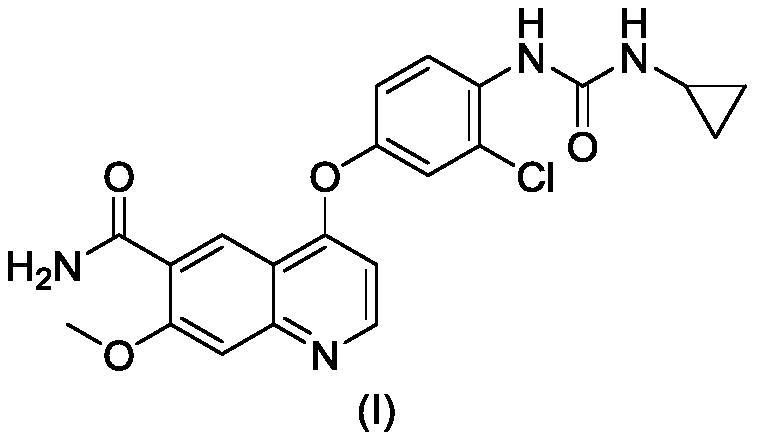
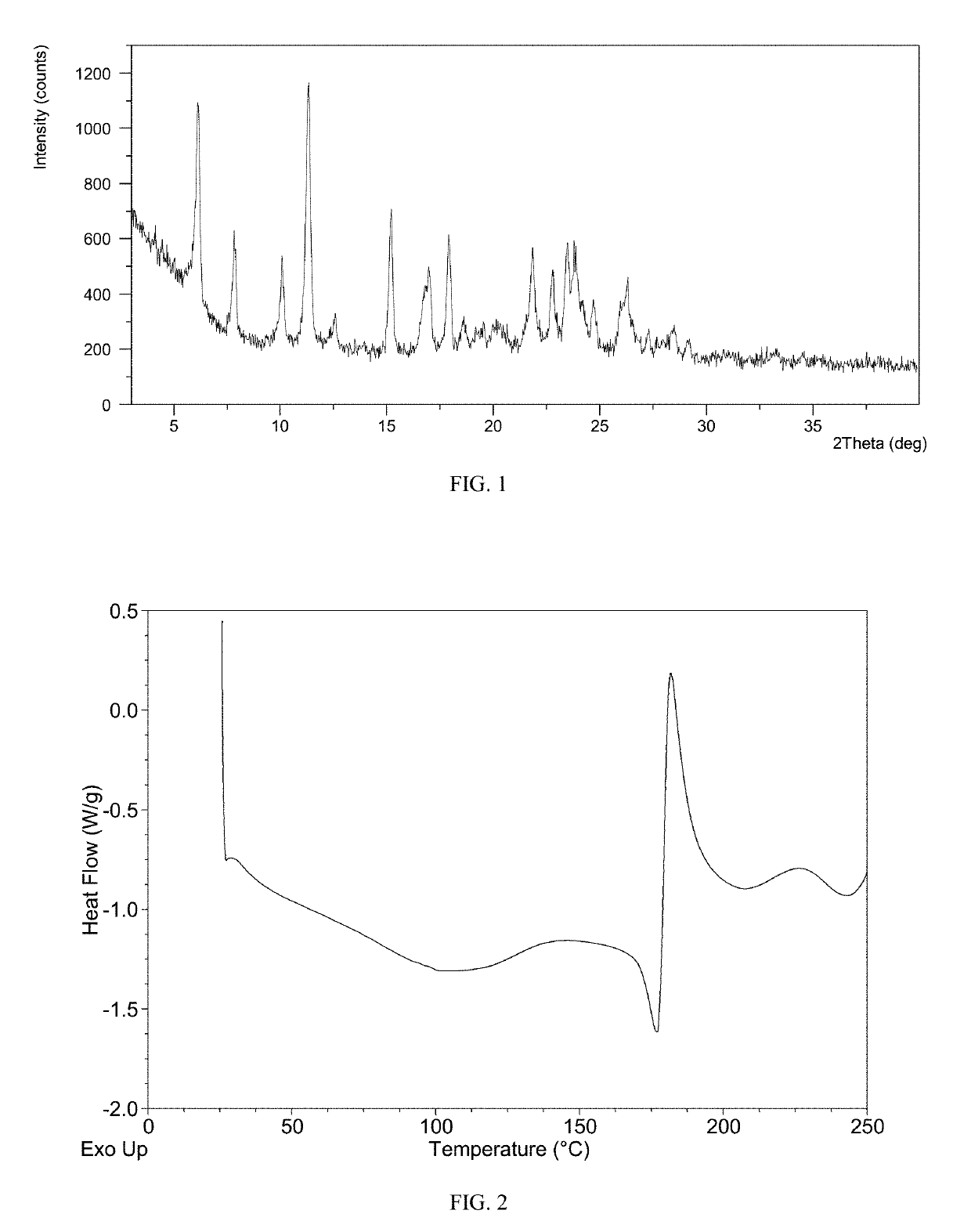
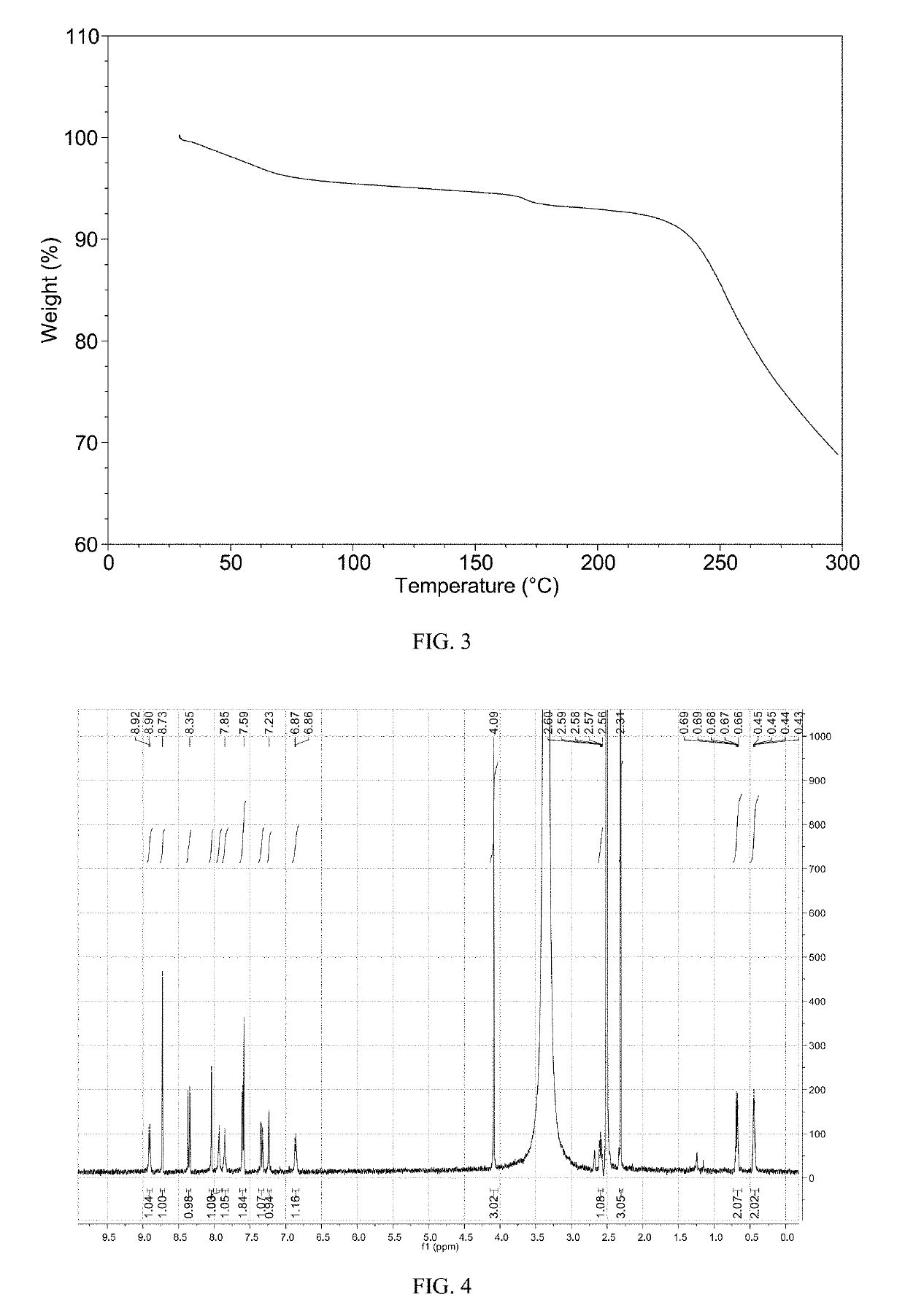
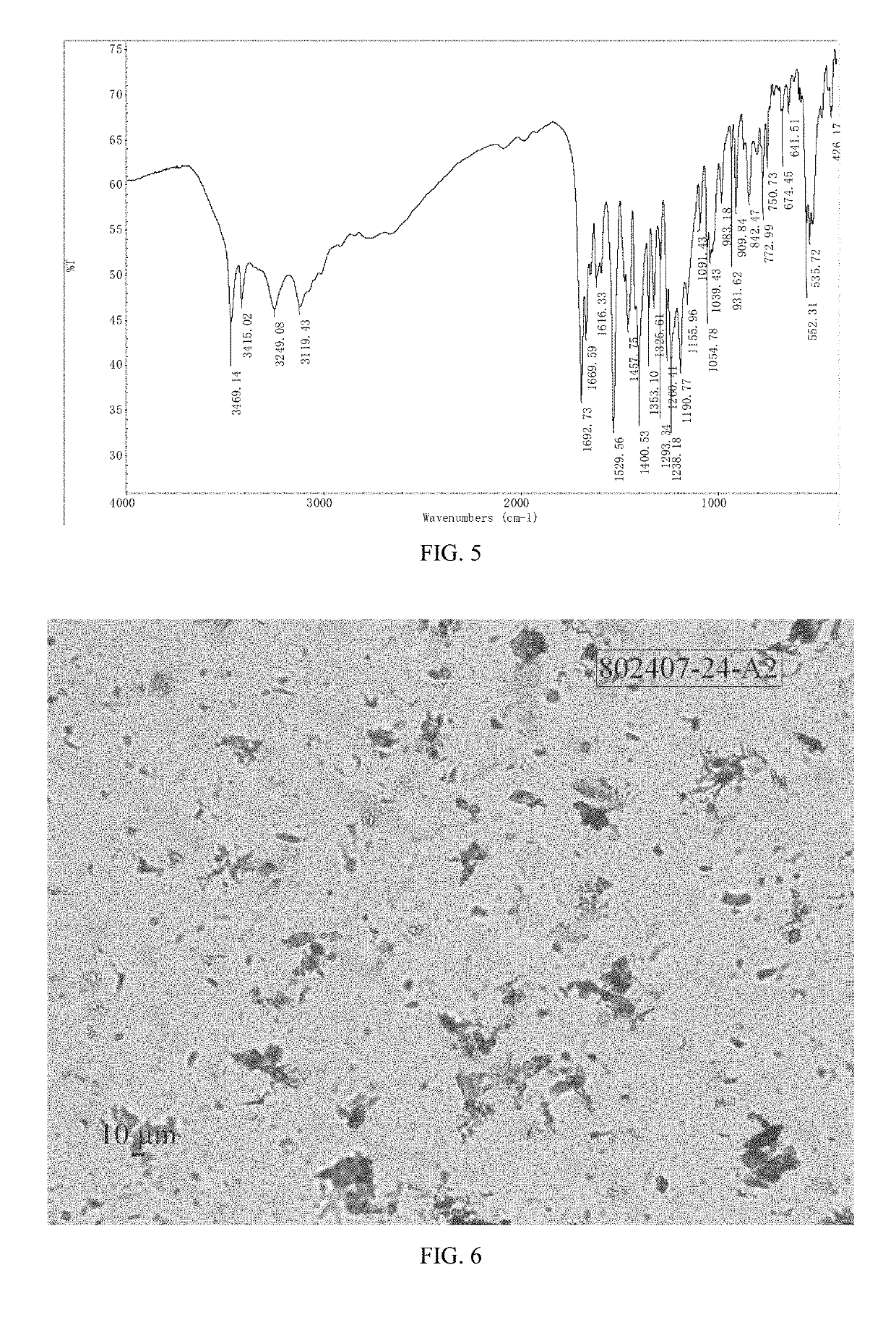
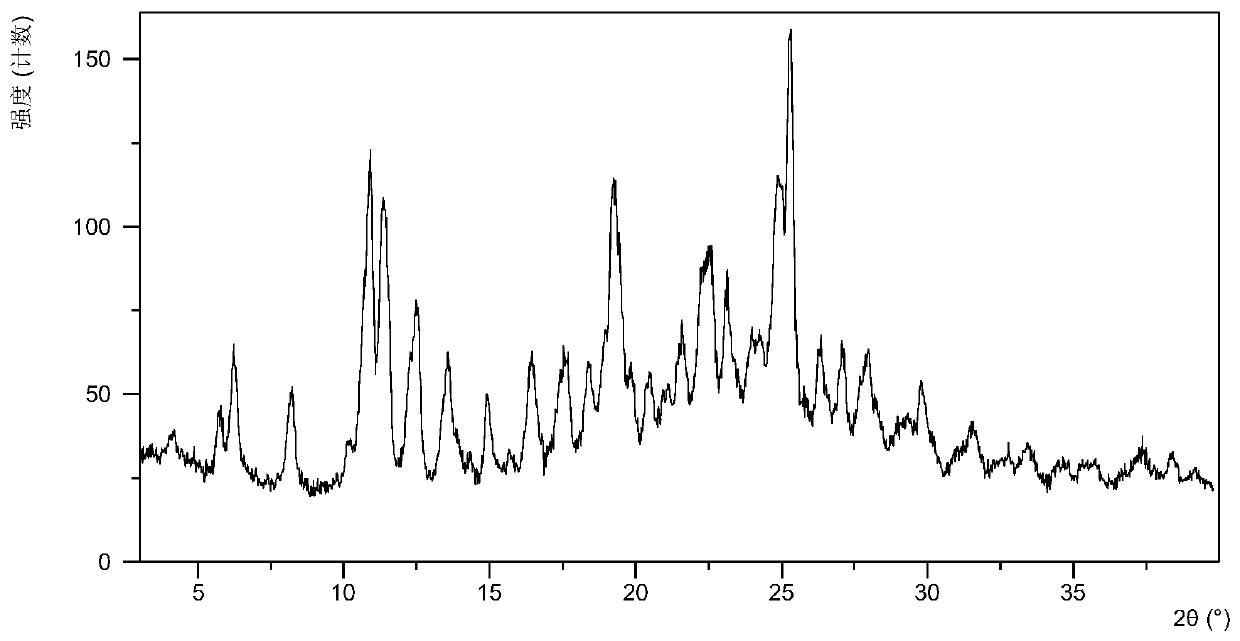
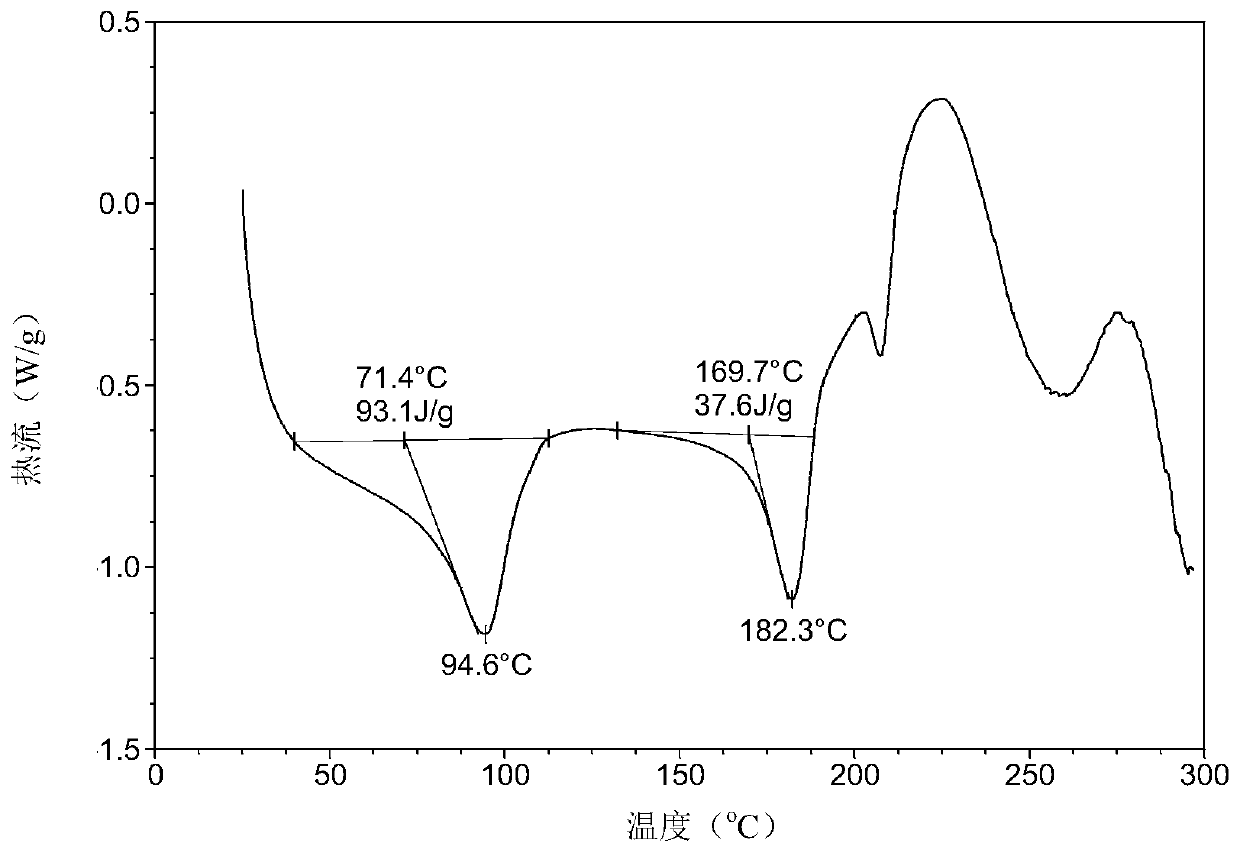
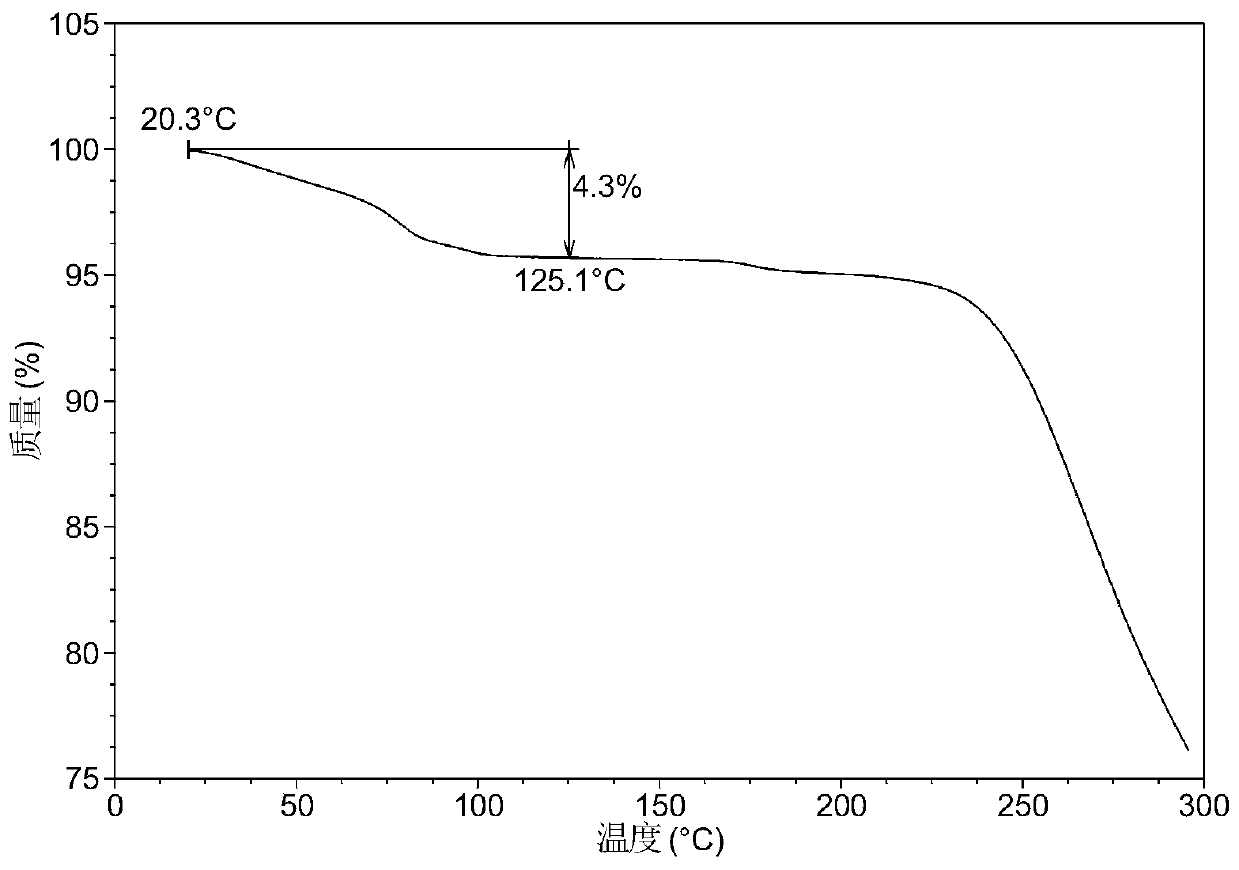
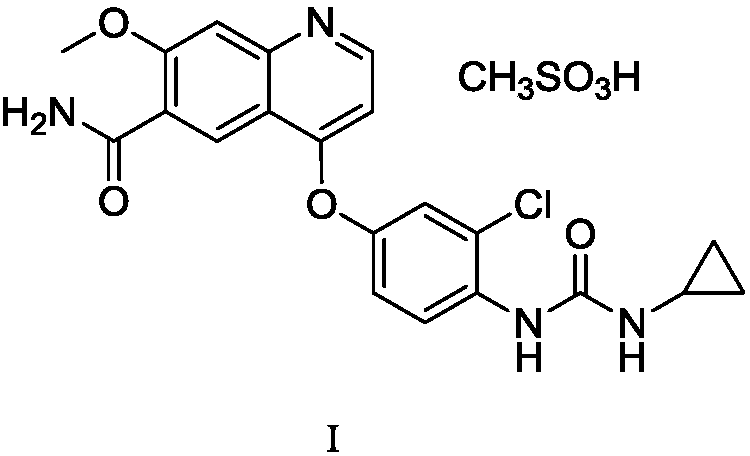
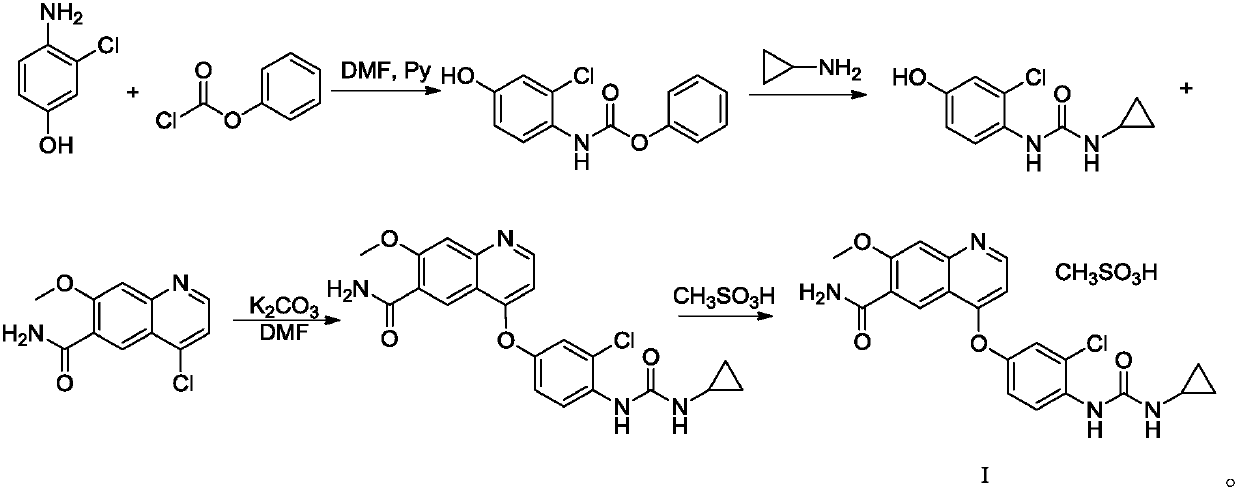
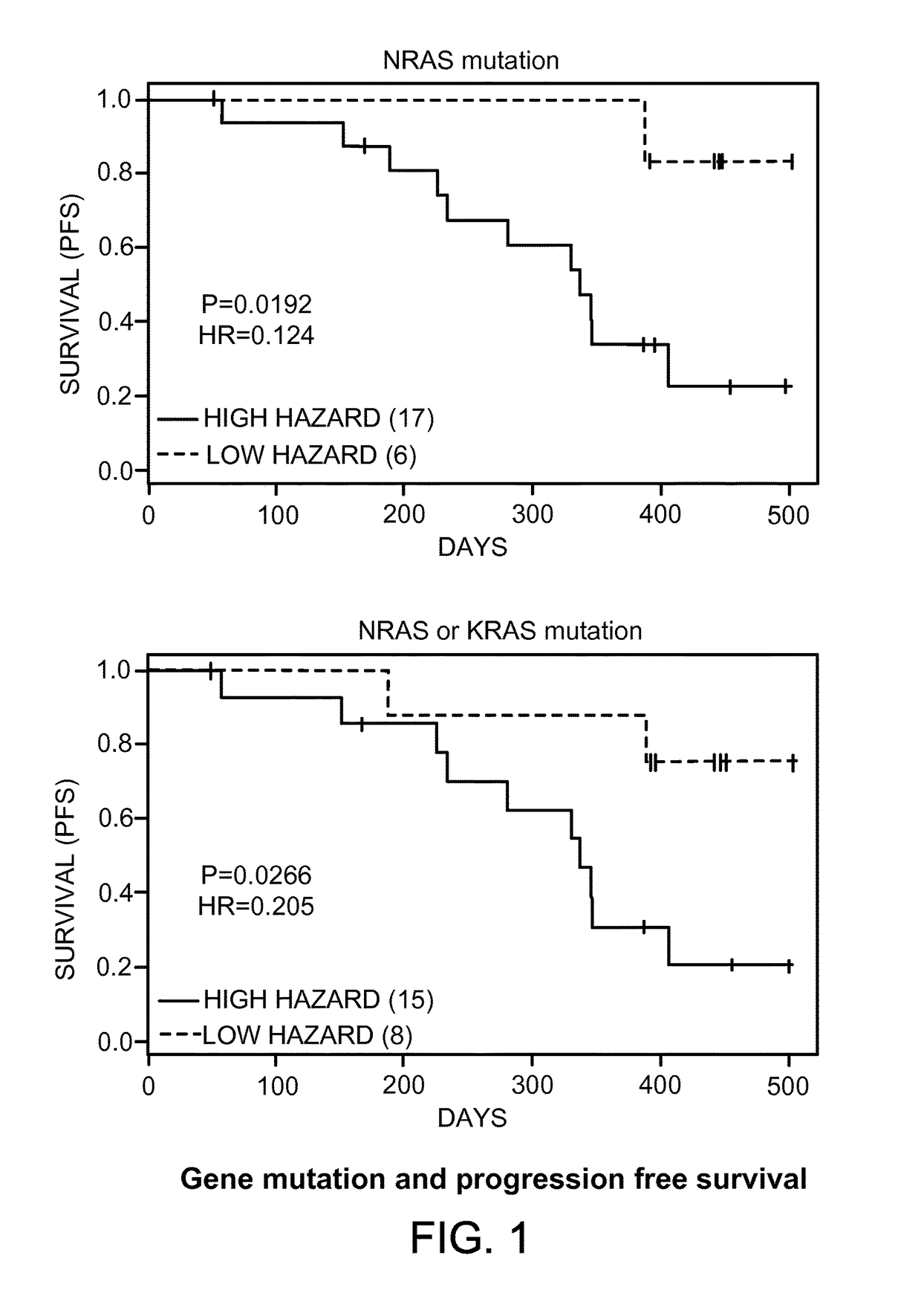
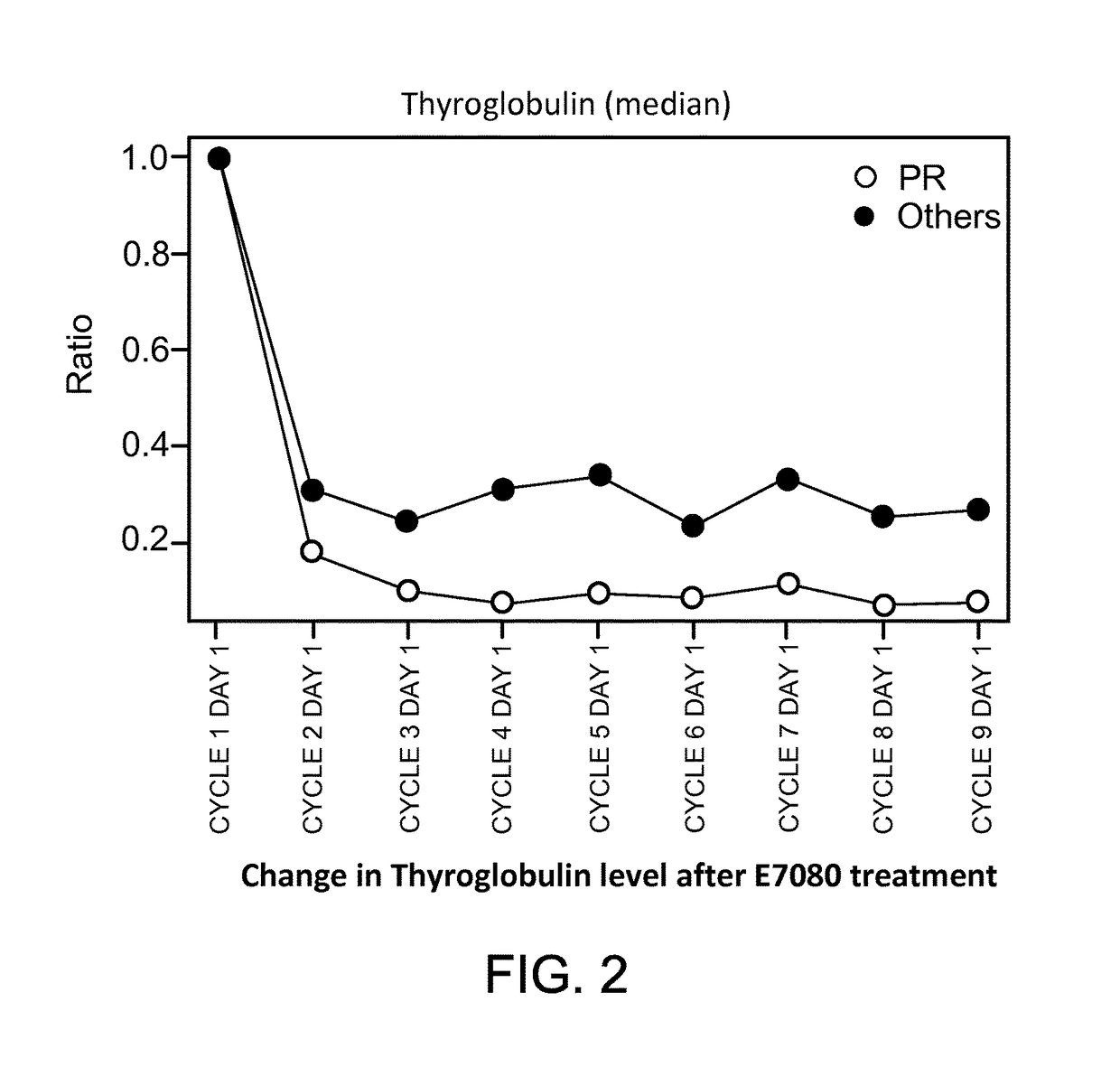
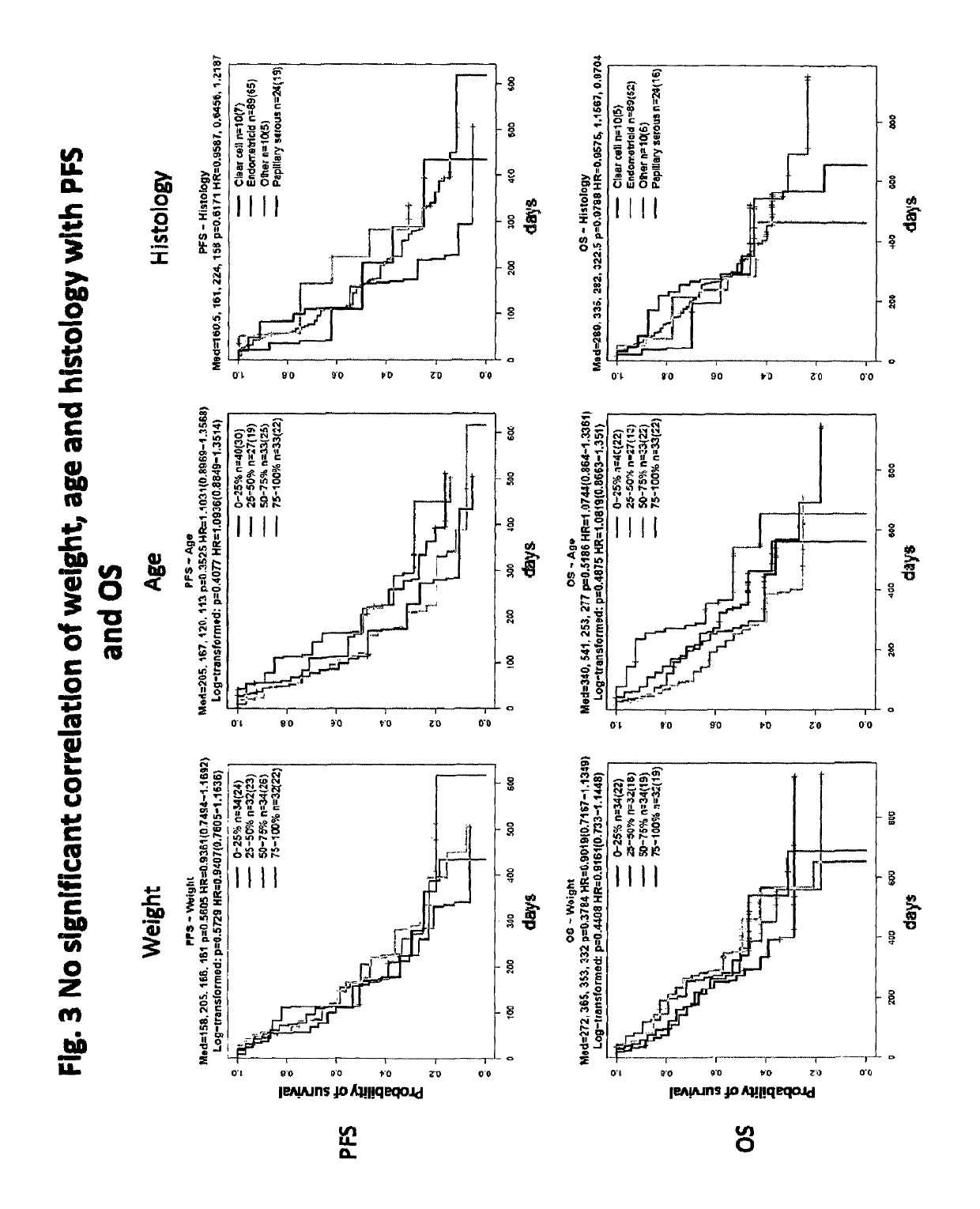
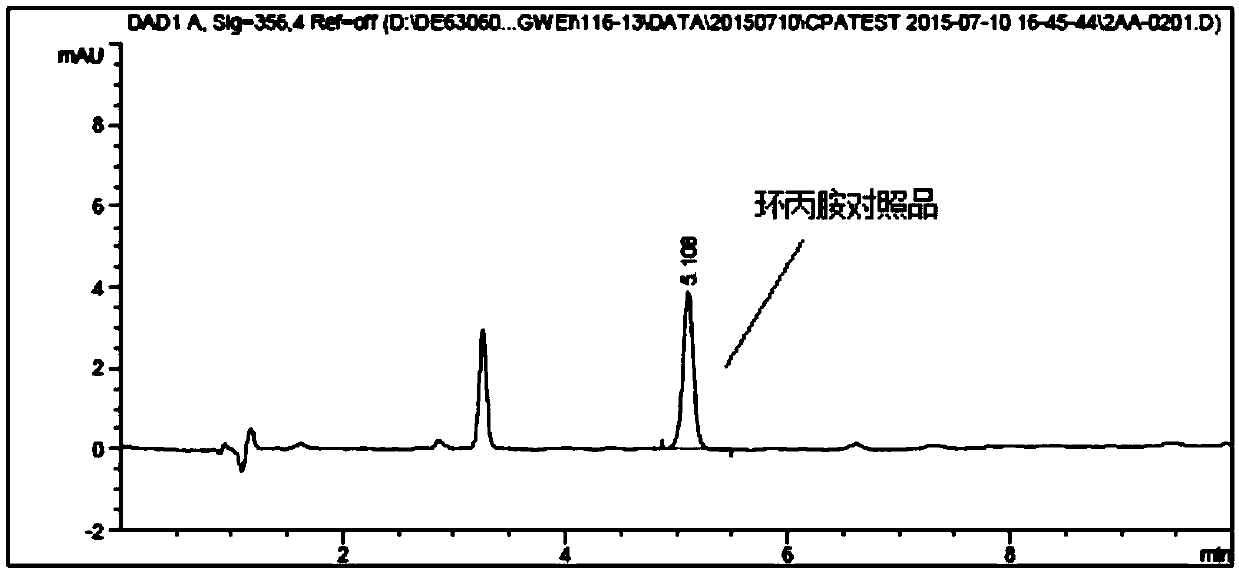
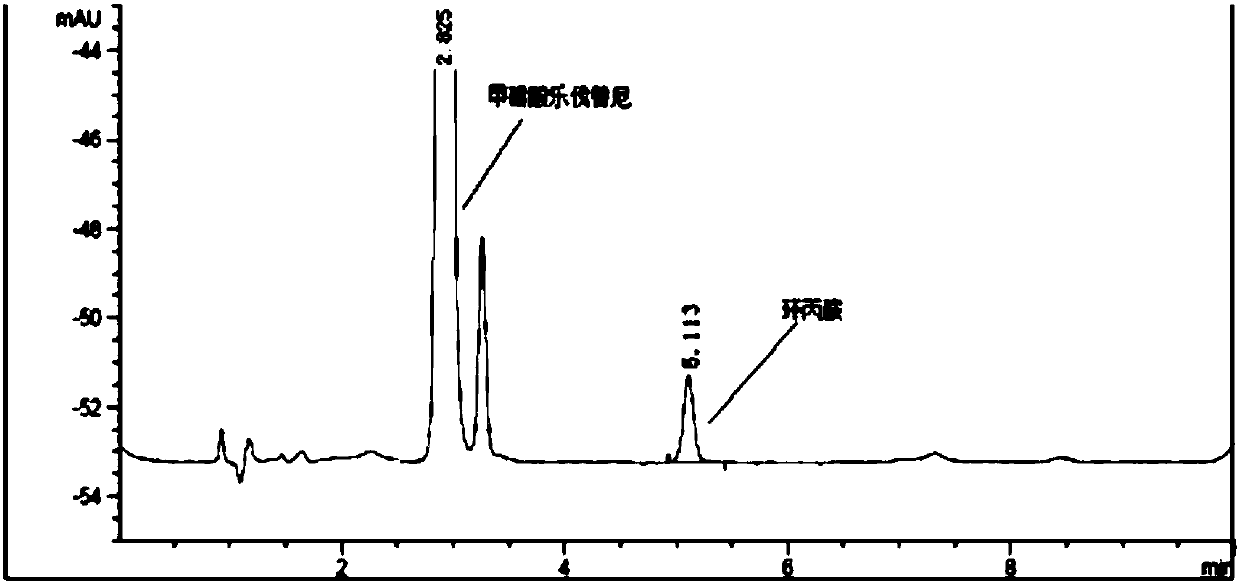
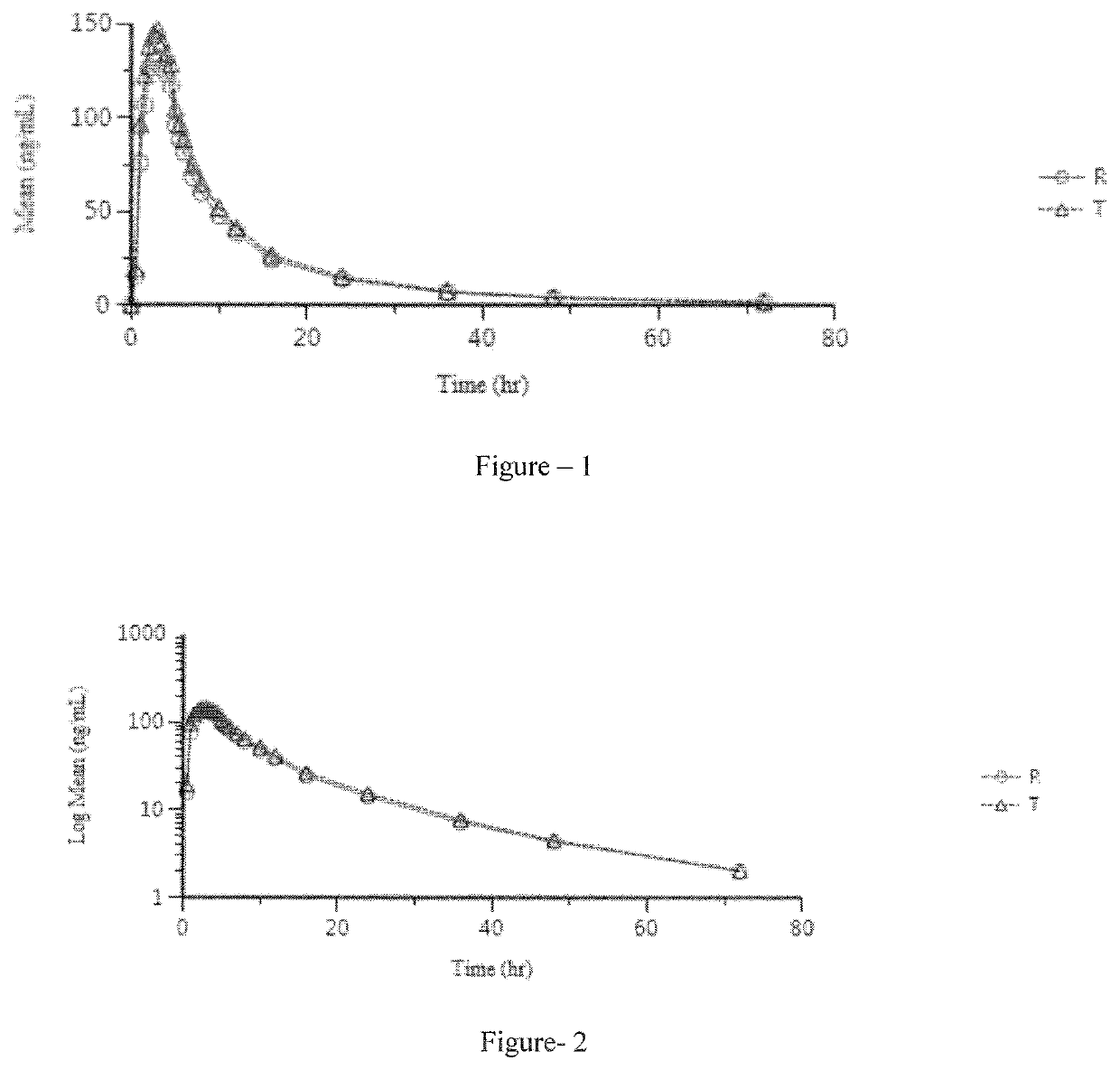
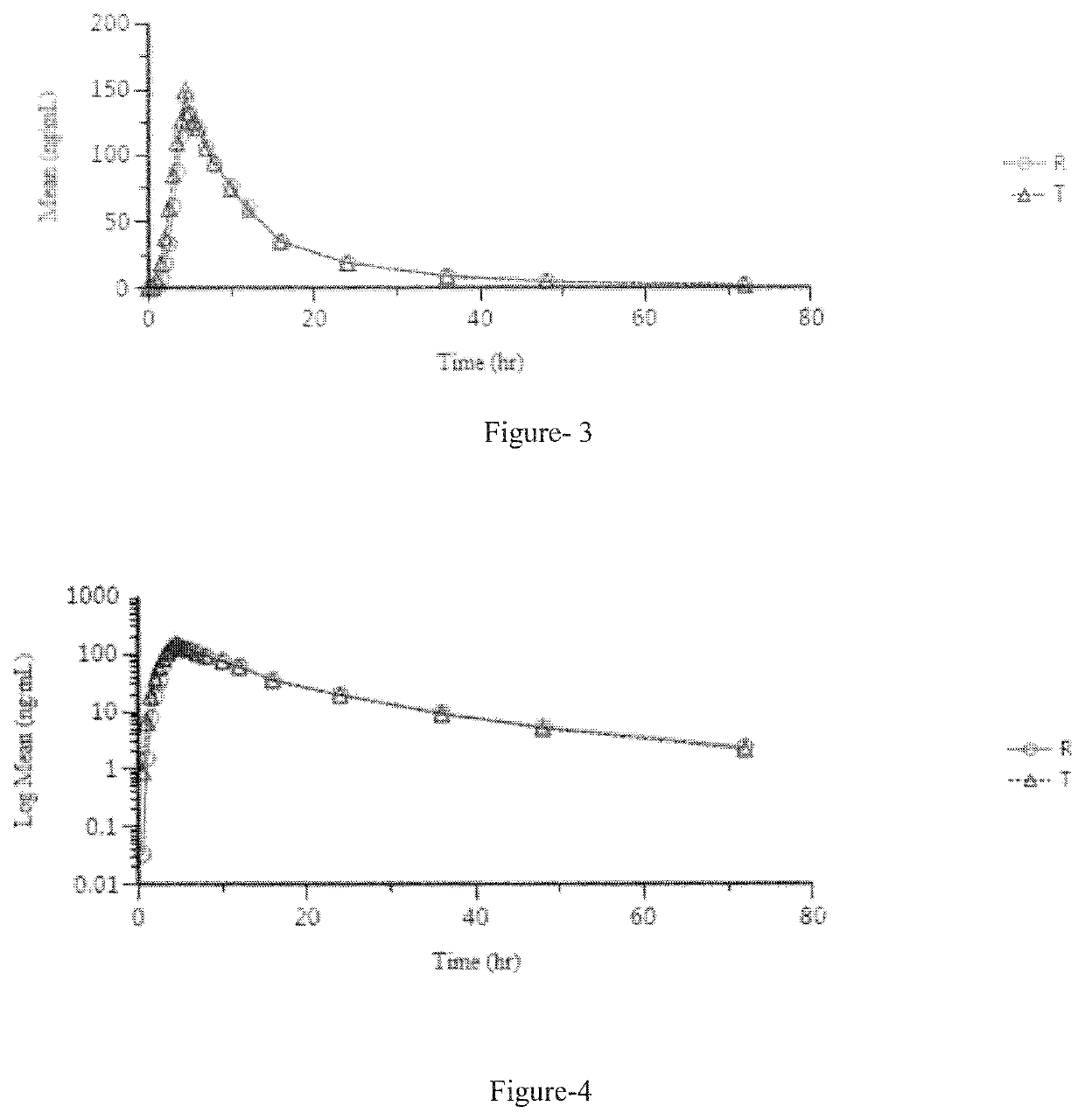
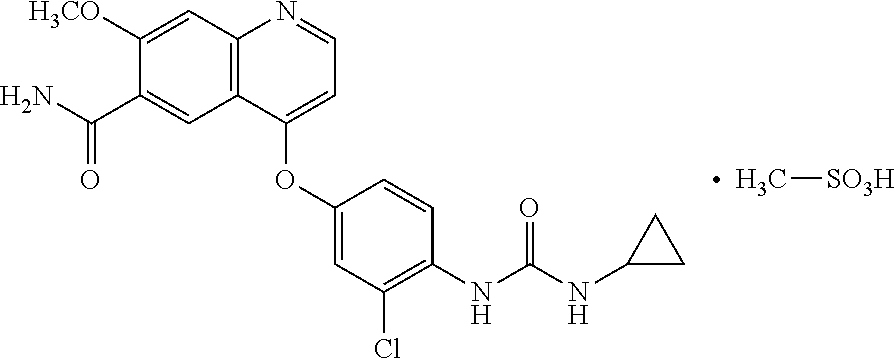
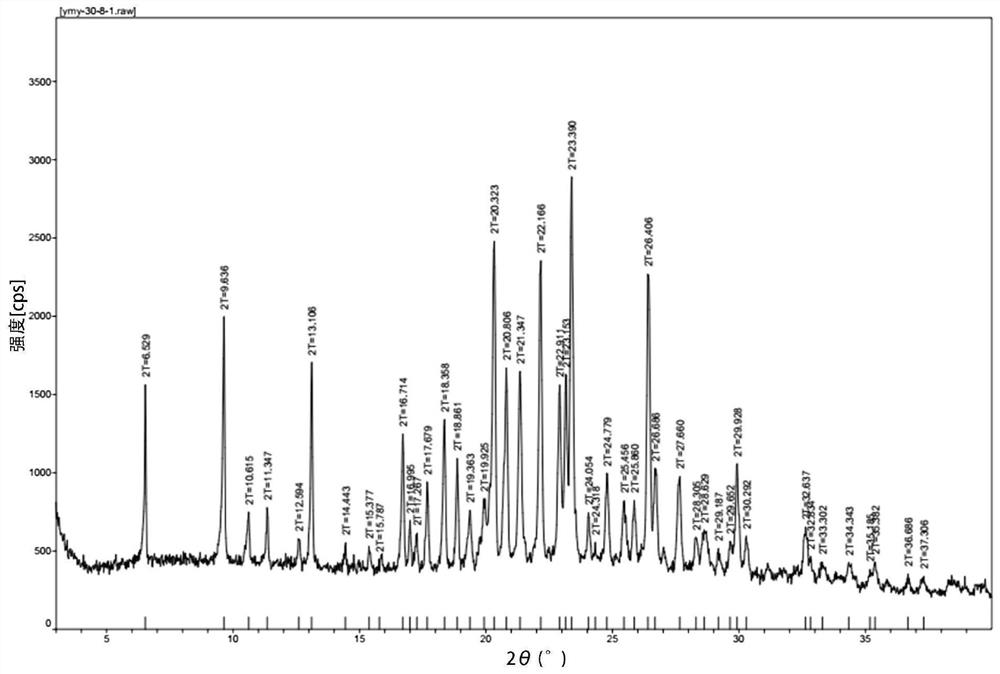
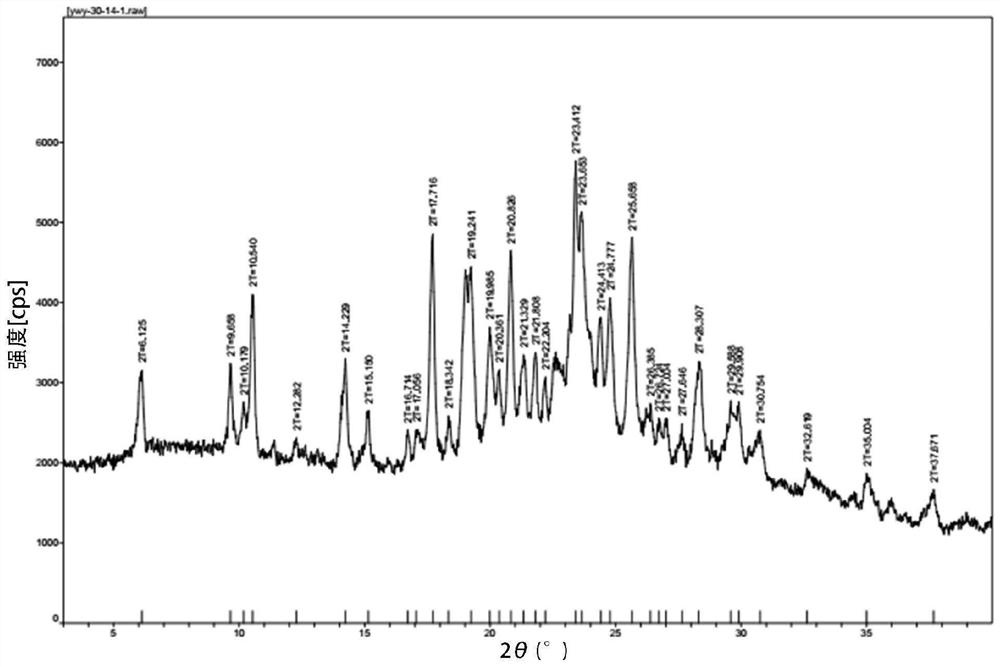
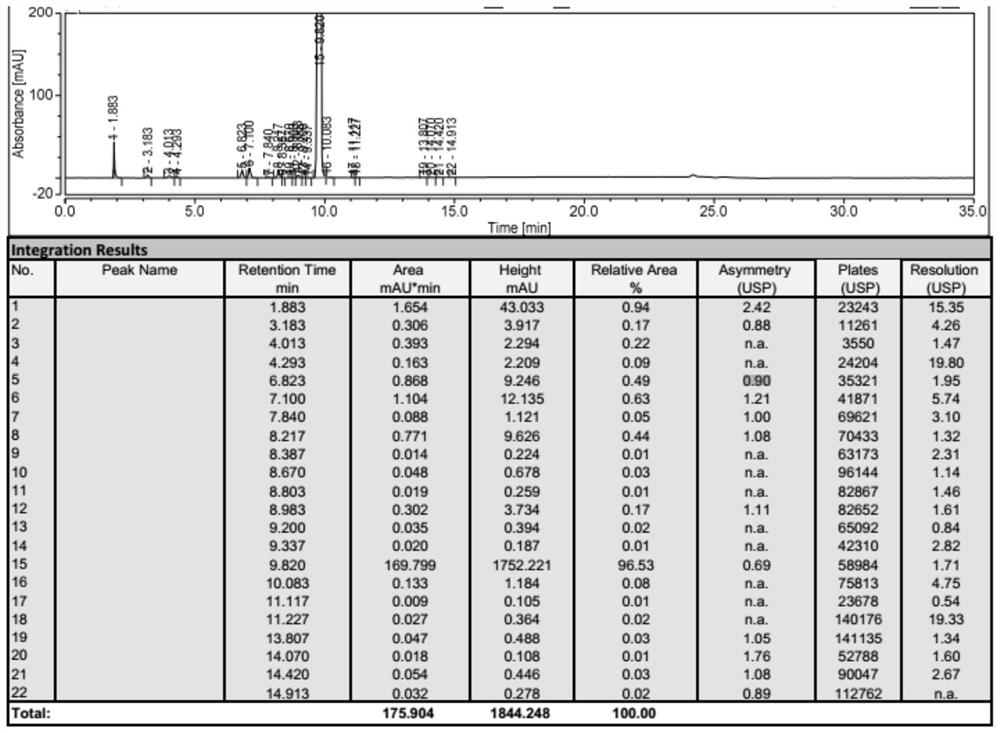
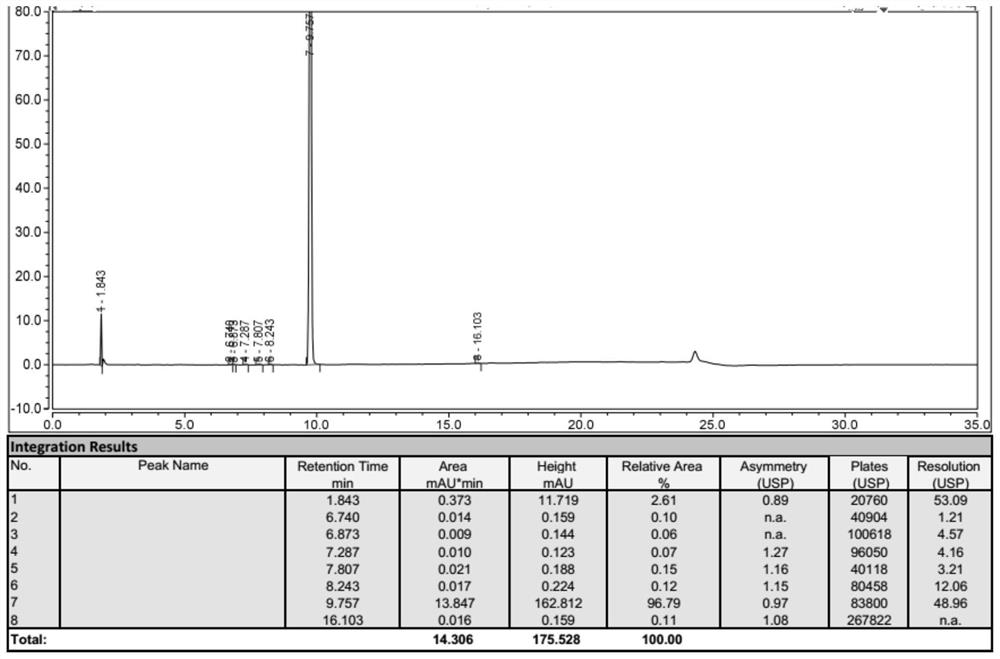
Patents
Literature
45 results about "Lenvatinib Mesylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A synthetic, orally available inhibitor of vascular endothelial growth factor receptor 2 (VEGFR2, also known as KDR/FLK-1) tyrosine kinase with potential antineoplastic activity. E7080 blocks VEGFR2 activation by VEGF, resulting in inhibition of the VEGF receptor signal transduction pathway, decreased vascular endothelial cell migration and proliferation, and vascular endothelial cell apoptosis.
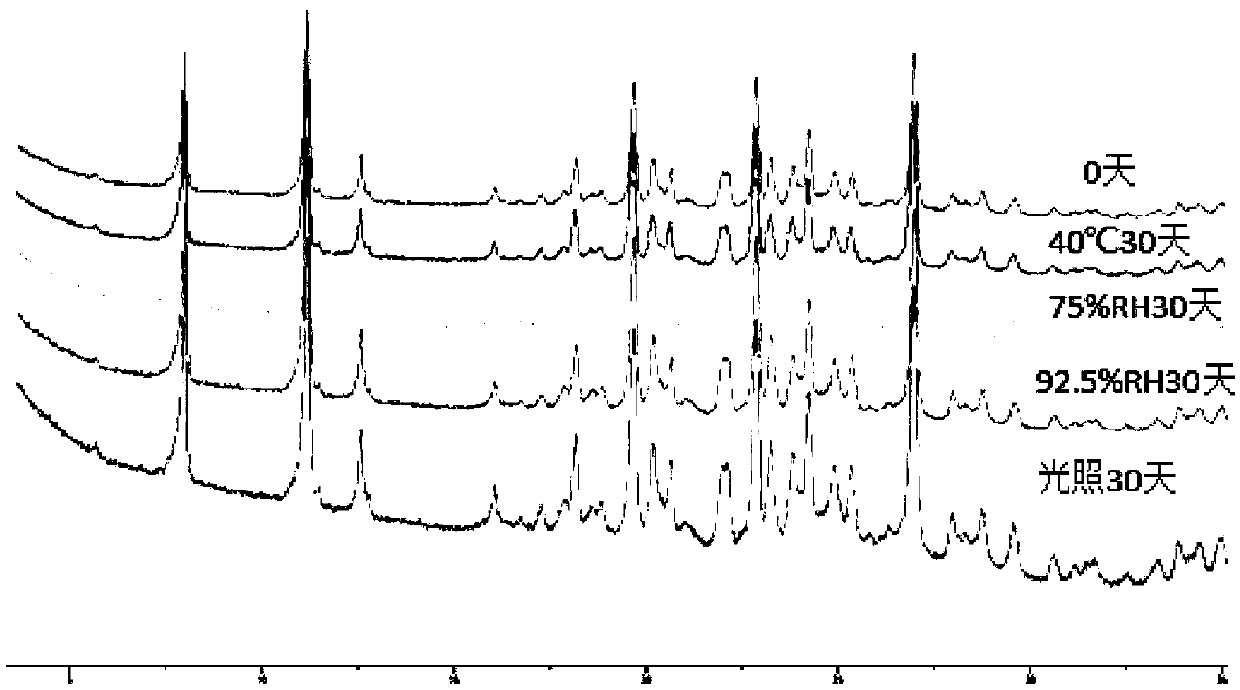
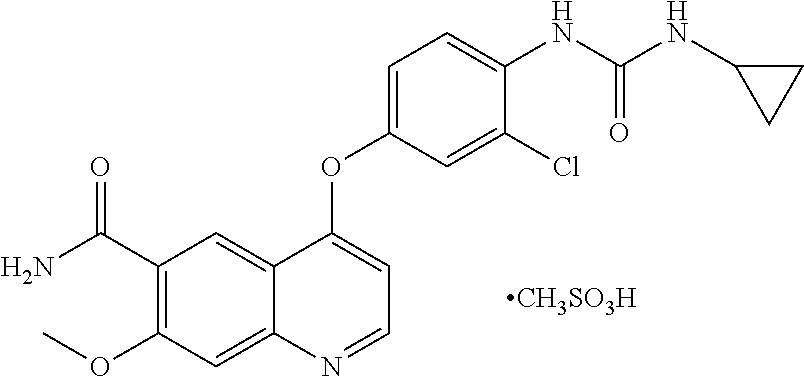
Preparation methods of lenvatinib mesylate drug impurities
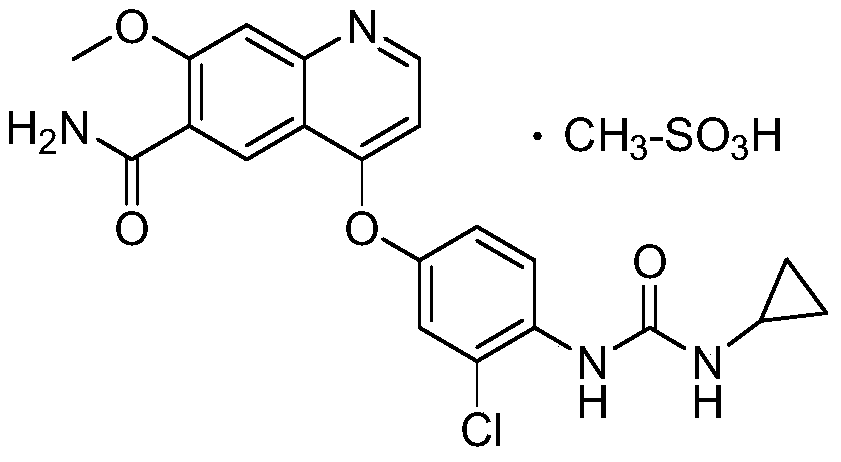
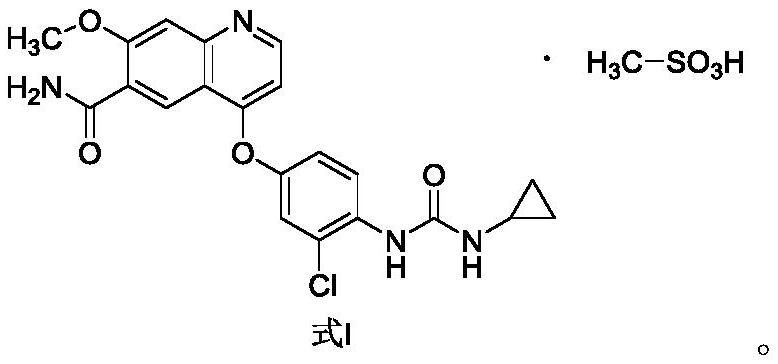
The invention belongs to the field of pharmaceutical synthesis, and relates to impurities in a raw medical material production process and preparation methods of the impurities, in particular to preparation methods of process impurities A, B and C of lenvatinib mesylate, namely, 4-[3-chloro-4-(N'-cyclopropylureido) phenoxy]-7-methoxyquinoline-6-carboxamide mesylate), as a drug for treating radioiodine-refractory thyroid cancer and an application of the impurities to quality research of lenvatinib mesylate. With adoption of the methods, the process impurities A, B and C are obtained through chemical synthesis for the first time, and the target compounds shown in the description can be obtained through efficient and rapid separation.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +2
Medicinal composition containing lenvatinib, and preparation method thereof
ActiveCN106551935AImprove uniformityImprove dissolution behaviorInorganic non-active ingredientsAntineoplastic agentsHydrogen phosphateLenvatinib Mesylate
The invention provides a medicinal composition containing lenvatinib. The medicinal composition comprises, by weight, 1-20% of lenvatinib mesylate, 20-60% of anhydrous calcium hydrogen phosphate, 10-55% of a filler, 5-35% of a disintegrating agent, 0-10% of an adhesive and 0.5-5% of a lubricant. Preferably, the particle size of the anhydrous calcium hydrogen phosphate is 200-600 meshes, or the D90 is about 20-75 [mu]m. The medicinal composition has good stability, has a dissolubility reaching 90% or above in 15 min, has effectively improved bioavailability, is easy to prepare, and is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
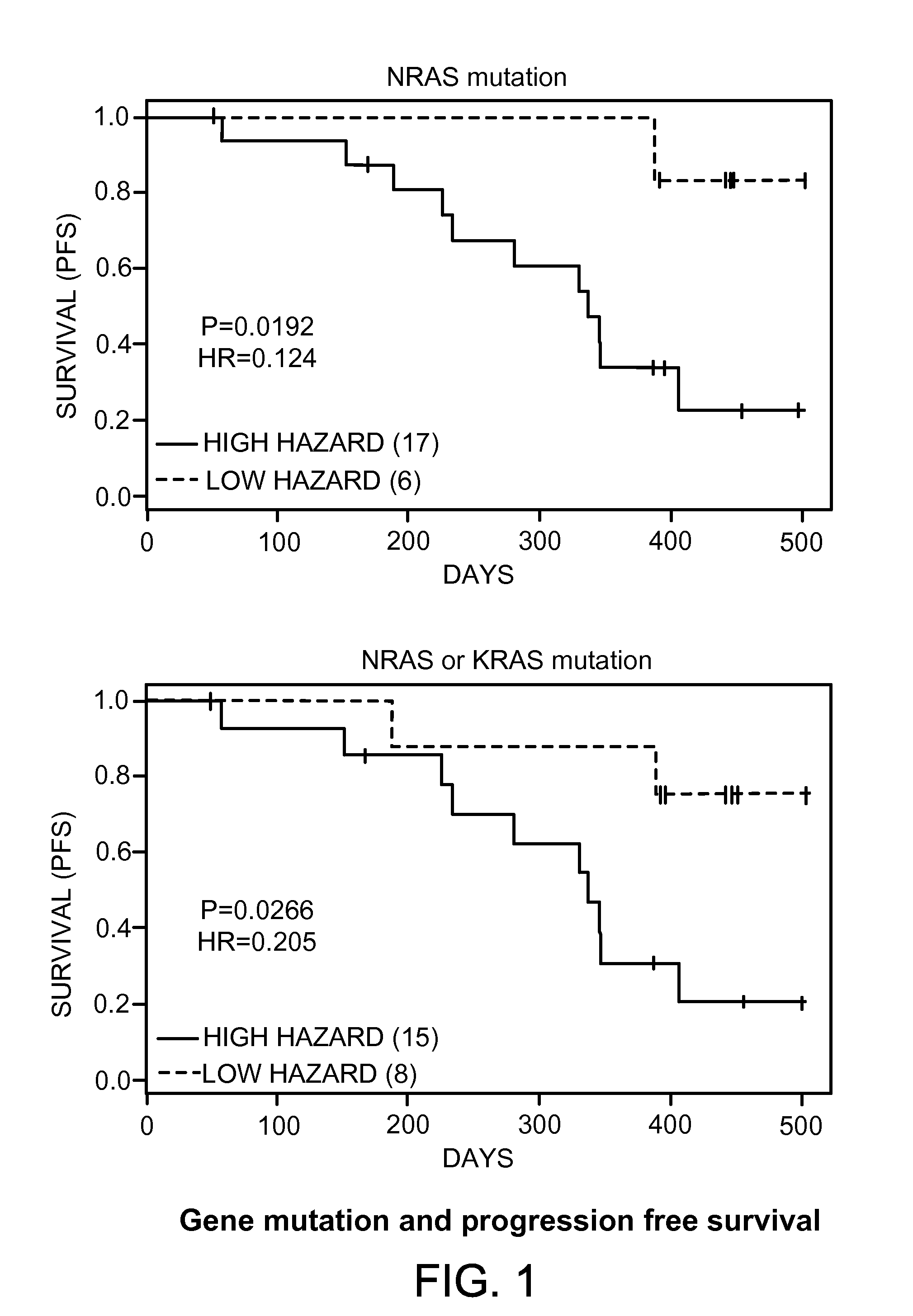
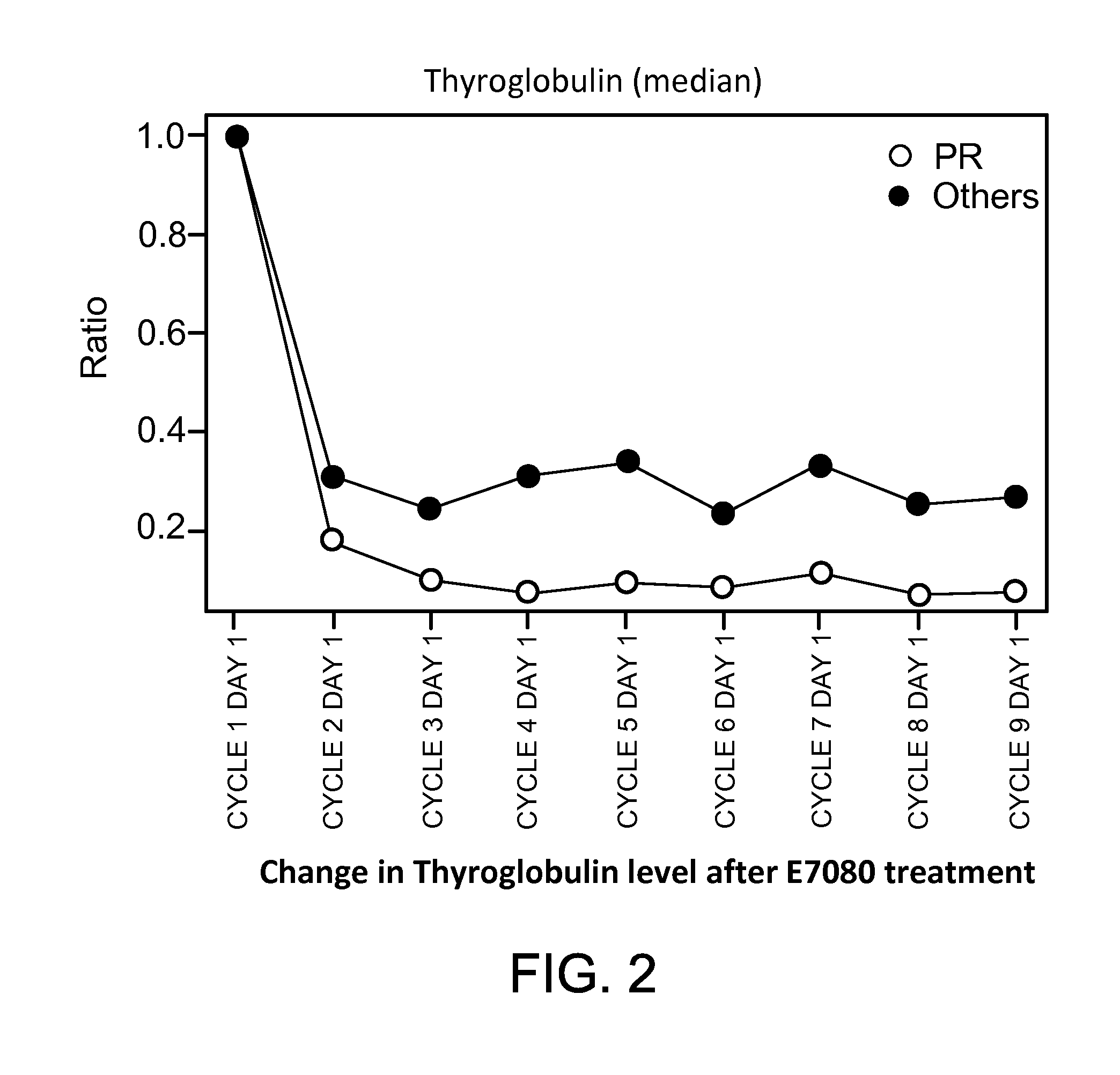
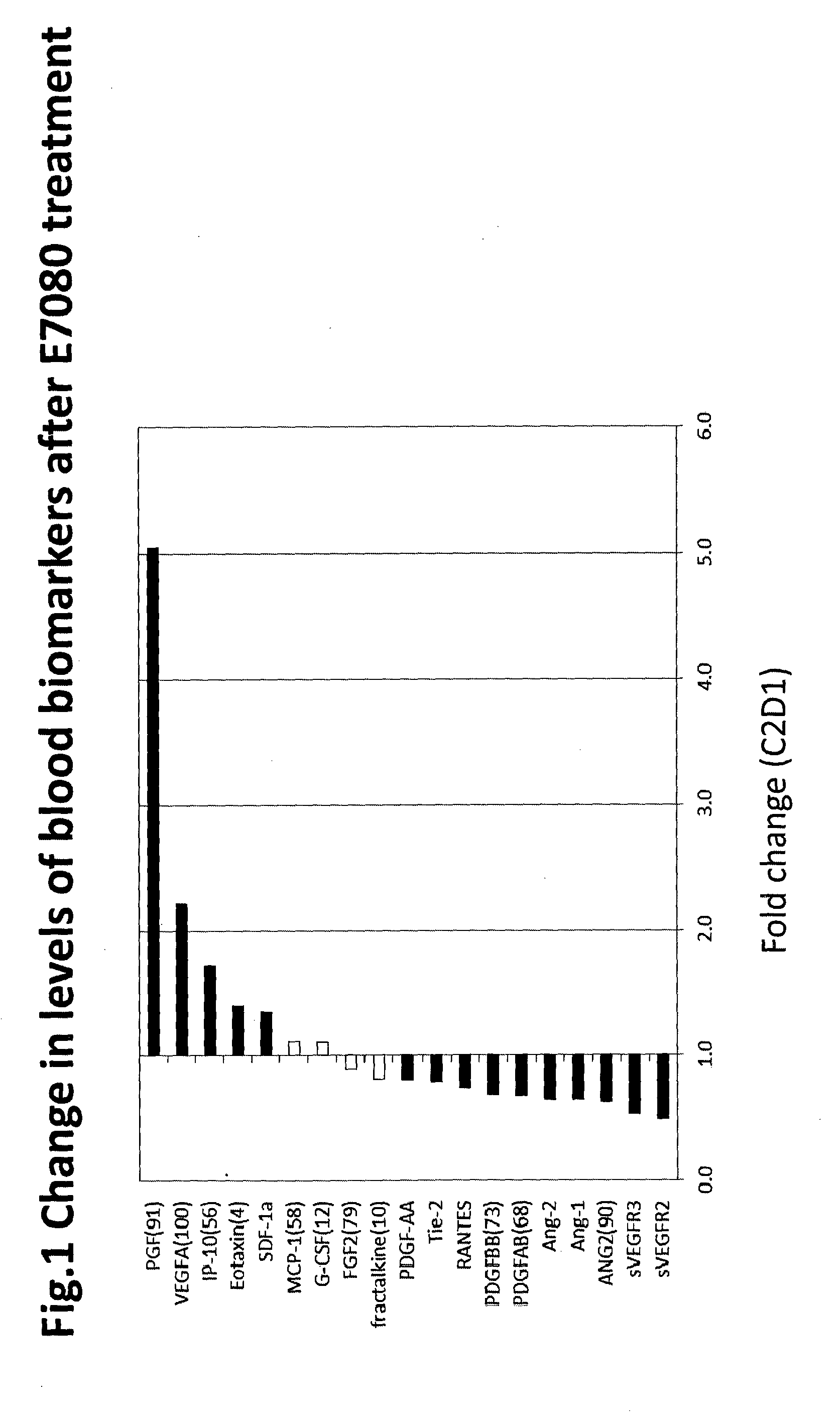
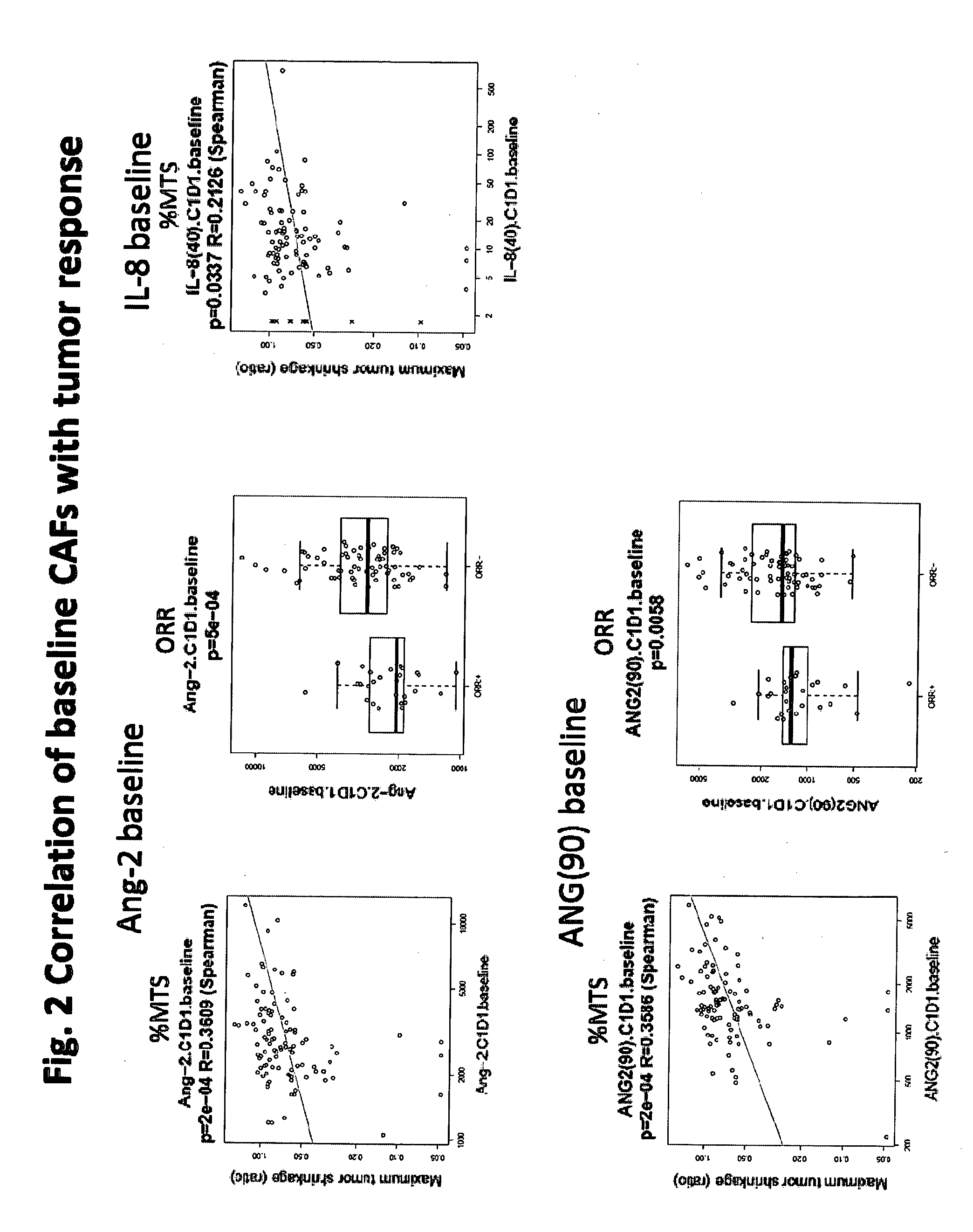
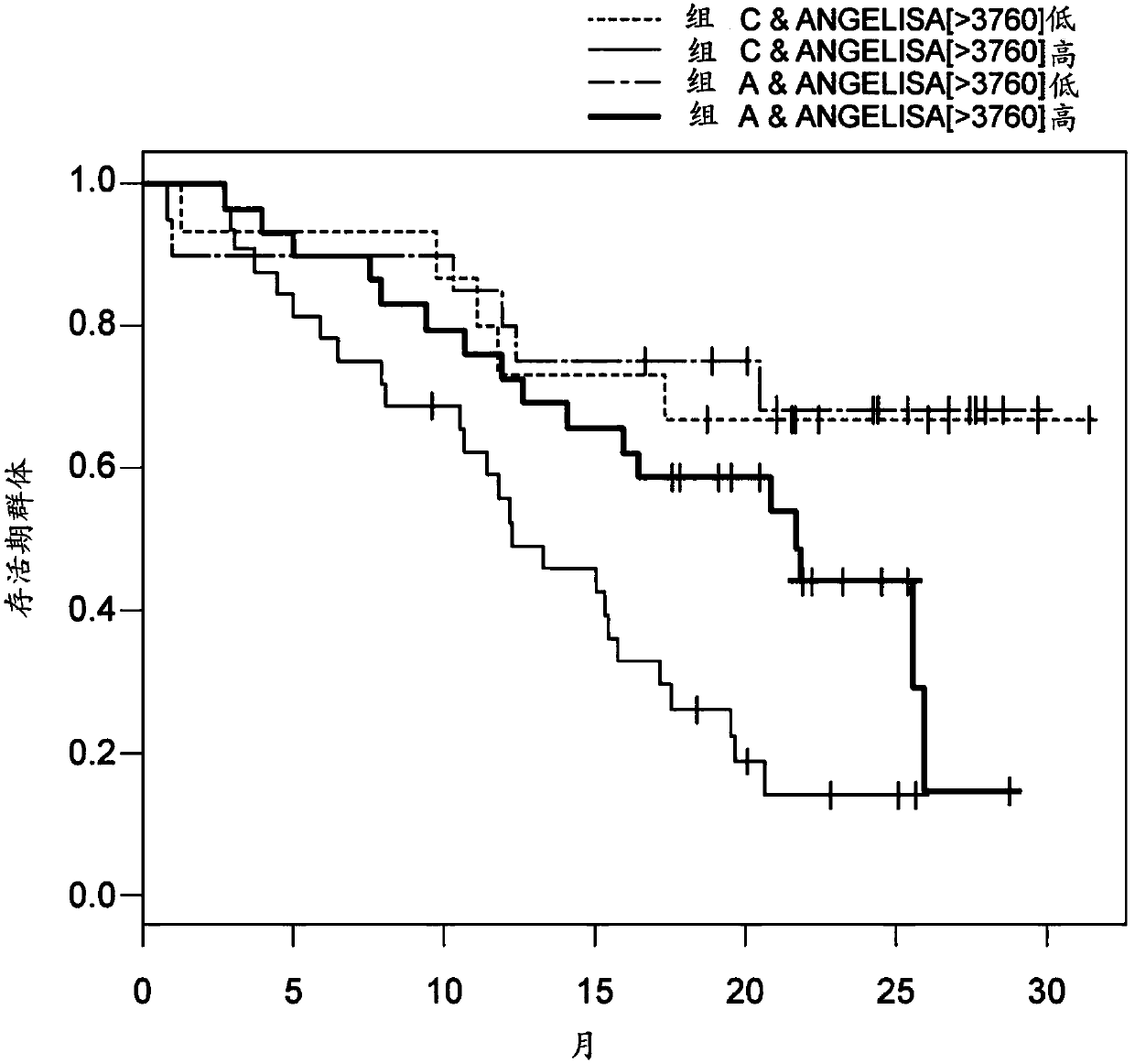
Biomarkers for predicting and assessing responsiveness of thyroid and kidney cancer subjects to lenvatinib compounds
ActiveUS20140187577A1Strong predictionReduce rateBiocideMicrobiological testing/measurementKidney cancerLenvatinib Mesylate
Biomarkers are provided that are predictive of a subject's responsiveness to a therapy comprising lenvatinib or a pharmaceutically acceptable salt thereof (e.g., lenvatinib mesylate). The biomarkers, compositions, and methods described herein are useful in selecting appropriate treatment modalities for a subject having cancer (e.g., thyroid cancer, kidney cancer), suspected of having cancer, or at risk of developing cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
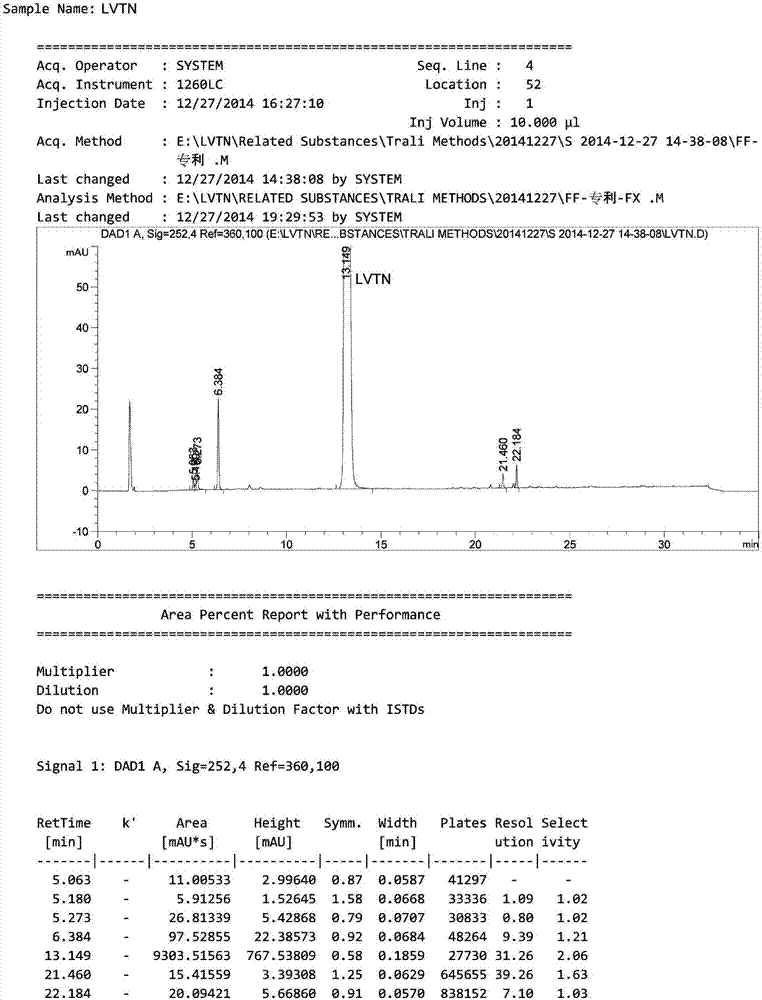
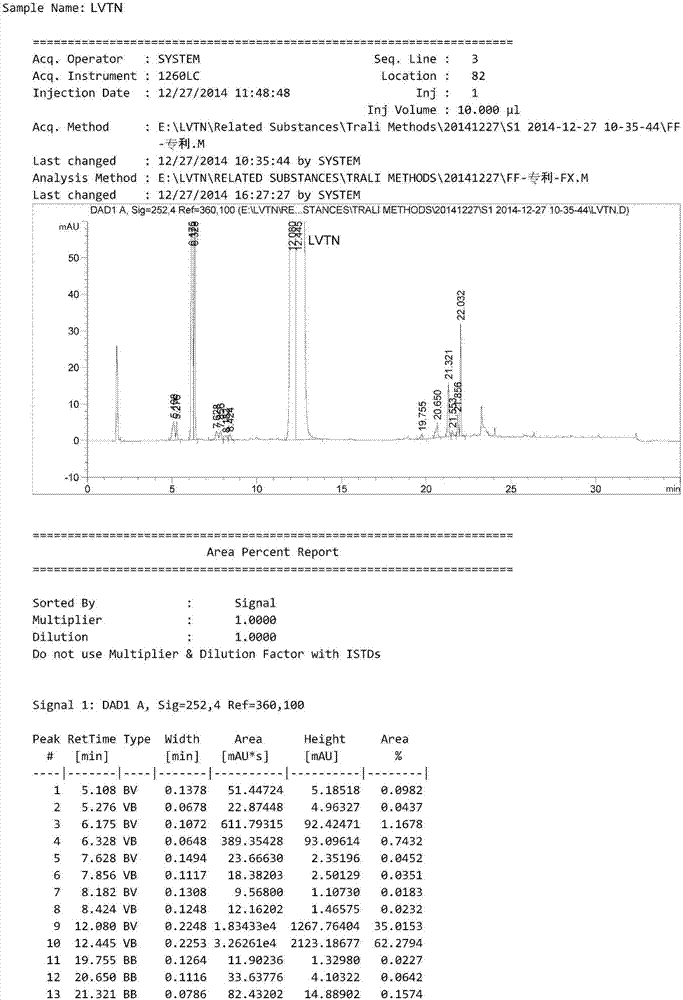
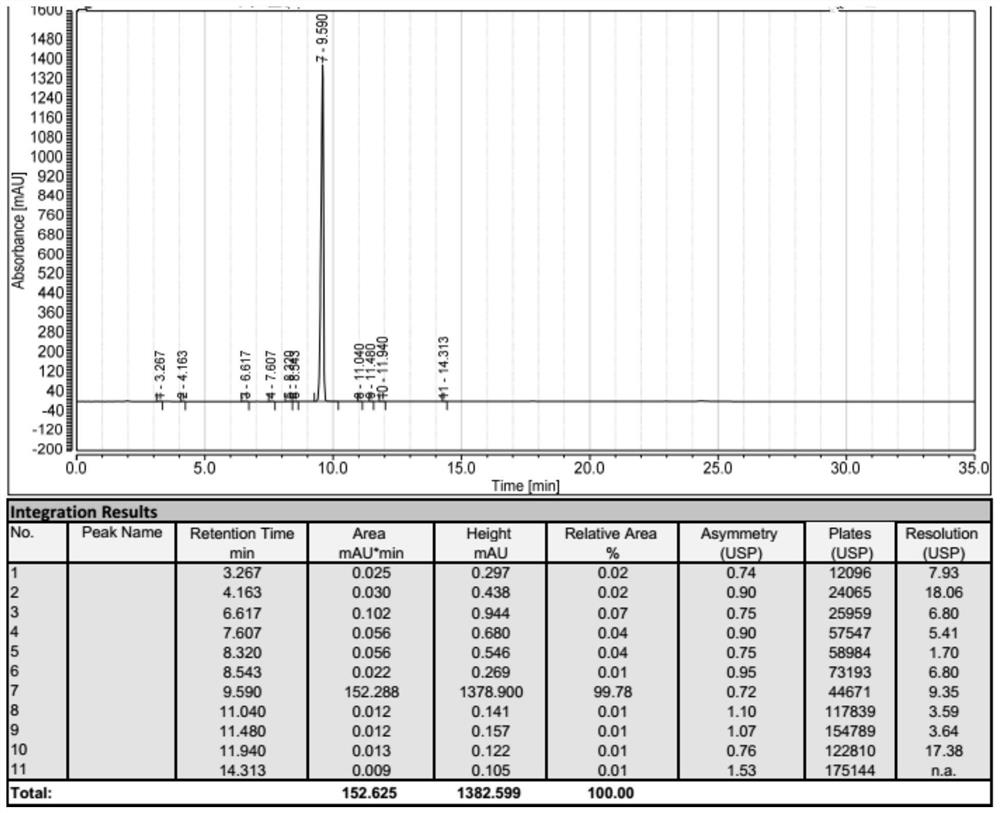
HPLC method for analyzing lenvatinib mesylate and preparation impurity thereof and application of impurity as reference standard
ActiveCN107305202AStrong specificityHigh precisionOrganic chemistryComponent separationCompound aLenvatinib Mesylate
The invention relates to a method for analyzing lenvatinib (4-(3-chloro-4-(N'-cyclopropylurea) phenoxy)-7-methoxyquinoline-6- carboxamide) or a preparation containing lenvatinib. The method comprises the step of determining an impurity in a sample through a chromatography, wherein the impurity is selected from one or more of compounds A-I and LVTN-1. The method comprises the following steps of (1) dissolving lenvatinib mesylate or a drug containing the lenvatinib mesylate into a solvent to prepare a sample solution; (2) dissolving one or more of the compounds A-I and a midbody-1 (LVTN-1) into the solvent to prepare a reference standard solution or a reference solution; (3) implementing a chromatography on the sample solution and the reference standard solution; and (4) determining presence of any one or more of the compounds A-I and the midbody-1 (LVTN-1) in the lenvatinib mesylate or a drug containing the lenvatinib mesylate through referring to one or more of the compounds A-I and the midbody-1 (LVTN-1) in the reference standard solution.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD
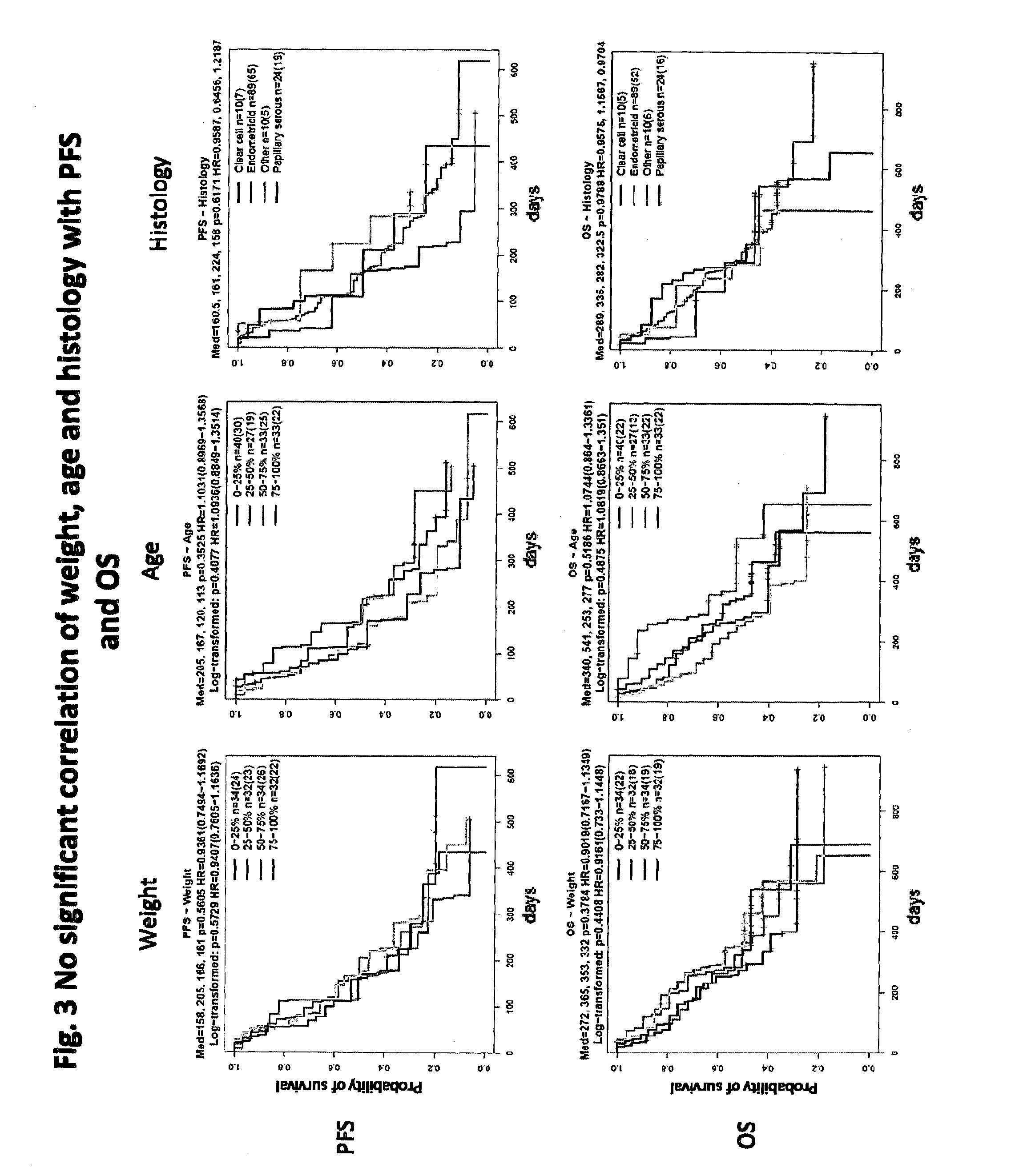
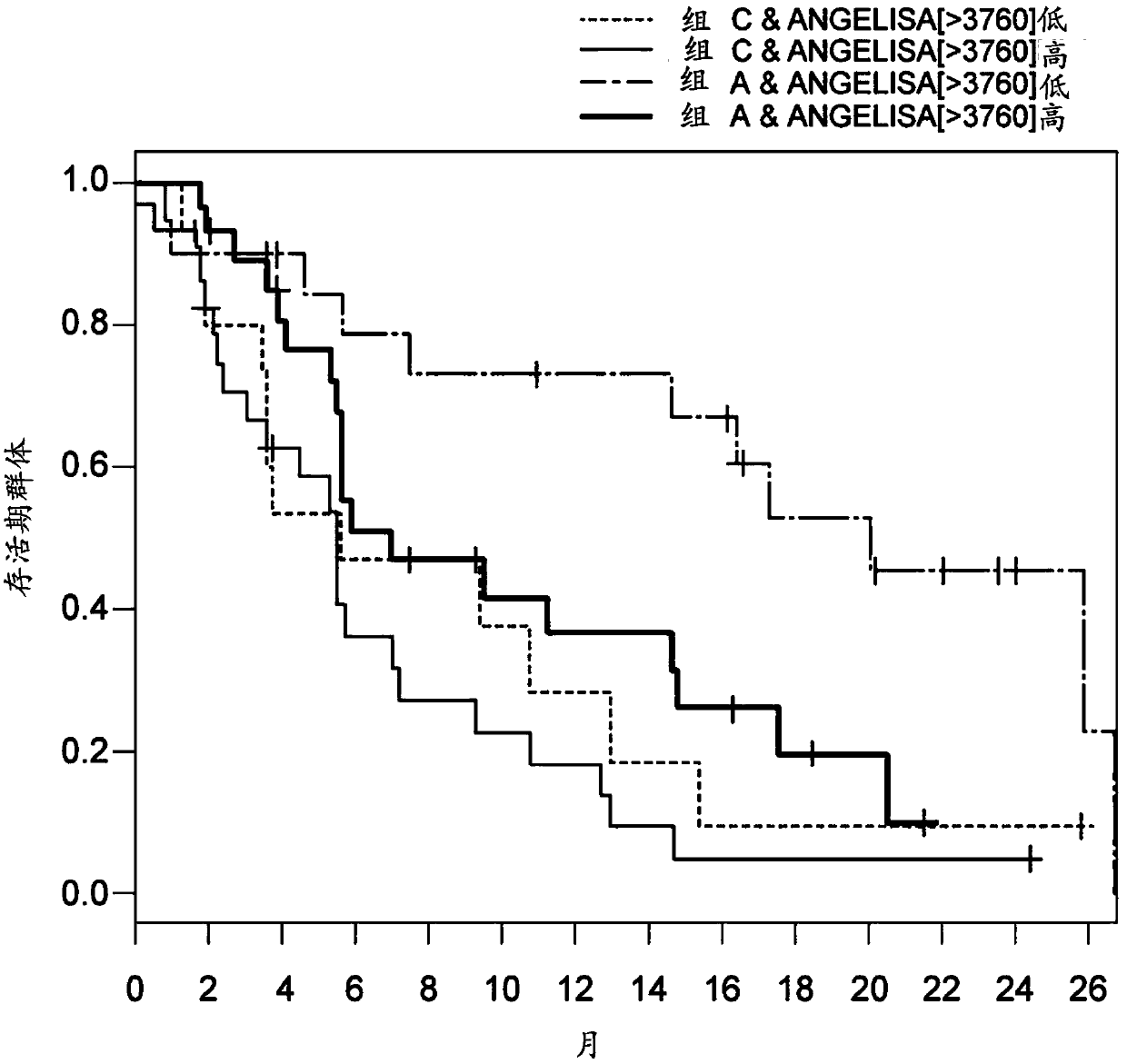
Biomarkers for predicting and assessing responsiveness of endometrial cancer subjects to lenvatinib compounds
Biomarkers are provided that predict whether a subject having endometrial cancer will or will not respond to a therapy comprising lenvatinib or a pharmaceutically acceptable salt thereof (e.g., lenvatinib mesylate). The biomarkers, compositions, and methods described herein are useful in selecting appropriate treatment modalities for and treating a subject having, suspected of having, or at risk of developing an endometrial cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
New crystal form of lenvatinib methanesulfonate salt and preparation method thereof
ActiveUS20180155291A1Improve solubilityImprove stabilityOrganic chemistry methodsAntineoplastic agentsSolubilityLenvatinib Mesylate
The present disclosure relates to a novel crystalline form of lenvatinib mesylate and the preparation method thereof. The novel crystalline form of mesylate of the present disclosure can be used for treating invasive and differentiated thyroid cancer. The novel crystalline form of mesylate of the present disclosure has good solubility, stability, and remarkable purification effect in process. The preparation method of this novel crystalline form is simple, low cost, and has an important value for future optimization and development of the drug.
Owner:CRYSTAL PHARMATECH CO LTD
Crystal form of lenvatinib methanesulfonate salt and preparation method thereof
ActiveUS10246418B2Improve solubilityLow costOrganic chemistry methodsAntineoplastic agentsSolubilityLenvatinib Mesylate
Owner:CRYSTAL PHARMATECH CO LTD
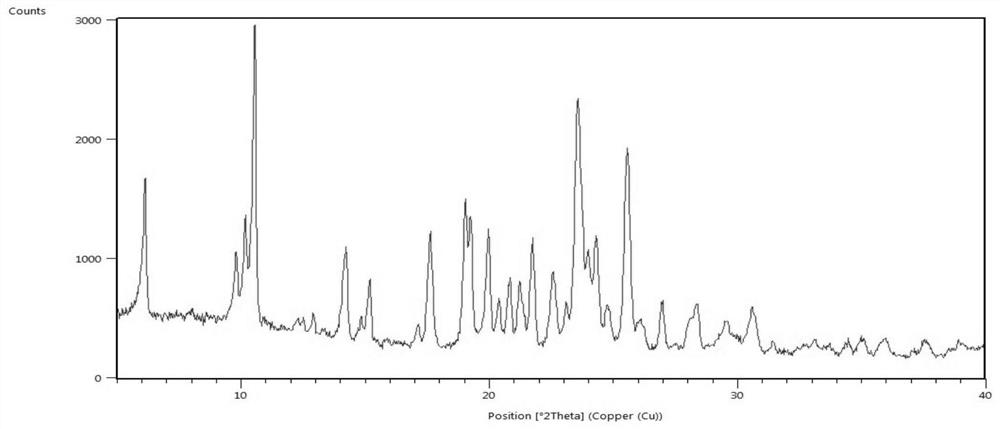
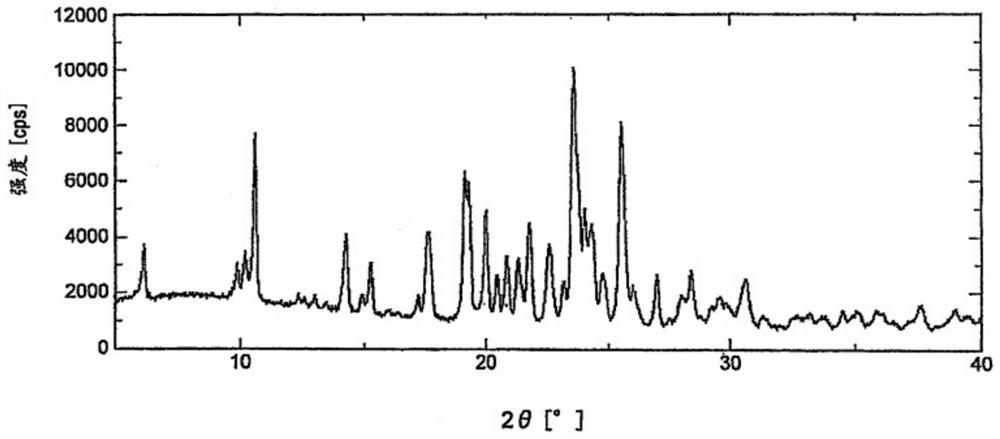
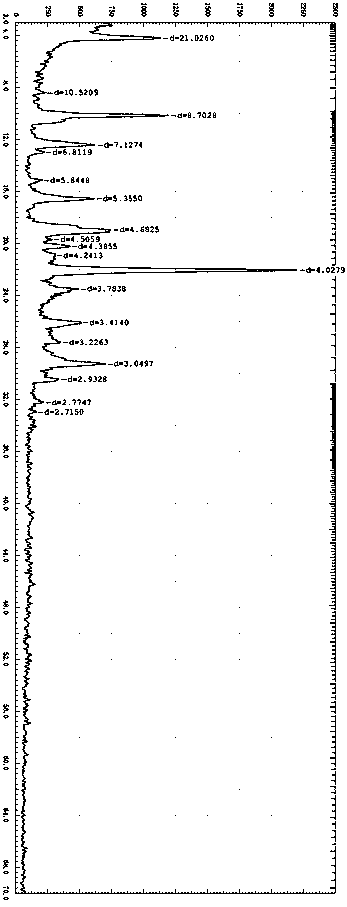
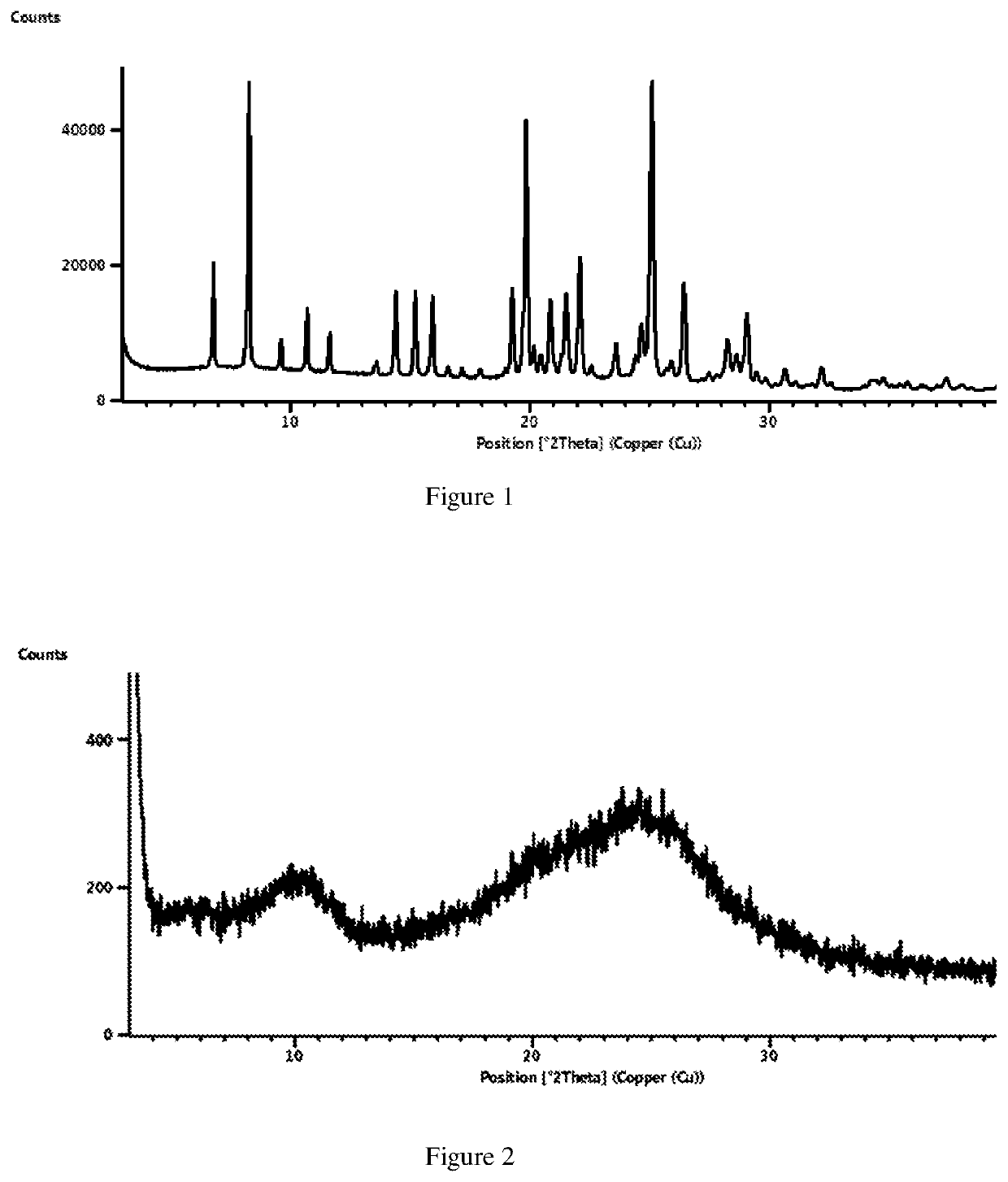
Crystal form of lenvatinib mesylate and preparation method thereof
PendingCN109988112ASimple manufacturing methodImprove solubilityOrganic chemistry methodsAntineoplastic agentsLenvatinib MesylateX-ray
The invention discloses a crystal form of lenvatinib mesylate, a preparation method of the crystal form, a pharmaceutical composition containing the crystal form and application of the crystal form inpreparation of medicines for treating invasive and differentiated thyroid cancer. An X-ray powder diffraction pattern of the crystal form of lenvatinib mesylate comprises peaks at a diffraction angle(2) of about 16.1+ / -0.2 degrees, 17.8+ / -0.2 degrees, 18.8+ / -0.2 degrees, 25.0+ / -0.2 degrees and 25.4+ / -0.2 degrees.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
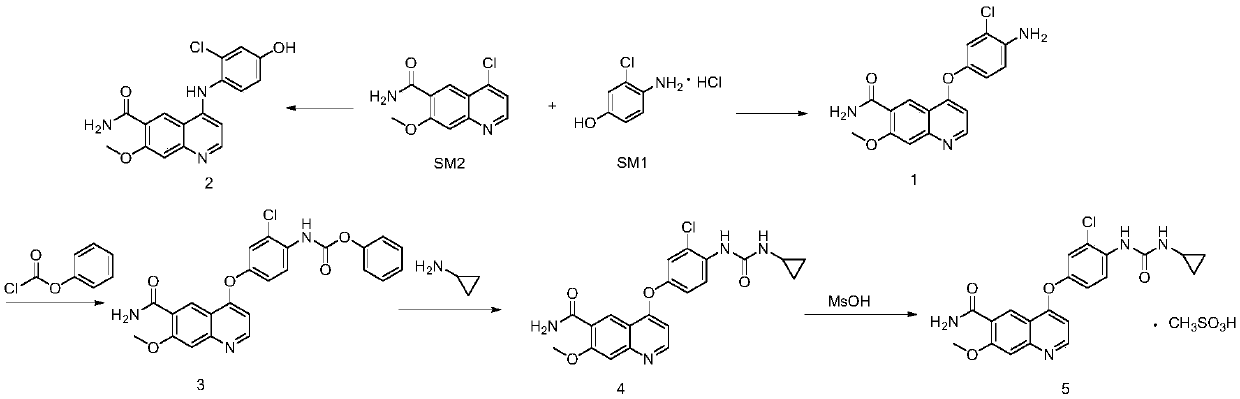
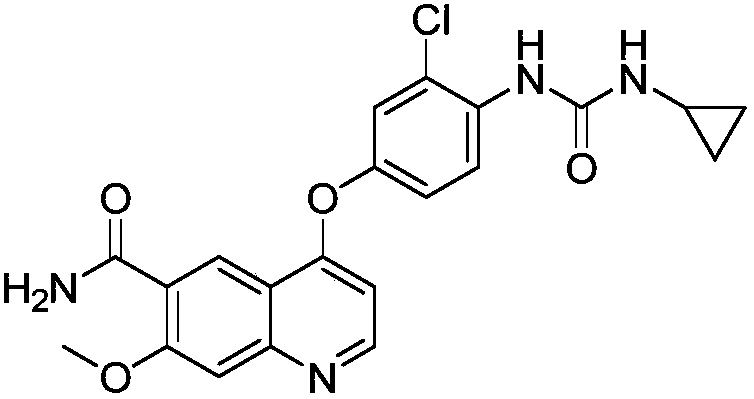
Synthesis method of lenvatinib and new intermediate
InactiveCN111349045AStarting materials are cheap and readily availableOrganic chemistryChlorobenzenePhenyl chloroformate
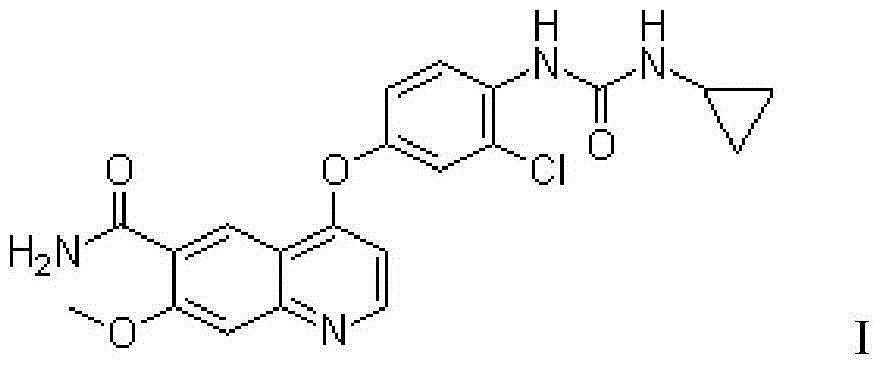
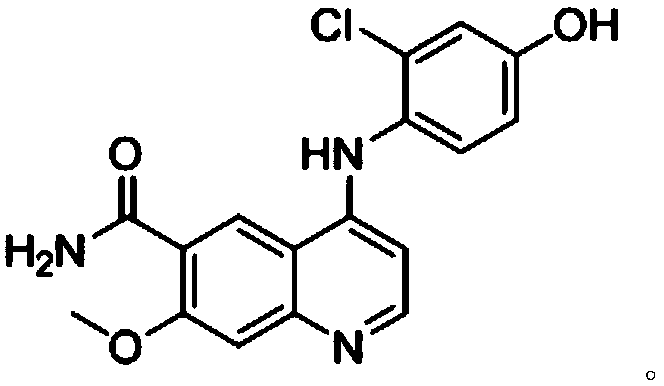
The invention discloses a synthesis method of lenvatinib and a new intermediate. The method comprises the following steps: step 1, taking 4-amino-3-chlorophenol hydrochloride and 4-chloro-7-methoxy-6-amido quinoline as initial raw materials, and carrying out a condensation reaction, so as to obtain a target product 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-carboxamide quinoline; carrying out amidation reaction on the obtained product and phenyl chloroformate to obtain 4-(4-(phenoxycarbonyl) amino-3-chloro-phenoxy)-7-methoxy-6-formamide quinoline, carrying out amidation reaction on the 4-(4-(phenoxycarbonyl) amino-3-chloro-phenoxy)-7-methoxy-6-formamide quinoline and cyclopropylamine to form urea to obtain lenvatinib, and carrying out salifying reaction with methanesulfonic acid to obtain lenvatinib mesylate. The synthesis method has few steps, is simple and convenient to operate, and omits Boc protection and deprotection steps by selecting a solvent. In the research process, it is foundthat for the reaction in the first step, a new compound 4-(4-hydroxy-2-chloro-anilino)-7-methoxy-6-carboxamide quinoline is obtained in a high-yield mode by replacing a solvent.
Owner:JIANGSU WANBANG BIOPHARMLS +1
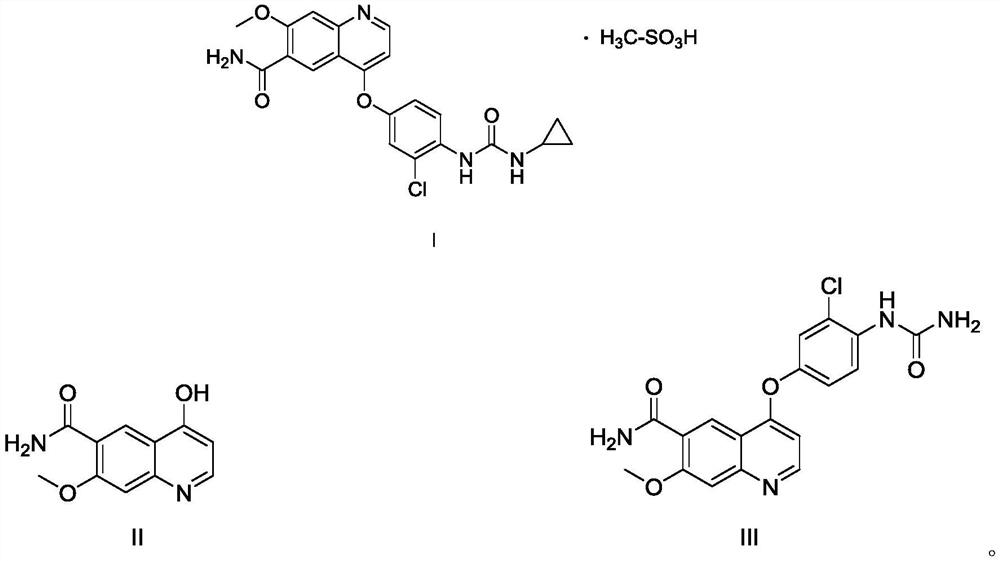
Preparation method of lenvatinib or lenvatinib mesylate impurity
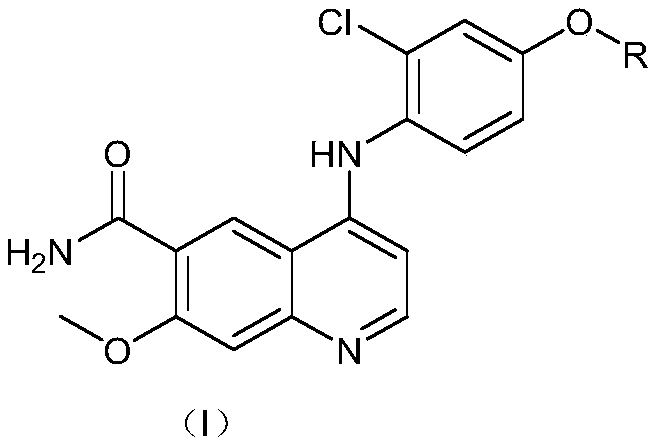
The invention discloses a preparation method of lenvatinib or a lenvatinib mesylate impurity. The preparation method comprises the following steps: taking 4-chloro-7-methoxyquinoline-6-amide as a rawmaterial to carry out a substitution reaction with 4-amino-3-chlorophenol hydrochloride under the action of a catalyst to obtain 4-((2-chloro-4-hydroxyphenyl)amino)-7-methoxyquinoline-6-formamide. Thelenvatinib or the lenvatinib mesylate impurity is as shown in a formula (I), wherein the definition of R is as shown in the specification. Through the preparation method, the lenvatinib or the lenvatinib mesylate impurity is obtained through chemical synthesis for the first time. In addition, a target compound can be obtained by efficient and quick separation.
Owner:YANGTZE RIVER PHARM GRP CO LTD
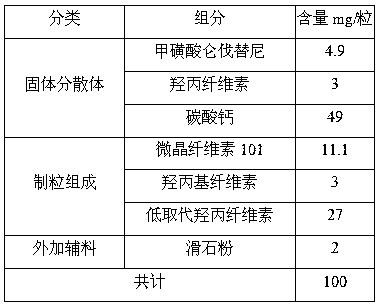
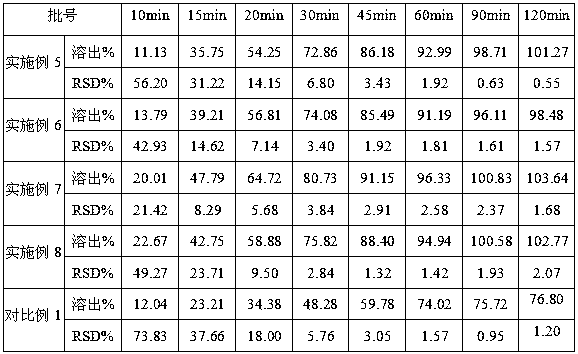
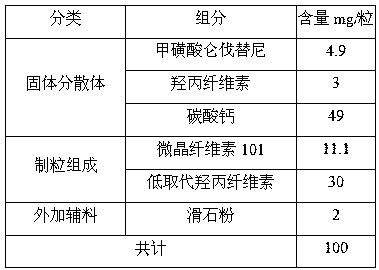
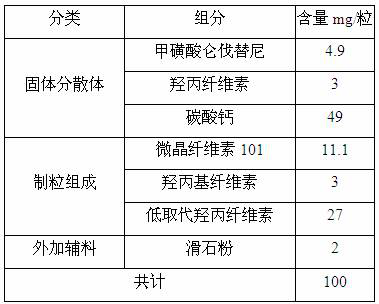
Lenvatinib mesylate solid dispersion and preparation method and application thereof
ActiveCN110693839AGood treatment effectPowder deliveryInorganic non-active ingredientsLenvatinib MesylateCombinatorial chemistry
The invention discloses a lenvatinib mesylate solid dispersion and a preparation method and application thereof, and belongs to the field of chemical pharmacy. The solid dispersion is obtained by thefollowing steps: dissolving lenvatinib mesylate into a solvent, then adding a cellulose derivative, performing stirring for dissolving, spraying the obtained solution onto a carrier, and removing a solvent by drying to obtain the lenvatinib mesylate solid dispersion. The carrier is one or a mixture of more than two of inorganic calcium salt, mannitol, sorbitol, microcrystalline cellulose and low-substituted hydroxypropyl cellulose in any proportion. The lenvatinib mesylate capsule prepared by the method disclosed by the invention improves the problems of the solubility, dissolution rate and stability of lenvatinib mesylate, and can better play a role in treatment.
Owner:LEPU PHARMACEUTICAL CO LTD
Preparation method of high-purity lenvatinib mesylate crystal form C
PendingCN111689897AHigh purityHigh yieldOrganic chemistry methodsSulfonic acids salts preparationLenvatinib MesylateCombinatorial chemistry
The invention belongs to the technical field of pharmaceutical chemicals and especially relates to a preparation method of a lenvatinib mesylate crystal form C. According to the method, the conditions of high temperature, acid serving as a solvent and the like are avoided, so that the content of impurities is greatly reduced, the purity is high, the yield is high, the purity is 99% or above and the yield is 95% or above. Therefore, the method is simple in process, convenient to operate, mild in condition, free of special reaction conditions, environmentally friendly and suitable for industrial production.
Owner:QILU PHARMA
Preparation method of lenvatinib mesylate crystal form X
PendingCN112174886AMeet product quality requirementsReduce usageOrganic compound preparationOrganic chemistry methodsAcetic acidLenvatinib Mesylate
A preparation method of a lenvatinib mesylate crystal form X comprises the following steps that lenvatinib is stirred in acetonitrile and purified water and pulped, wherein the mass volume ratio of lenvatinib to acetonitrile to purified water is 1 g: (2-6 ml): (2-6 ml), and the stirring and pulping temperature is 20-30 DEG C; then, a mixed solution of acetic acid, acetonitrile and methanesulfonicacid is dropwise added, wherein the mass volume ratio of lenvatinib to acetic acid to acetonitrile is 1 g: (1-3 ml): (2-6 ml), and the mass ratio of lenvatinib to methanesulfonic acid is 1 g: (0.22-0.45 g); and after the system is clarified, 0-5% by mass of a seed crystal in a crystal form X is added, acetonitrile is dropwise added into the solution, the mass volume ratio of acetonitrile to lenvatinib is (1-6 ml: 1 g), the temperature is kept at 15-30 DEG C, the heat preservation stirring time is 12-24 h, and suction filtration and drying are conducted to obtain solid powder. The method is simple in preparation process, better in product quality and suitable for industrial production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Novel Crystalline Form of Lenvantinib Mesylate and Process of Preparation Thereof
ActiveUS20190218184A1Improve solubilityImprove stabilityOrganic chemistry methodsAntineoplastic agentsSolubilityLenvatinib Mesylate
The present disclosure relates to a novel crystalline form of lenvatinib mesylate and the preparation method thereof. The novel crystalline form of mesylate of the present disclosure can be used for treating invasive and differentiated thyroid cancer. The novel crystalline form of mesylate of the present disclosure has good solubility, stability, and remarkable purification effect in process. The preparation method of this novel crystalline form is simple, low cost, and has an important value for future optimization and development of the drug.
Owner:CRYSTAL PHARMATECH CO LTD
New crystal form of lenvatinib mesylate, and preparation method thereof
InactiveCN110903239AOrganic chemistry methodsSulfonic acids salts preparationLenvatinib MesylatePharmaceutical drug
The invention relates to a new crystal form of lenvatinib mesylate, and a preparation method thereof. The new crystal form of mesylate is good in stability, remarkable in process purification effect,simple in preparation method and low in cost, and has important value in optimizing and developing of medicine in the future.
Owner:CRYSTAL PHARMA CO LTD
Method for refining Lenvatinib mesylate
ActiveCN110818634AHigh purityHigh refining yieldOrganic chemistryOrganic compound preparationAcetic acidLenvatinib Mesylate
The invention discloses a method for refining Lenvatinib mesylate. The method for refining the Lenvatinib mesylate, provided by the invention, comprises the following step: subjecting a solution formed by an organic solvent and crude Lenvatinib mesylate to cooling and crystallization, thereby obtaining the Lenvatinib mesylate, wherein the organic solvent is a mixed solvent of ethylene glycol di-C1-C4 alkyl ether and C1-C4 alkyl alcoholic solvent or a mixed solvent of ethylene glycol mono-C1-C4 alkyl ether and acetic acid C1-C4 alkyl ester solvent; and the crude Lenvatinib mesylate has an HPLCpurity of 95.0% to 99.0%. The product prepared by the method disclosed by the invention is high in purity, the HPLC purity is higher than 99.80%, the content of a single impurity is lower than 0.10%,the yield of refining is high and reaches 81% to 87%, the production cost is low, and thus, the method is applicable to industrial production.
Owner:上海新礼泰药业有限公司
Biomarkers for predicting and assessing responsiveness of thyroid and kidney cancer subjects to lenvatinib compounds
ActiveUS9945862B2Strong predictionReduce rateMicrobiological testing/measurementAntineoplastic agentsLenvatinib MesylateKidney cancer
Owner:EISIA R&D MANAGEMENT CO LTD
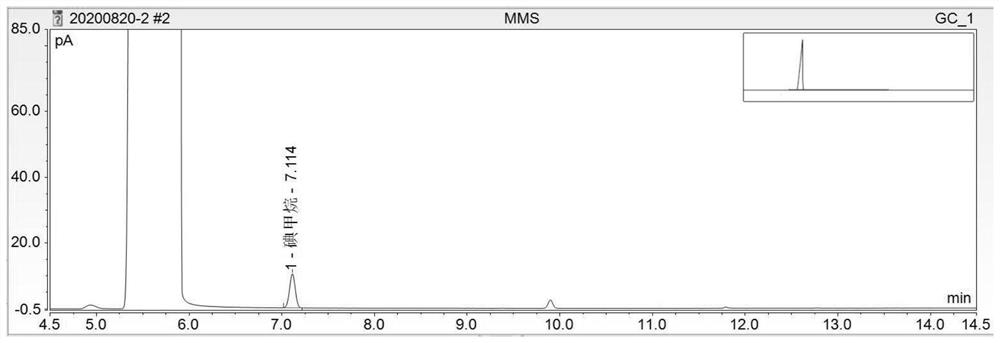
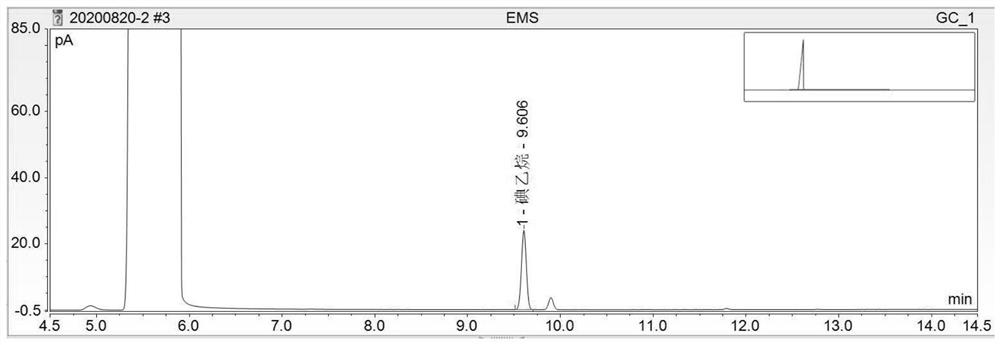
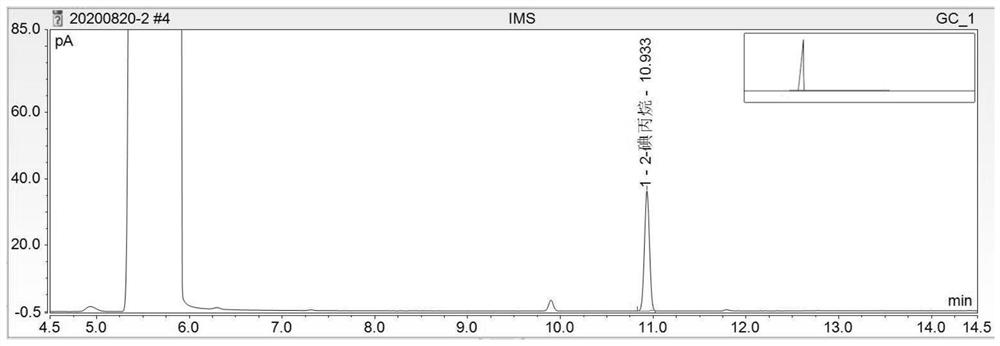
Method for detecting methanesulfonate genotoxic impurities in lenvatinib mesylate by gas chromatography
PendingCN112684066AEffective deduction of detection interferenceReduce matrix interferenceComponent separationVitamin CGas liquid chromatographic
The invention discloses a method for detecting methanesulfonate genotoxic impurities in lenvatinib mesylate by gas chromatography, the methanesulfonate genotoxic impurities may be contained in an antitumor drug lenvatinib mesylate, and at present, the methanesulfonate genotoxic impurities are generally detected by liquid chromatography or gas chromatography-mass spectrometry; according to the method, a sample is derivatized through a derivatization solution of sodium iodide and vitamin C, methanesulfonate substances in lenvatinib mesylate are detected by adopting a gas chromatograph, methanesulfonate impurities are calculated by adopting a standard addition method, the interference of a test sample can be effectively reduced, the method is superior to a common external standard method and a common internal standard method in accuracy, methodology verification proves that the method has good sensitivity, precision and accuracy, and the method is low in detection cost, high in operability and superior to a conventional liquid chromatography-mass spectrometry or gas chromatography-mass spectrometry method.
Owner:南京佰麦生物技术有限公司
Biomarkers for predicting and assessing responsiveness of endometrial cancer subjects to lenvatinib compounds
ActiveUS10517861B2Easy to detectDisease diagnosisAntineoplastic agentsLenvatinib MesylateTreatment modality
Biomarkers are provided that predict whether a subject having endometrial cancer will or will not respond to a therapy comprising lenvatinib or a pharmaceutically acceptable salt thereof (e.g., lenvatinib mesylate). The biomarkers, compositions, and methods described herein are useful in selecting appropriate treatment modalities for and treating a subject having, suspected of having, or at risk of developing an endometrial cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
HPLC method for detecting cyclopropylamine in lenvatinib mesylate
ActiveCN111257491AThe content cannot be accurately determinedAccurate detectionComponent separationHplc methodLenvatinib Mesylate
The invention discloses an HPLC (High Performance Liquid Chromatography) method for detecting cyclopropylamine in lenvatinib mesylate, The method is characterized in that the method adopts a reversed-phase chromatographic column and uses derivatization reagent to perform derivatization treatment of a to-be-detected sample, and a mobile phase is selected from salt buffer solution containing amine substances and organic solvent. The method can be used for accurately detecting the content of cyclopropylamine in lenvatinib mesylate, is simple and feasible, is low in analysis cost, is accurate andreliable in result, and is convenient for controlling the product quality in the production and quality control process.
Owner:SIMCERE ZAIMING PHARM CO LTD
Pharmaceutical compositions of lenvatinib
ActiveUS10583133B2Oral administration is convenientInorganic non-active ingredientsGranular deliveryCalcium hydroxideLenvatinib Mesylate
The present invention relates to a pharmaceutical composition comprising lenvatinib mesylate and a stabilizer in an amount of about 10% to about 20% based on the total weight of the composition, wherein the stabilizer is selected from the group consisting of calcium hydroxide and potassium hydroxide; and its process for preparation thereof.
Owner:SHILPA MEDICARE LTD
Preparation method of high-purity crystal
PendingCN113999173ANo seeding inductionReduce self-decompositionOrganic compound preparationOrganic chemistry methodsLenvatinib MesylateSolvent
The invention provides a preparation method of a high-purity crystal, and particularly relates to a preparation method of a mesylate lenvatinib crystal C. The preparation method comprises the following steps: 1) dissolving a lenvatinib free alkali in a good solvent to obtain a solution of the lenvatinib free alkali; 2) clarifying and filtering the solution obtained in the step 1), adding methanesulfonic acid and a poor solvent I or a solution of methanesulfonic acid in the poor solvent I, and carrying out a salt forming reaction to obtain a crude product of the mesylate lenvatinib; 3) putting the obtained crude product of the mesylate into a poor solvent II, and pulping to obtain a mesylate crystal C. The purity of the obtained mesylate crystal C is not lower than 99.5%, the purity of any single impurity is not higher than 0.10%, and the total impurity is not higher than 0.5%. The purity of the lenvatinib mesylate crystal C prepared by the preparation method of the lenvatinib mesylate crystal C is higher, wherein, the contents of an impurity A and an impurity B in the lenvatinib mesylate crystal C are greatly reduced.
Owner:2Y CHEM
Preparation method of amorphous quinoline carboxamide derivative
ActiveCN113087666AFluffy appearanceReduce moisture contentOrganic chemistryOrganic compound preparationLenvatinib MesylateQuinoline
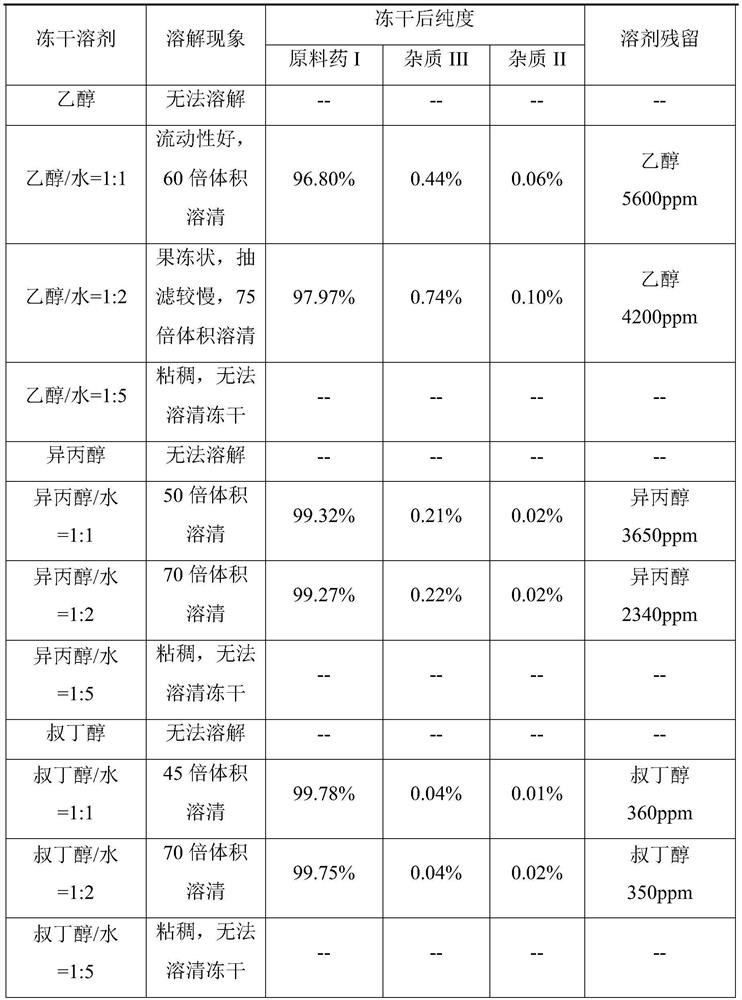
The invention relates to a preparation method of an amorphous quinoline carboxamide derivative, which comprises the following steps: dissolving a crystal form C of lenvatinib mesylate in a tert-butyl alcohol / water mixed solvent, stirring, filtering, collecting filtrate, and freeze-drying to obtain the amorphous quinoline carboxamide derivative. The amorphous form obtained by the method is high in purity, uniform in freeze-drying, fluffy in sample appearance, low in water content, qualified in solvent residue, small in damage of a freeze-drying solvent to instruments and equipment, safe to operate and suitable for industrial production, the obtained amorphous form does not generate a gel phenomenon in a preparation process, and capsules prepared from the amorphous form as a raw material have the advantage of being fast in dissolution.
Owner:NANJING CHIA TAI TIANQING PHARMA
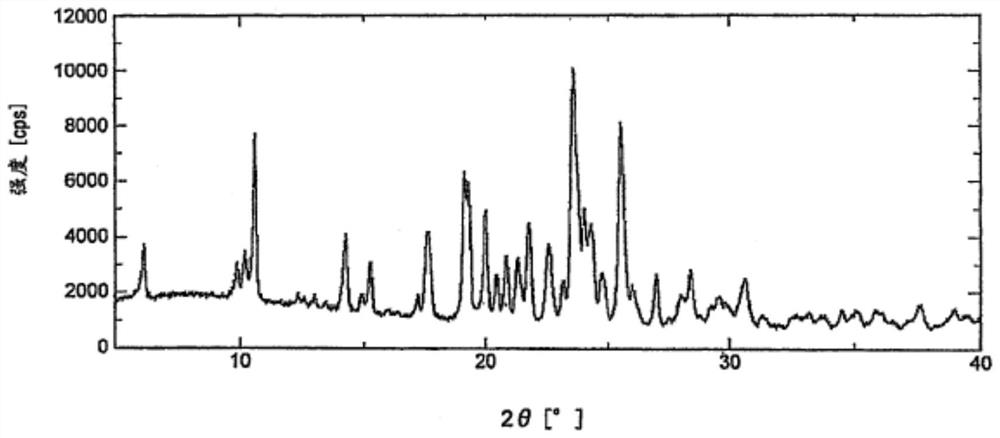
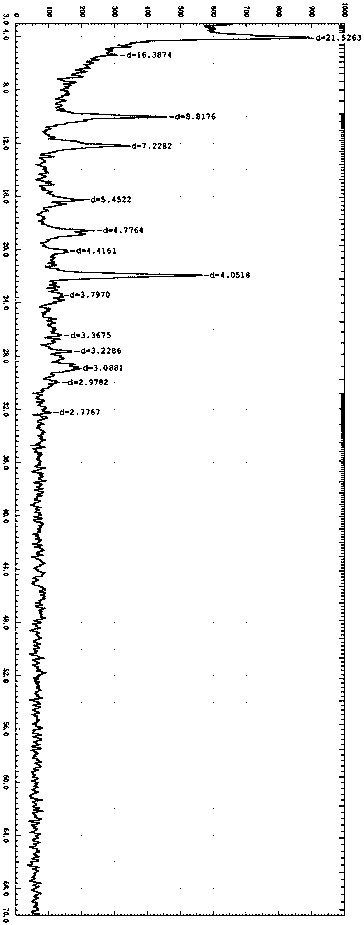
Novel crystal form of lenvatinib mesylate
PendingCN110563644AOrganic compound preparationOrganic chemistry methodsSolubilityLenvatinib Mesylate
The invention discloses a novel crystal form of lenvatinib mesylate. In an X-ray powder diffraction pattern, obtained through determination using Cu-Ka ray, of the new crystal form, at least followingcharacteristic peaks are contained: diffraction angles 2 theta are at 4.19 + / -0.2 DEG, 10.15 + / -0.2 DEG, 12.40 + / -0.2 DEG, 18.93 + / -0.2 DEG, 22.05 + / -0.2 DEG and 29.26 + / -0.2 DEG. The obtained new crystal form has good solubility and stability, and is suitable for industrial production. In addition, the invention also provides a preparation method and a medicinal composition of the new crystal form.
Owner:北京赛思源生物医药技术有限公司
Biomarkers for combination therapy comprising lenvatinib and everolimus
Owner:EISIA R&D MANAGEMENT CO LTD
Solid state forms of lenvatinib mesylate
The present invention provides a crystalline form of Lenvatinib Mesylate, processes for the preparation of crystalline form of lenvatinib Mesylate and pharmaceutical compositions thereof. The crystalline form of lenvatinib Mesylate designated as Form VN1 is characterized by powder X-ray diffraction pattern. The present invention further provides a process for the preparation of amorphous form of lenvatinib Mesylate. The amorphous form is characterized by powder X-ray diffraction pattern.
Owner:DR REDDYS LAB LTD
The preparation method of amorphous quinoline carboxamide derivative
ActiveCN113087666BFluffy appearanceReduce moisture contentOrganic chemistryOrganic compound preparationLenvatinib MesylateQuinoline
The invention relates to a preparation method of an amorphous quinoline carboxamide derivative, which comprises dissolving lenvatinib mesylate crystal form C in a tert-butanol / water mixed solvent, stirring, filtering, collecting the filtrate, and freeze-drying to obtain an amorphous . The amorphous obtained by the method has high purity, uniform freeze-drying, fluffy sample appearance, low water content, qualified residual dissolution, little damage to instruments and equipment by the freeze-drying solvent, safe operation and suitable for industrial production, and the obtained amorphous is not stable in the preparation process. Gel phenomenon will occur, and capsules made of amorphous materials have the advantage of fast dissolution.
Owner:NANJING CHIA TAI TIANQING PHARMA
A kind of lenvatinib mesylate solid dispersion and its preparation method and application
ActiveCN110693839BGood treatment effectPowder deliveryInorganic non-active ingredientsLenvatinib MesylateMannitol
The invention discloses a lenvatinib mesylate solid dispersion, a preparation method and application thereof, and belongs to the field of chemical pharmacy. The solid dispersion is obtained by the following method, dissolving lenvatinib mesylate in a solvent, then adding a cellulose derivative, stirring to dissolve, spraying onto a carrier, and removing the solvent by drying to obtain methanesulfonate A lenvatinib solid dispersion, the carrier is one or a mixture of two or more in any proportion among inorganic calcium salt, mannitol, sorbitol, microcrystalline cellulose and low-substituted hydroxypropyl cellulose. The lenvatinib mesylate capsule prepared by the method of the invention improves the solubility, dissolution rate and stability of the lenvatinib mesylate, so that it can better exert its therapeutic effect.
Owner:LEPU PHARMACEUTICAL CO LTD
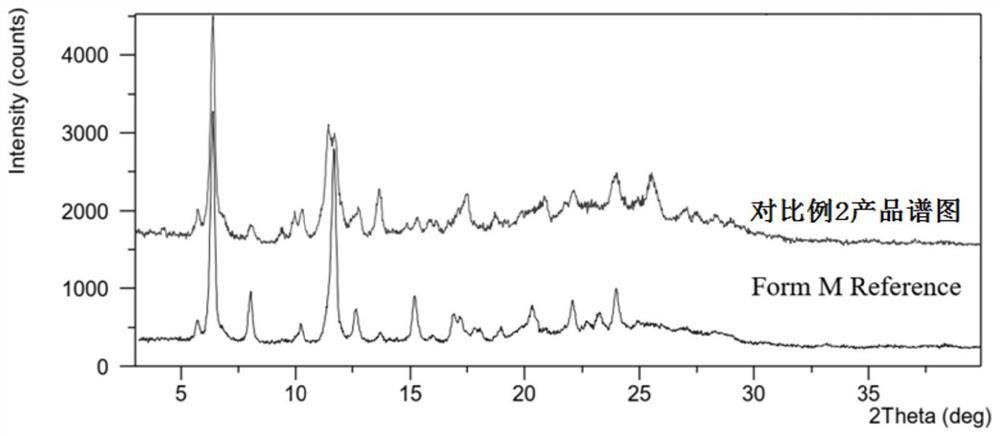
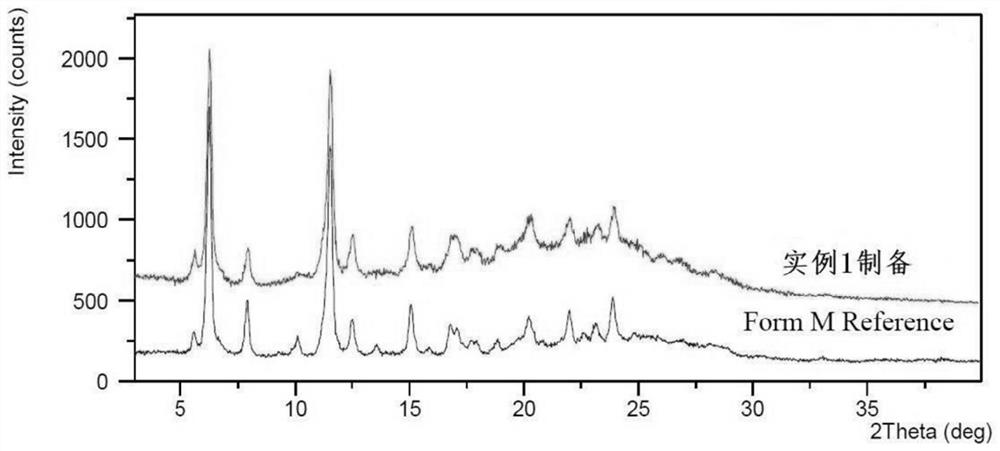
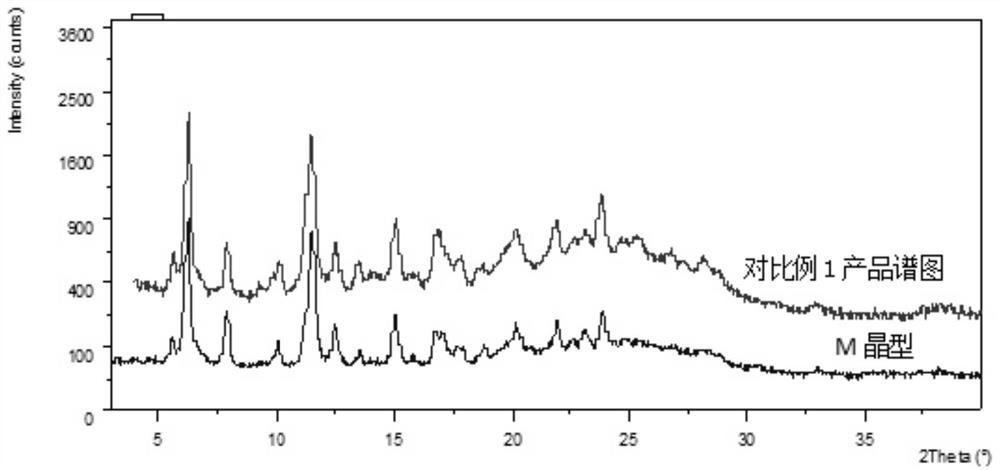
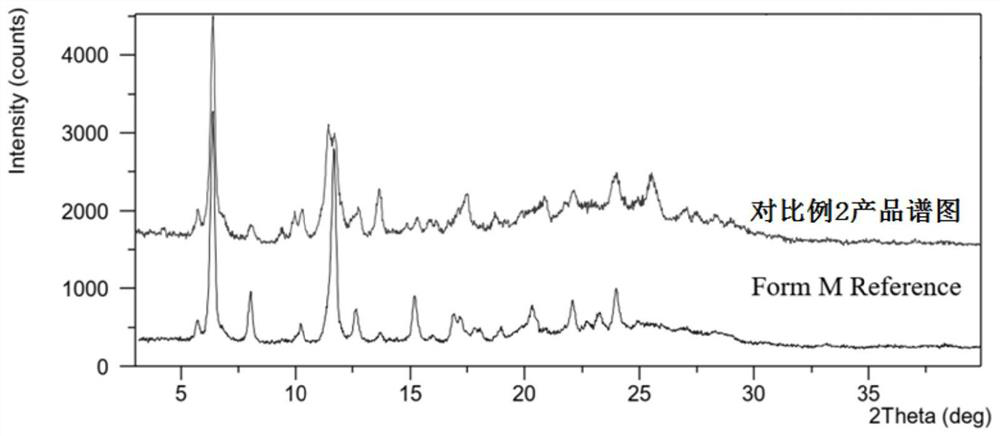
The preparation method of lenvatinib mesylate M crystal form
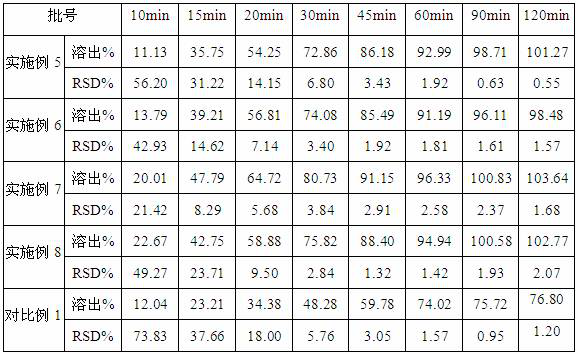
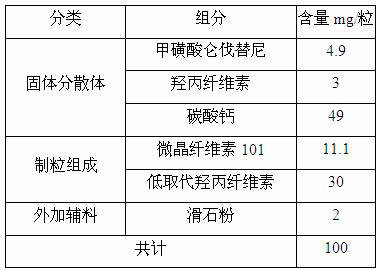
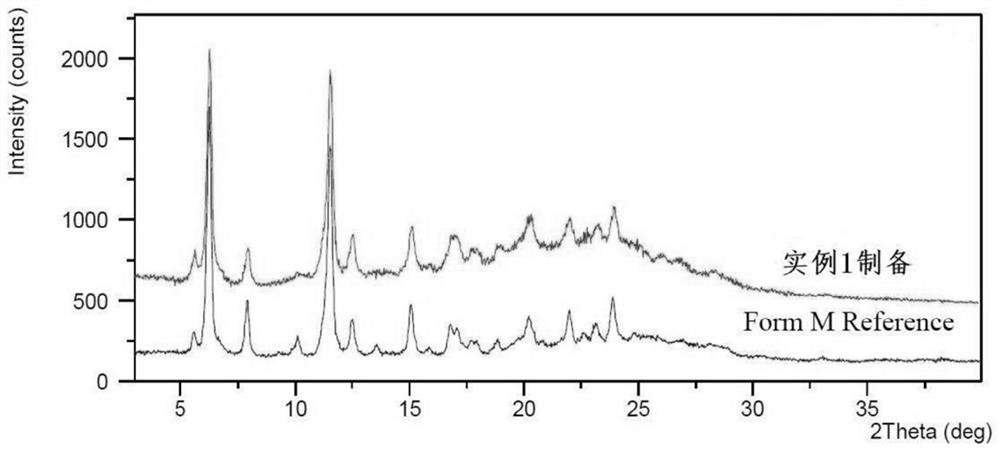
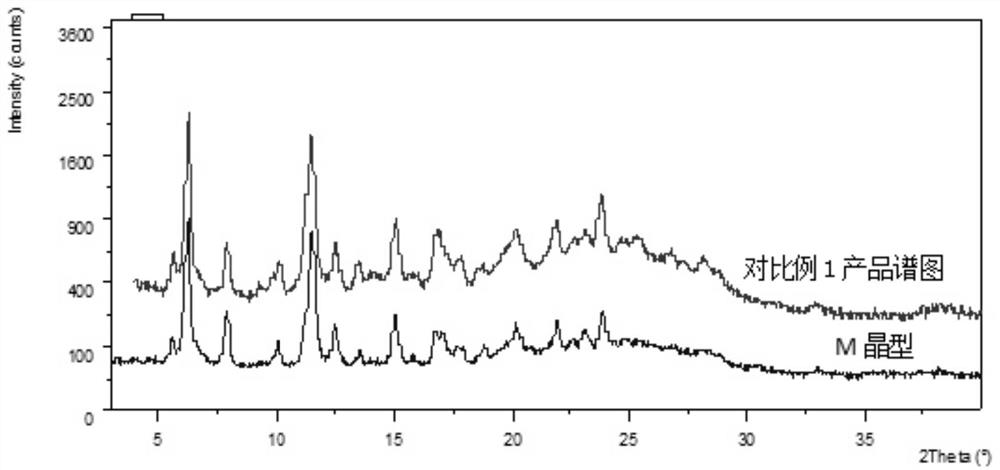
ActiveCN112939859BGood process reproducibilityHigh yieldOrganic compound preparationOrganic chemistry methodsLenvatinib MesylateMethanesulfonic acid
The invention discloses a preparation method of lenvatinib mesylate M crystal form. The preparation method comprises the following steps: (1) salt-forming reaction; (2) separating the wet product of the salt; (3) drying to obtain the salt (4) Dry products replenish water. The preparation method has good process reproducibility, high yield, high crystal form and chemical purity, the quality meets the quality control requirements, and can be stably scaled up to the kilogram level.
Owner:南京方生和医药科技有限公司
Preparation method of lenvatinib mesylate M crystal form
ActiveCN112939859ASuppress generationGuaranteed purityOrganic compound preparationOrganic chemistry methodsLenvatinib MesylatePhysical chemistry
The invention discloses a preparation method of a lenvatinib mesylate M crystal form. The preparation method comprises the following steps: (1) conducting salt forming reaction; (2) separating a wet product of salt; (3) conducting drying to obtain a dry product of salt; and (4) supplementing water to the dried product. The preparation method is good in process reproducibility, high in yield and high in crystal form and chemical purity, and the quality meets the quality control requirement and can be stably amplified to a kilogram level.
Owner:南京方生和医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com