Preparation method of lenvatinib or lenvatinib mesylate impurity
A technology of lenvatinib and mesylate, applied in the field of preparation of process impurities in the production process of lenvatinib or mesylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
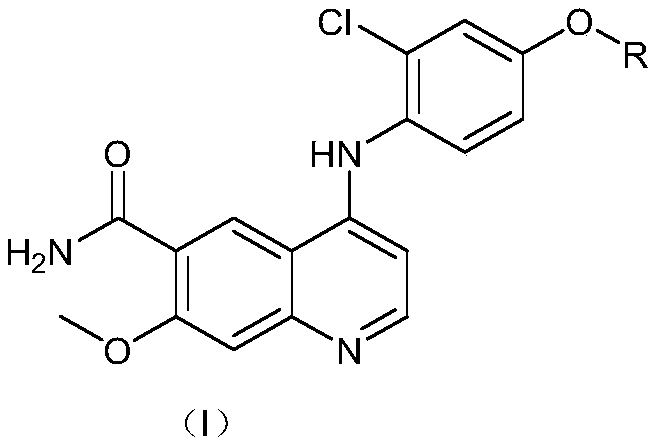
[0050] Lenvatinib mesylate impurity—preparation of 4-((2-chloro-4-hydroxyphenyl)amino)-7-methoxyquinoline-6-carboxamide
[0051] Add 4-chloro-7-methoxyquinoline-6-amide (10g) and 4-amino-3-chlorophenol hydrochloride (11.5g), potassium iodide (25g) into the reaction flask, add ethanol (150ml) Under stirring, heated to reflux, stirred and reacted for 20 hours, and TLC monitored that the reaction was complete (the developing solvent was dichloromethane:methanol=10:1, 4-((2-chloro-4-hydroxyphenyl)amino)-7-methanol The Rf value of oxyquinoline-6-carboxamide is 0.3), the reaction solution is cooled to room temperature, and water (450ml) is added to stir and crystallize for 2 hours, and 13.5g of the target product is obtained by filtration (mass yield 135%), HPLC purity 98.7 %. MS (ESI): 342.3 [M-H] - .
Embodiment 2
[0053] Lenvatinib mesylate impurity-4-(4-((6-carbamoyl-7-methoxyquinolin-4-yl)amino)-3-chlorophenoxy)-7-methoxy Preparation of quinoline-6-carboxamide
[0054] Weigh 1ml of purified water and add it to the reaction flask, and at the same time weigh potassium hydroxide (0.71g) and add it to the reaction flask, stir until it dissolves, add 9ml of DMSO, and stir to dissolve 4-((2-chloro-4-hydroxybenzene Base) amino)-7-methoxyquinoline-6-formamide (2.18g) and 4-chloro-7-methoxyquinoline-6-amide (1.0g) were added to the reaction flask successively, and the temperature was raised to Stir the reaction at 110°C for 2 hours, and TLC monitors that the reaction is complete (the developing solvent is dichloromethane:isopropanol=20:1,4-(4-((6-carbamoyl-7-methoxyquinoline-4- Base) amino)-3-chlorophenoxy)-7-methoxyquinoline-6-carboxamide Rf value 0.2), add 3ml acetone and 27ml purified water in reaction solution, cool to room temperature, stir and crystallize 2 hour, filtered, the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com