Patents
Literature
40results about How to "Oral administration is convenient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interpenetrating network polymer type super porous aquogel, its prepn. method and application
InactiveCN1757662AOral administration is convenientHigh mechanical strengthPharmaceutical non-active ingredientsCarrier-bound/immobilised peptidesCross-linkFoaming agent
A super-porous aqueo-gel of an interpenetrating network polymer used for the orally applying system of protein polypeptide to suppress proteinase and break the close linking between epithelial cells contains two polymers: a cross-linked polymer and a cross-linked polyose polymer. Its preparing process includes such steps as mixing at least one unsaturated enylmonomer, at least one polyenyl cross-linking agent, a linear polyose polymer and a foaming agent to generate the super-porous aqueo-gel of semi-interpenetrating network polymer, and cross-linking with linear polyose.
Owner:FUDAN UNIV
Ultra porous hydrogel complex substance, preparing method and use in pharmaceutics thereof
InactiveCN1488331AHigh mechanical strengthGood bioadhesionPeptide/protein ingredientsPharmaceutical delivery mechanismCross-linkFoaming agent
The invention discloses an ultra-multiaperture hydrogel compound, making method and application in pharmacy. It contains cross-linked polymer and Carbomer. At least one unsaturated alkene monomer and polyene cross-linking agent polymerize to develop it. The making steps: mix at least one unsaturated alkene monomer, one polyene cross-linking agent, Carbomer and one foaming agent; the mixture develops it on the condition of polymerizing and foaming. It can be used in stomach floating preparation and protein polypeptide oral medicine supplying system.
Owner:FUDAN UNIV
Methods for oral administration of active drugs
InactiveUS20090155363A1Oral administration is convenientBiocidePowder deliveryCo administrationOral medication
The present invention relates to methods that facilitate the oral administration of active drugs to a patient. Specifically, the methods of the present invention may utilize compositions comprising an active drug and a gelling agent that provides an easily consumable gel dosage form and the active drug is homogenously mixed within the gel.
Owner:MAIBACH TODD
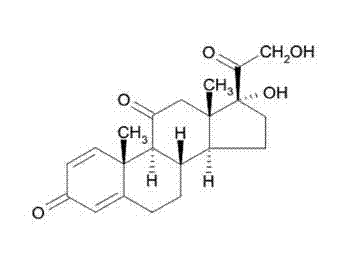
Oral prednisone time-selecting release preparation and preparation method thereof
InactiveCN103690545AGive full play to the therapeutic effectImprove balanceOrganic active ingredientsAntipyreticCelluloseFormulary
The invention discloses an oral prednisone time-selecting release preparation and a preparation method thereof. The oral prednisone time-selecting release preparation provided by the invention mainly consists of 0.3-5 parts of prednisone and derivatives thereof, 10-50 parts of glyceryl behenate and 3-30 parts of hydroxypropyl cellulose, and can further contain a disintegrating agent and other pharmaceutically acceptable excipients. The preparation method is as below: extruding tablet cores or granules containing the drug according to the formula by a tablet press or a dry granulator; and coating the tablet cores or particles containing the drug by a coating pan or a fluidized bed to attach the coating film to the tablet cores or particles containing the drug, so as to obtain the oral prednisone time-selecting release preparation. The oral prednisone time-selecting release preparation provided by the invention can achieve a good balance between the biological rhythm of the patients and the curative effects, and is safer, more convenient and effective compared with a traditional preparation. The oral prednisone time-selecting release preparation is prepared by an extrusion-coating process, which is simple for operation, and the obtained time-selecting release preparation has the advantages of drug stability and high reproducibility.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Pharmaceutical compositions of cholesteryl ester transfer protein inhibitors
InactiveUS7235259B2Improve aqueous concentrationEnhance solubilityPowder deliveryBiocideCholesteryl esterChemistry
A pharmaceutical composition comprises a solid amorphous dispersion of a cholesteryl ester transfer protein inhibitor and a concentration-enhancing polymer.
Owner:PFIZER INC +1
Polypeptide protein oral nano particle preparation
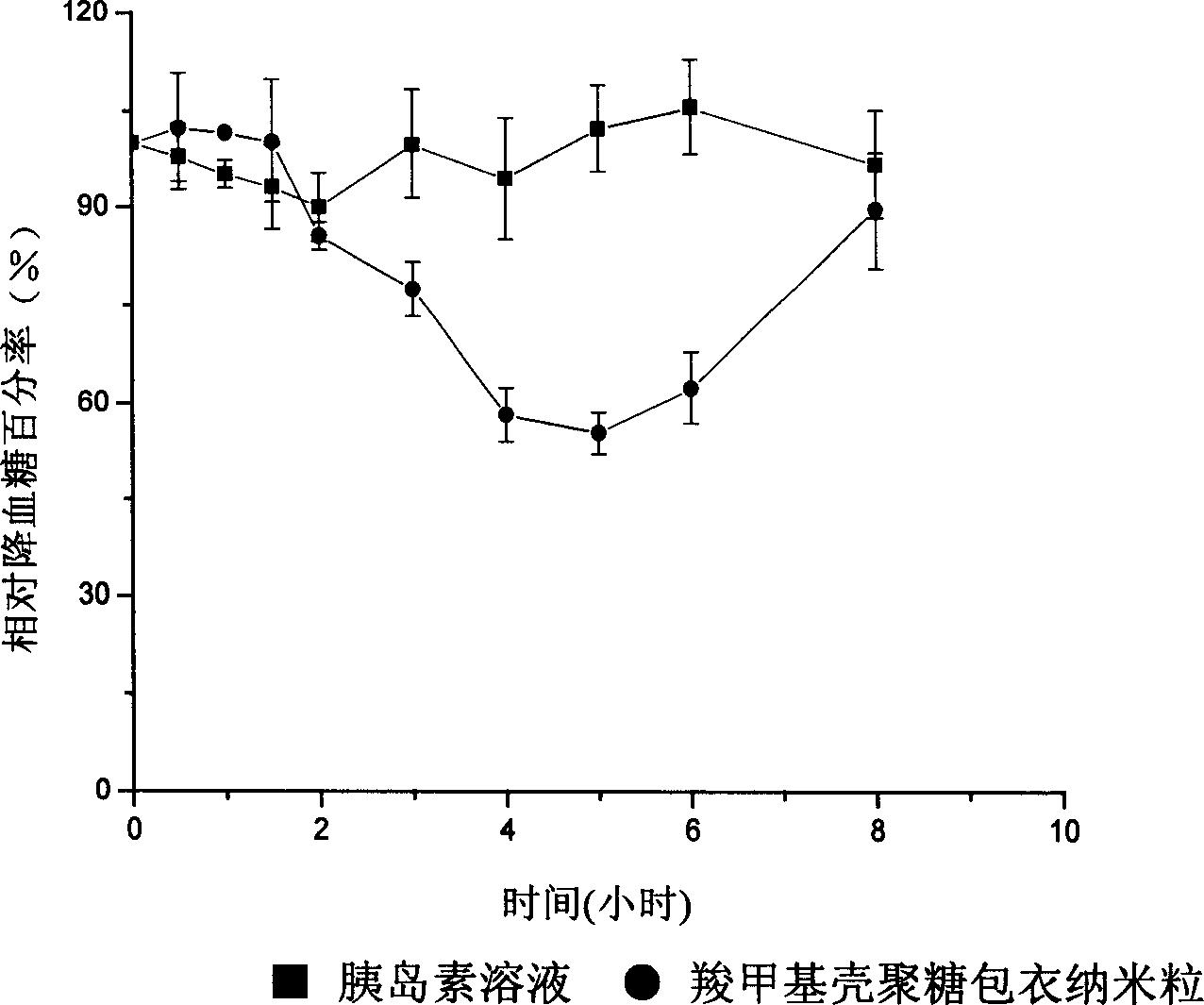
InactiveCN1454588AImprove bioavailabilityOral administration is convenientPowder deliveryPeptide/protein ingredientsChitosan coatingPolyester
The invention is a polypeptide protein type oral nano granular preparation. It uses polymer or amphiphatic compound as coating material, and uses the hydrogenous bonds formed between the surfactants absorbed on the interface of the coating material and the oil-water, for examples, the amido in the swelling chitosan molecule in the chitosan coating polyester nano granule and the oxygen atom or the alcohol hydroxyl in the hydrophilic ehenoxy group form the hydrogenous bonds to make it; or the chitosan directly makes the gelation reaction with the multivalent anions to make the nano-granular preparation.
Owner:SHENYANG PHARMA UNIVERSITY
Proteolysis targeting chimeric body, prodrug molecule for improving oral bioavailability of protein hydrolysis targeting chimeric body and application thereof
ActiveCN111909155ASignificant induction periodSignificantly induce apoptosisOrganic chemistryAntineoplastic agentsStage melanomaCancer cell
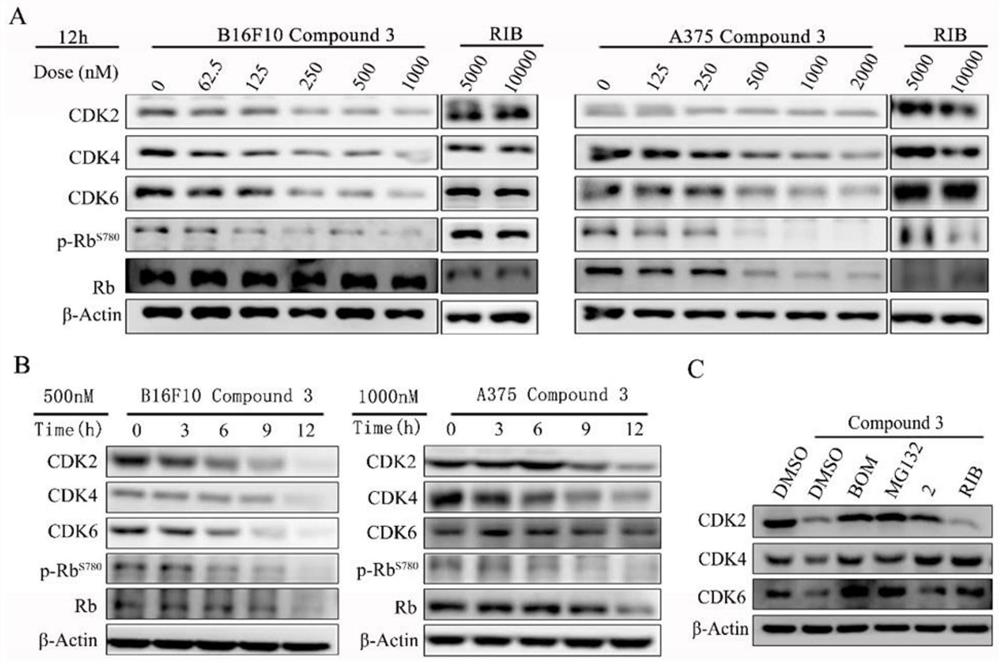
The invention provides a proteolytic targeting chimeric body, a prodrug molecule for improving oral bioavailability of the proteolytic targeting chimeric body and application thereof. According to thetechnical scheme, a novel PROTAC degradation agent compound is developed on the basis of a ribociclib derivative and a CRBN ligand. Small molecules can effectively degrade CDK2 / 4 / 6 and compounds thereof in malignant melanoma at the same time; the cell cycle can be quickly reset, and apoptosis of various cancer cells, especially melanoma cells, can be induced. A mechanism should be explained as that CDK 2 / 4 / 6 deficiency may lead to synthetic lethal effects of malignant melanoma in the presence of a compound. The results show that the combination of CDK2 / 4 / 6 is expected to become a kinase target for treating solid tumors. In addition, the invention also develops a prodrug with high oral bioavailability for the first time, and is convenient for oral administration in animal tests. A universal solution is provided for oral administration of PROTAC molecules with CRBN ligands.
Owner:DONGGUAN UNIV OF TECH
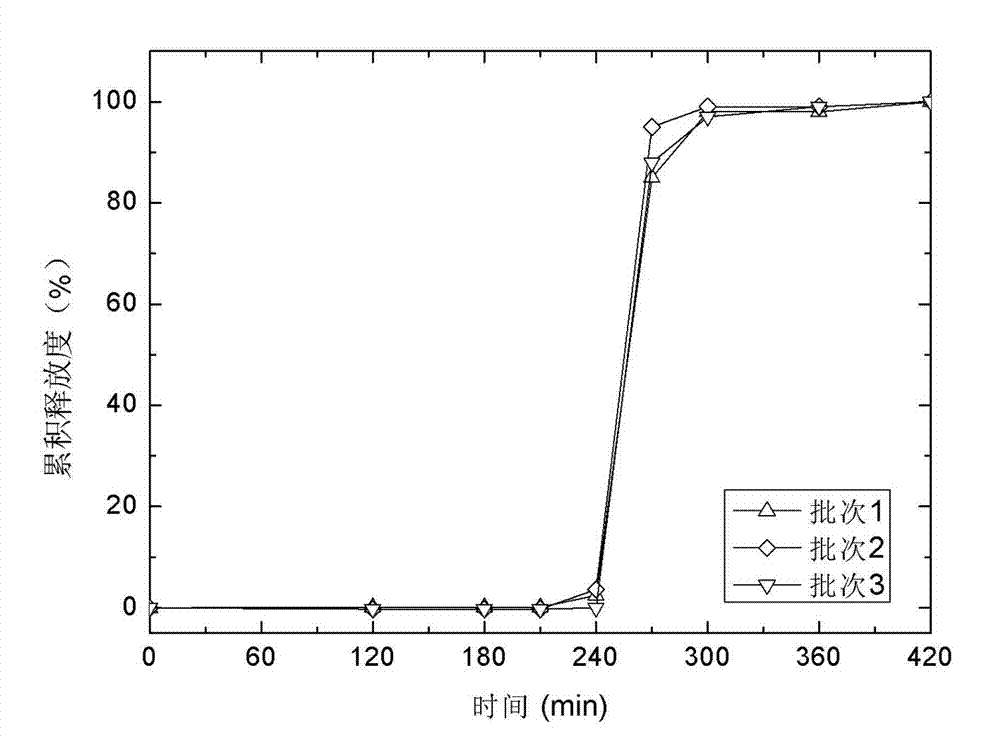
Method for Producing Pancreatin Pellets
ActiveUS20120213857A1Improve propertiesImproved and economical for productionPowder deliveryPeptide/protein ingredientsMaterials scienceResidual moisture
In order to avoid compromising the pharmacological effect of pancreatin caused by the addition of auxiliary materials or binding agents, a pancreatin pellet having a 100% pancreatin content consists exclusively of pancreatin with a residual moisture content of less than 3% by weight, preferably less than 1% by weight or less than 0.5% by weight.
Owner:NORDMARK PHARMA GMBH
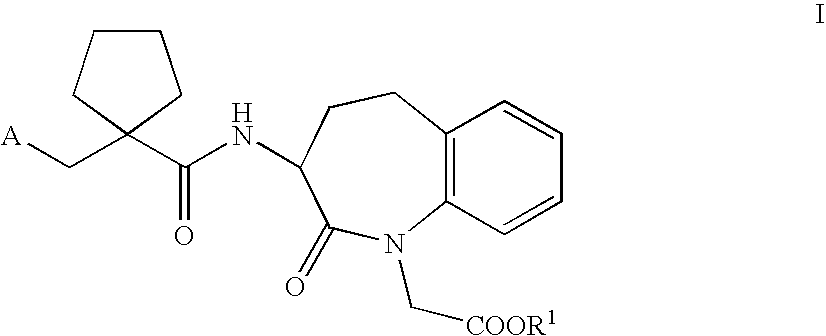
Synthesized micromolecule compound capable of conveying bioactivator and application thereof
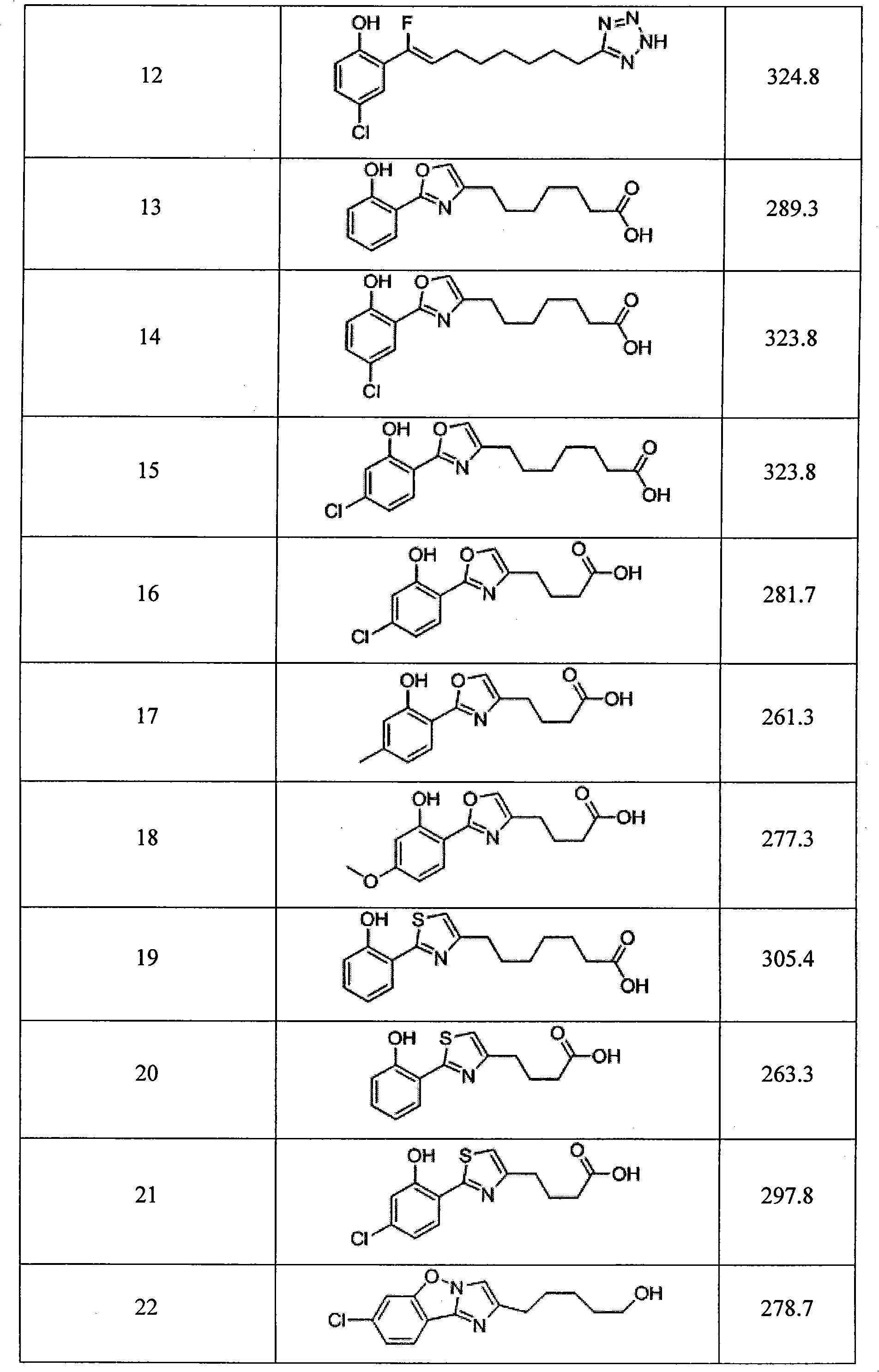
ActiveCN101555212AImprove stabilityImprove absorption rateOrganic chemistryPharmaceutical non-active ingredientsTetrazoleCarboxylic acid
The invention relates to a synthesized micromolecule compound capable of conveying bioactivator and an application thereof. The micromolecule compound of the invention or any medicinal salt thereof are provided with the following general formula: A-B-X-C-D-E; wherein, A is C6-C10 aromatic ring or C2-C9 hetero-aromatic ring, B is C6-C10 aromatic ring or C2-C9 hetero-aromatic ring, X is O,NH, S or CH2 and C is -(CH2)n-, C3-C6 naphthene base, C6-C10 aromatic ring or C2-C9 hetero-aromatic ring. D is carboxylic acid group (COOH) or CONHOH, when C stands for -(CH2)n-; when C is C6-C10 aromatic ring or C2-C9 hetero-aromatic ring, D is -(CH2)n-, and E is negative charged carboxyl group (COOH), -CONHOH, tetrazole, OH or -SO3H.
Owner:SANSURE (SHANGHAI) GENE TECH LTD
Stabilized oral pharmaceutical composition
InactiveUS20050112197A1Oral administration is convenientIncrease drug concentrationBiocideNervous disorderPolyethylene glycolCyclooxygenase
An orally deliverable pharmaceutical composition is provided comprising an aminosulfonyl-comprising drug, for example a selective cyclooxygenase-2 inhibitory drug such as celecoxib, and a solvent liquid comprising a polyethylene glycol and one or more free radical-scavenging antioxidants. At least a substantial part of the drug is in dissolved form in the solvent liquid. The composition has rapid-onset properties and is useful in treatment of cyclooxygenase-2 mediated conditions and disorders.
Owner:GAO PING +8
Pharmaceutical compositions containing dually acting inhibitors of neutral endopeptidase for the treatment of sexual dysfunction
InactiveUS20050267072A1Improve human erectile functionReduce ET- formationBiocideAnimal repellantsMedicineSexual dysfunction
The present invention relates to the novel medicinal use of dually acting compounds capable of inhibiting neutral endopeptidase (=NEP) and human soluble endopeptidase (=hSEP) in the prophylaxis and / or treatment of sexual dysfunction in mammals and humans.
Owner:SOLVAY PHARMA GMBH
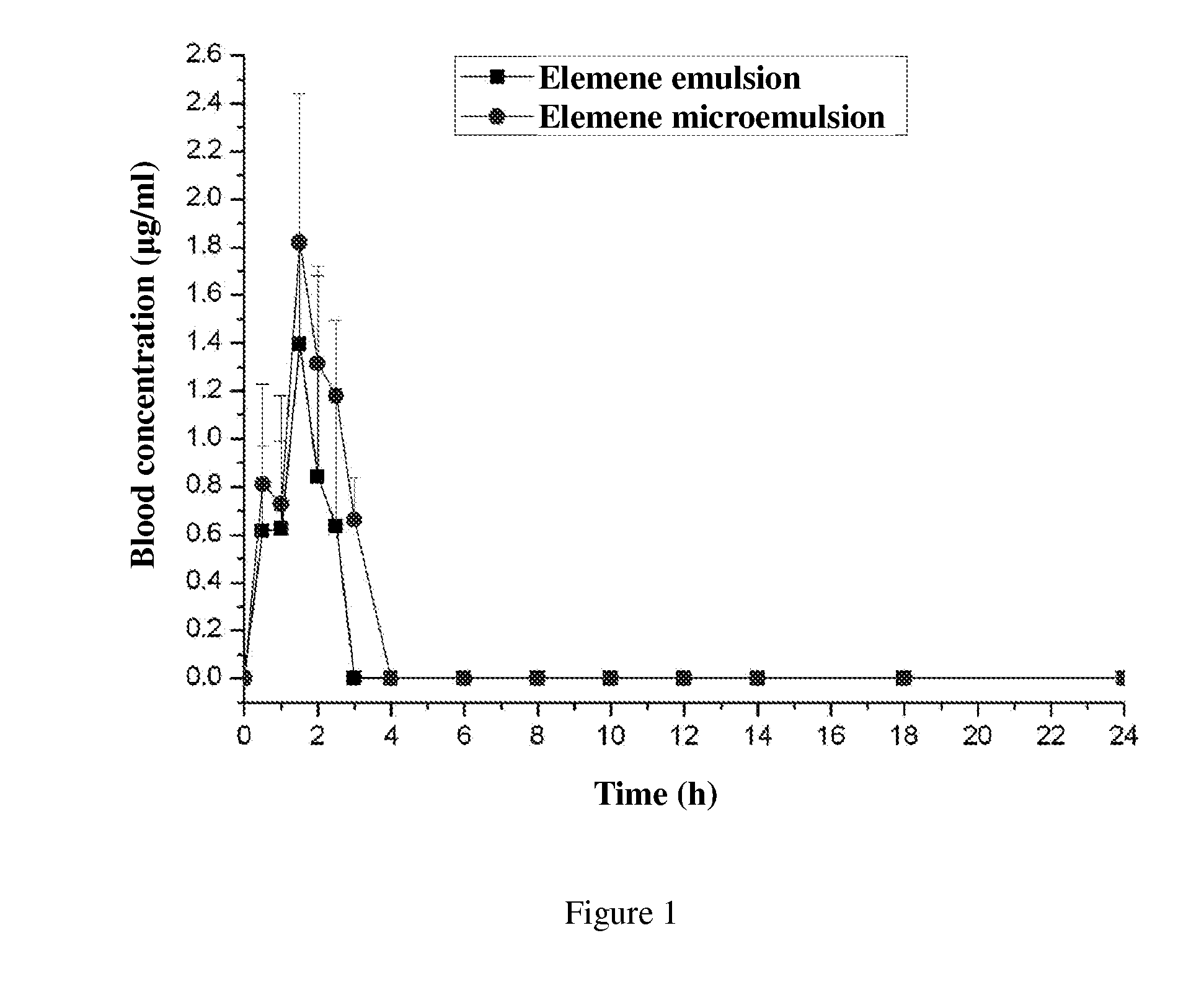
Oral microemulsion of elemene
InactiveUS20120322892A1Eliminate side effectsSave raw materialsBiocideOrganic active ingredientsPolyoxyethylene castor oilPolyethylene glycol
Owner:XIE TIAN
Pharmaceutical Compositions of Cholesteryl Ester Transfer Protein Inhibitor
InactiveUS20060211654A1Increase concentrationImprove solubilityAntibacterial agentsPowder deliveryCholesterylester transfer proteinCholesteryl ester
A pharmaceutical composition comprises a solid amorphous dispersion of a cholesteryl ester transfer protein inhibitor and a concentration-enhancing polymer.
Owner:BEND RES
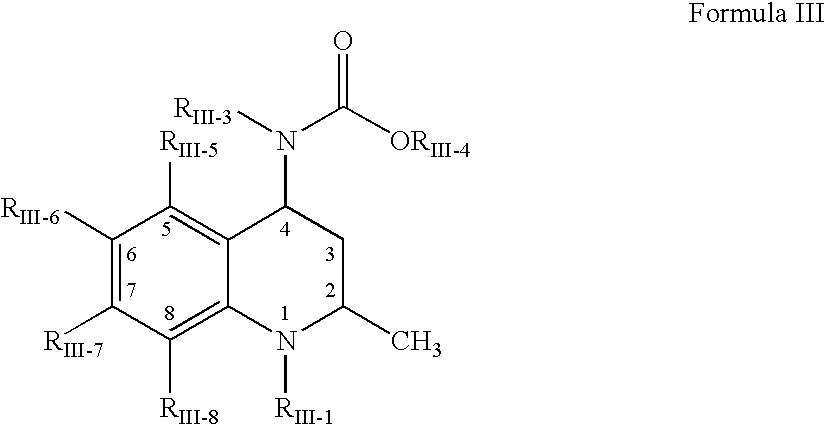
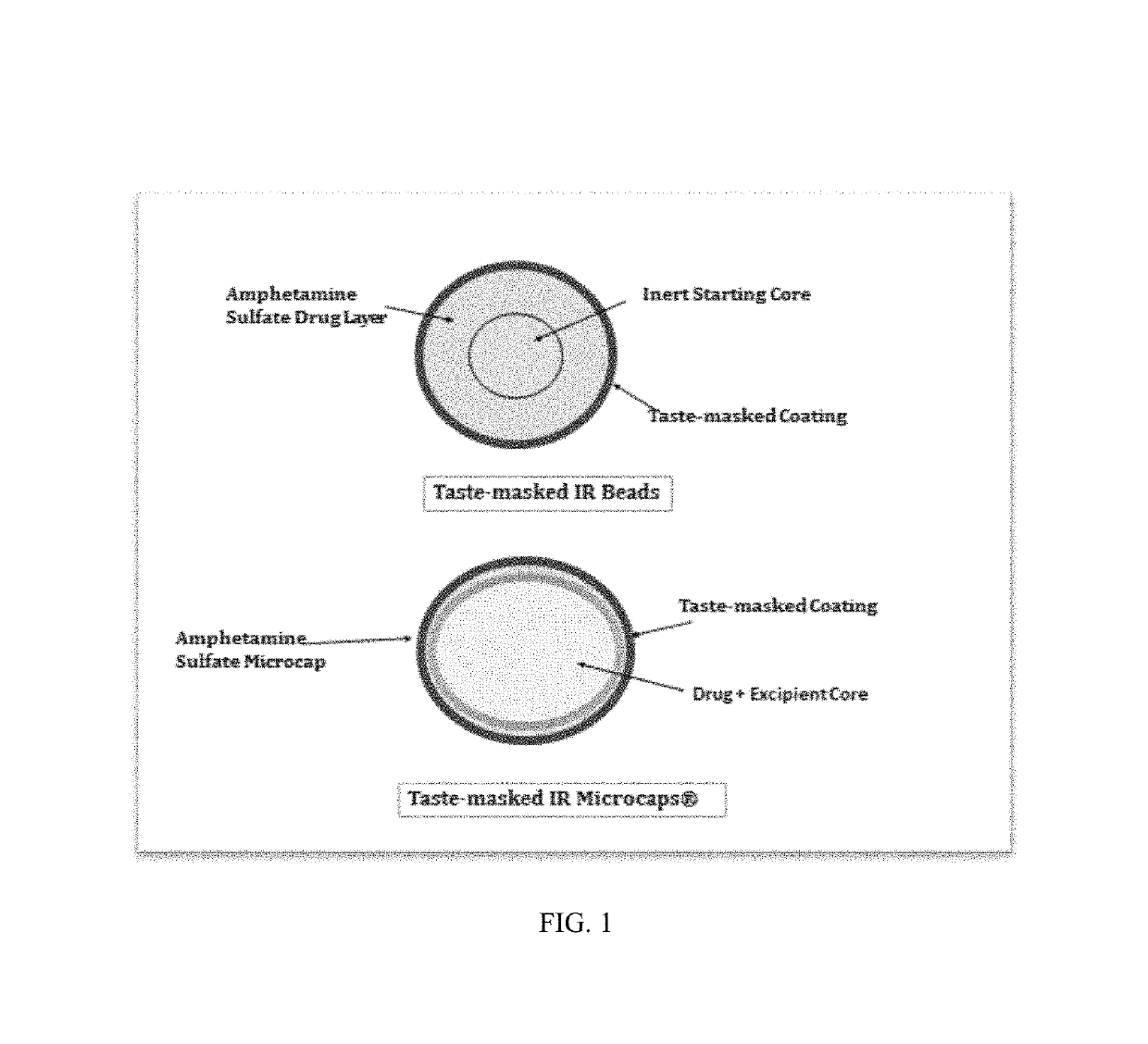
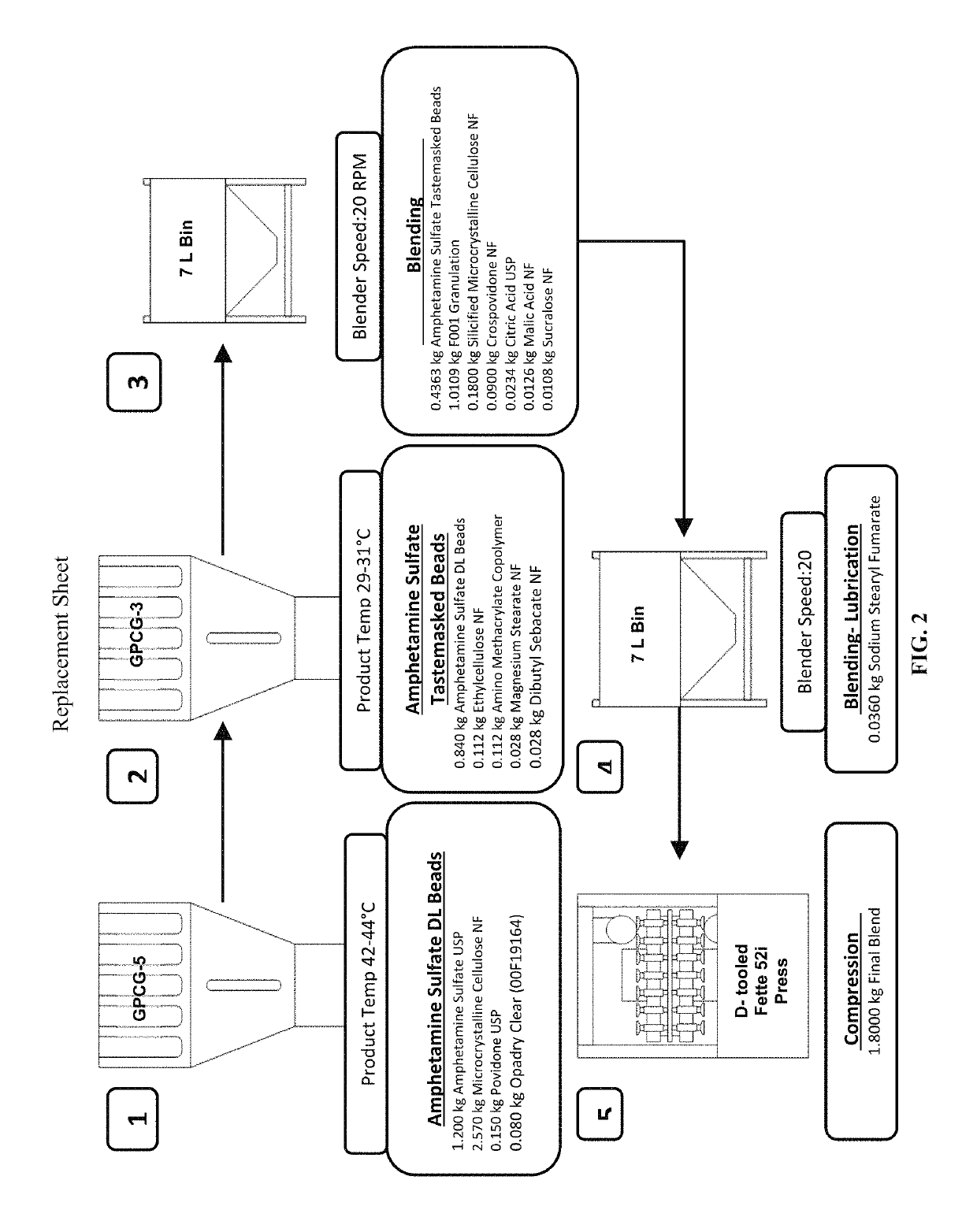
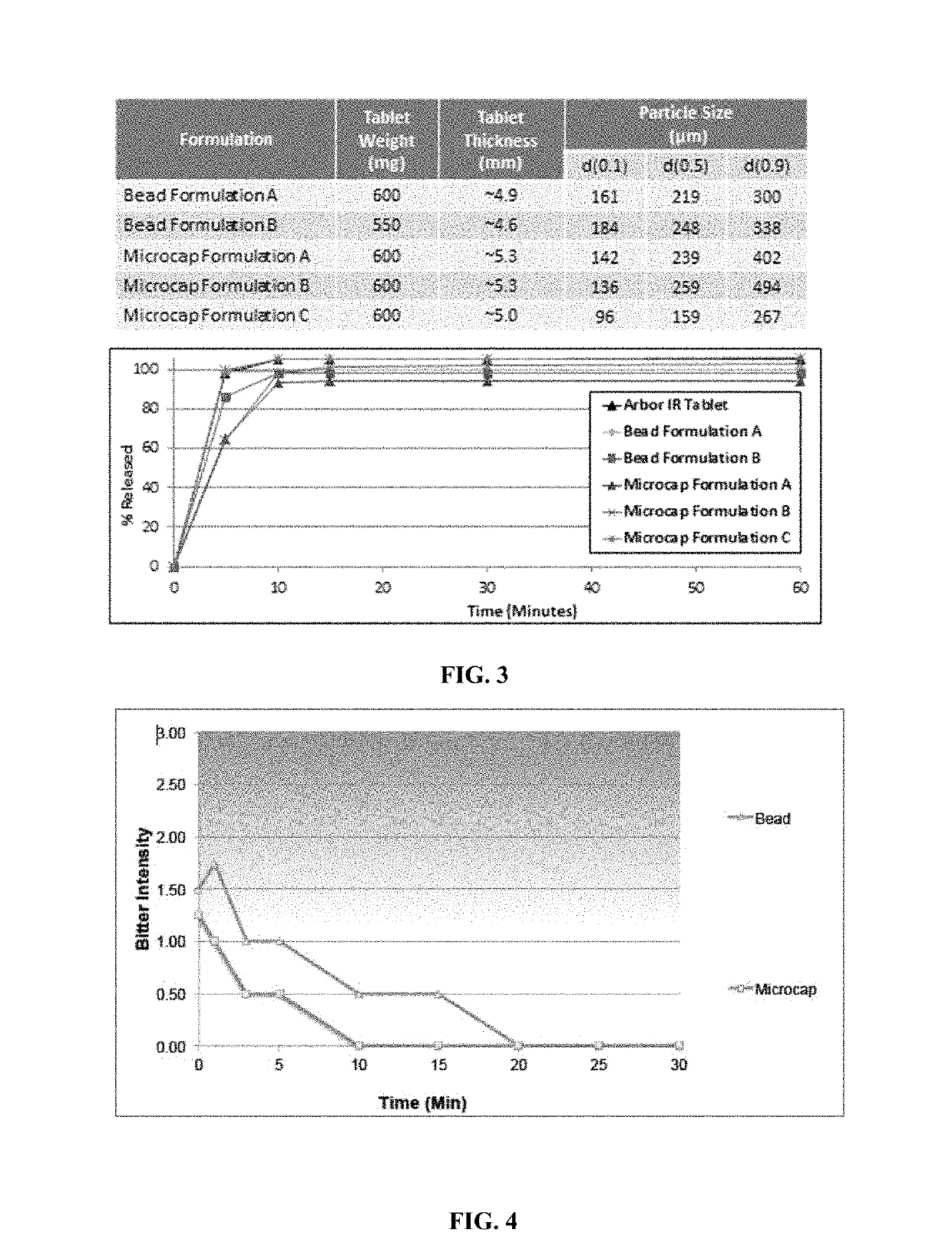
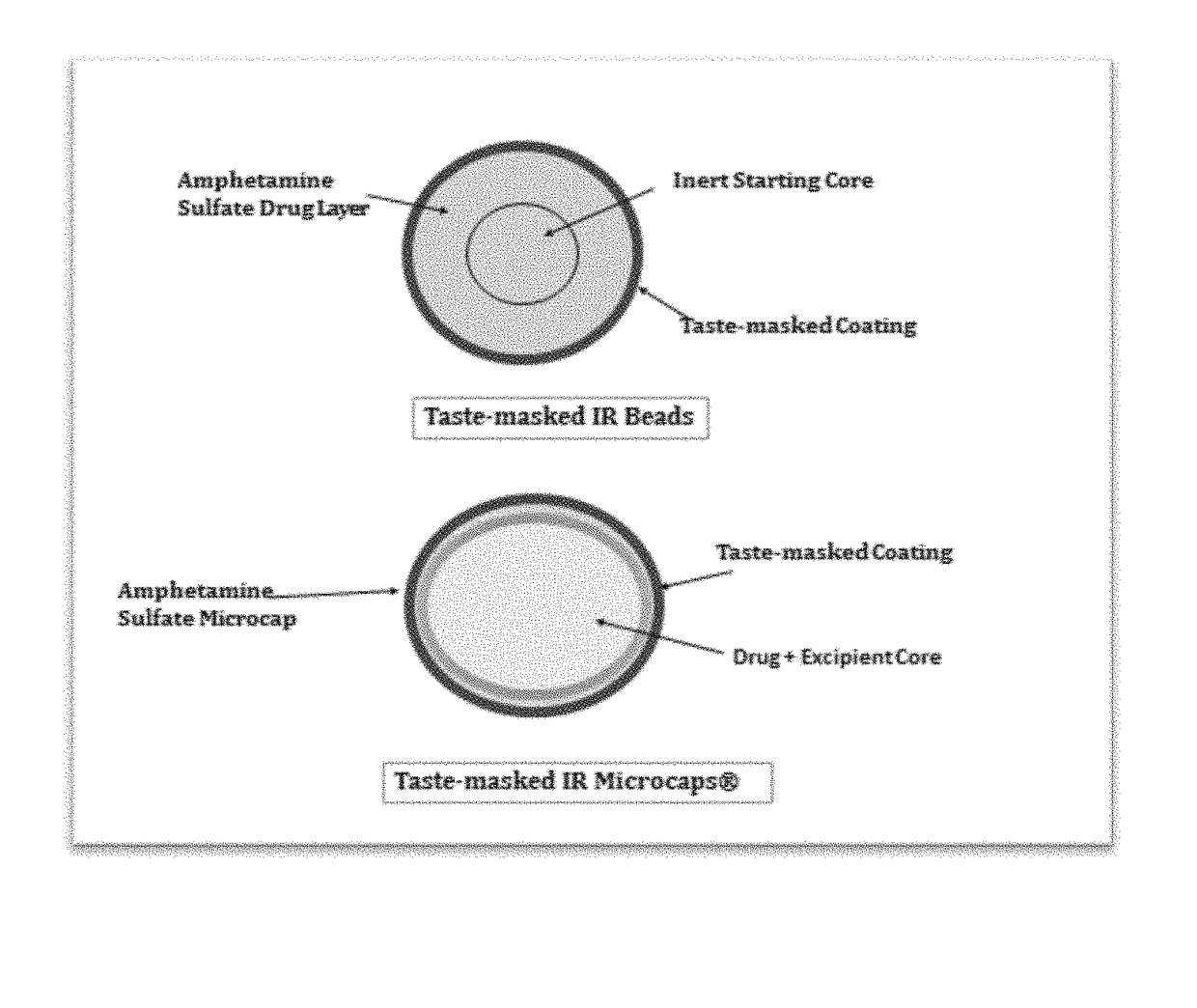
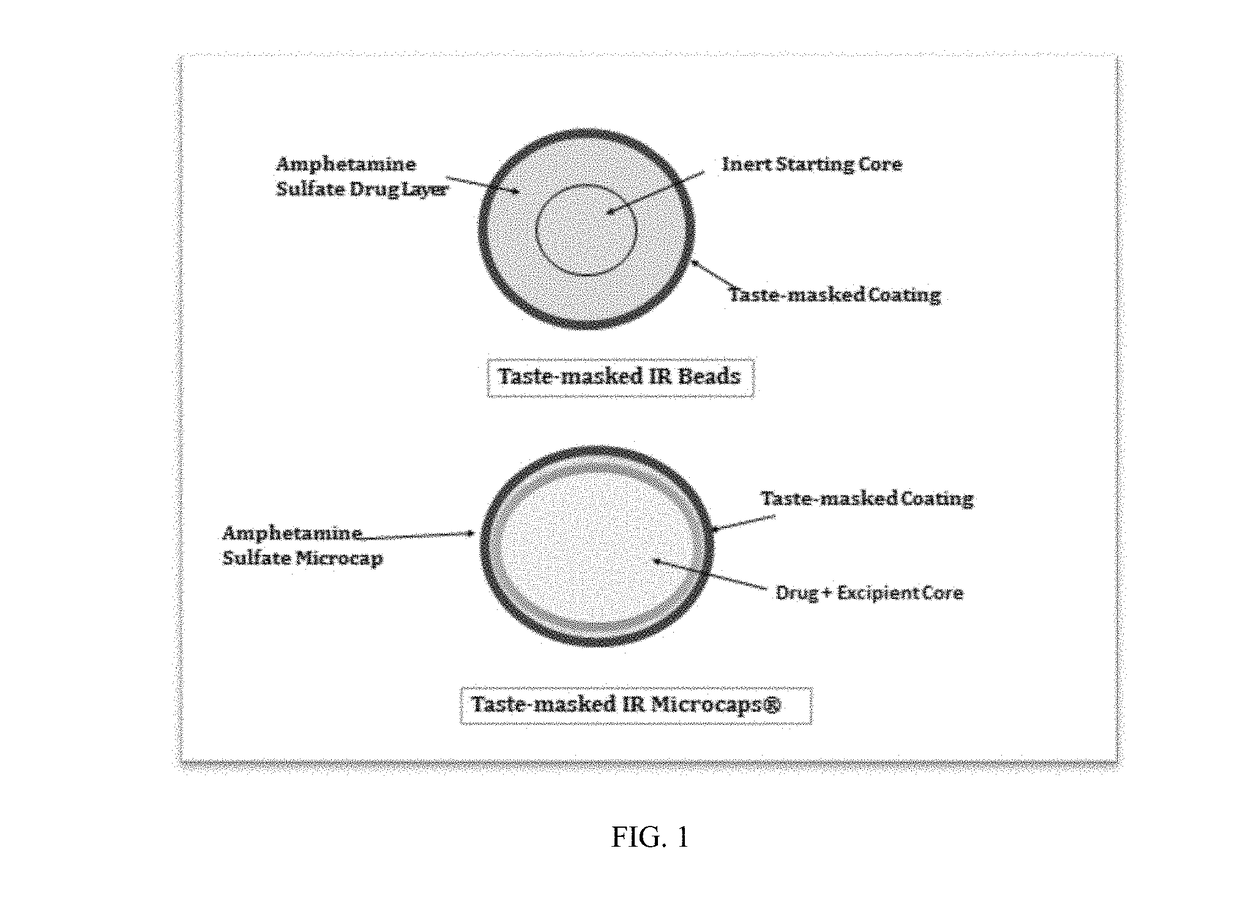
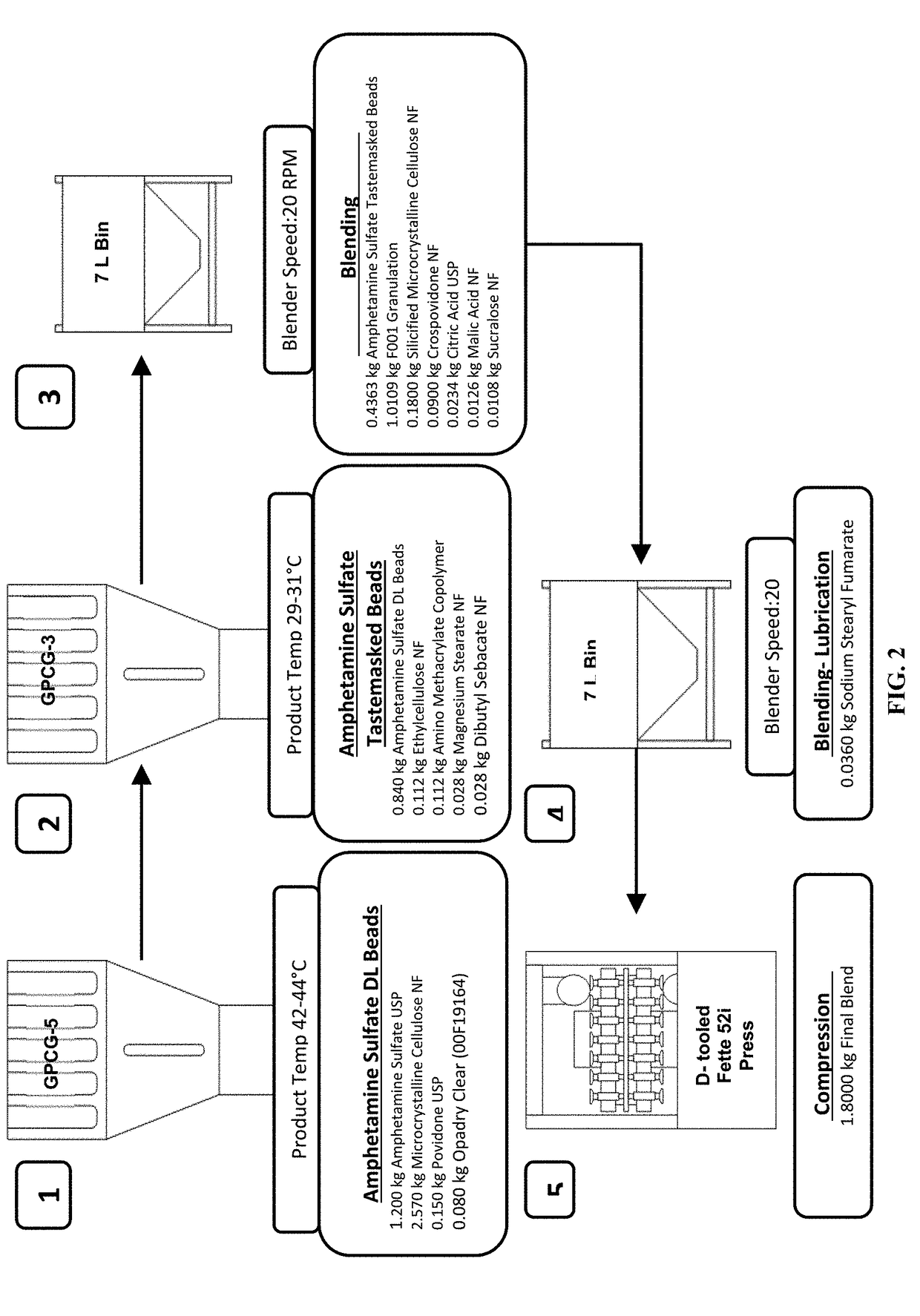
Oral amphetamine composition
ActiveUS10441554B2Oral administration is convenientRapid onsetOrganic active ingredientsPill deliveryAmphetamine SulfateWater soluble
In various embodiments, the present invention is directed to oral pharmaceutical compositions. For example, in some embodiments, the present invention is directed to taste-masked compositions. In some embodiments, the taste masked compositions comprise a highly water soluble drug such as amphetamine, e.g., in the form of a salt such as amphetamine sulfate. In various embodiments, the present invention is directed to taste-masked, orally disintegrating compositions.
Owner:ADARE PHARM INC
Remdesivir tablet and preparation method thereof
InactiveCN112675143AOral administration is convenientImprove securityOrganic active ingredientsAntiviralsOrganic acidBlood concentration
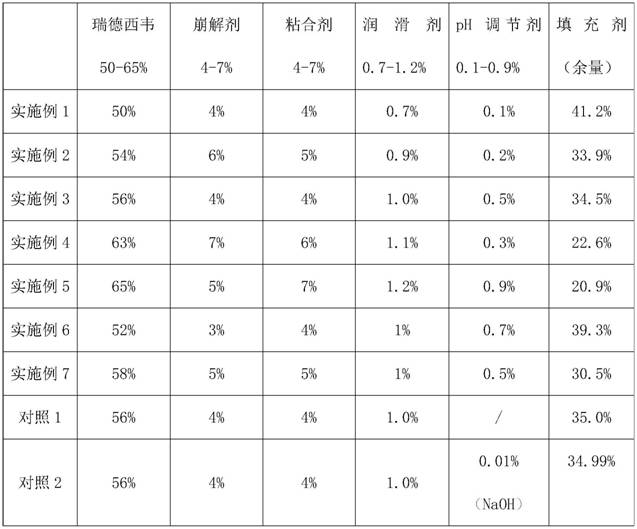
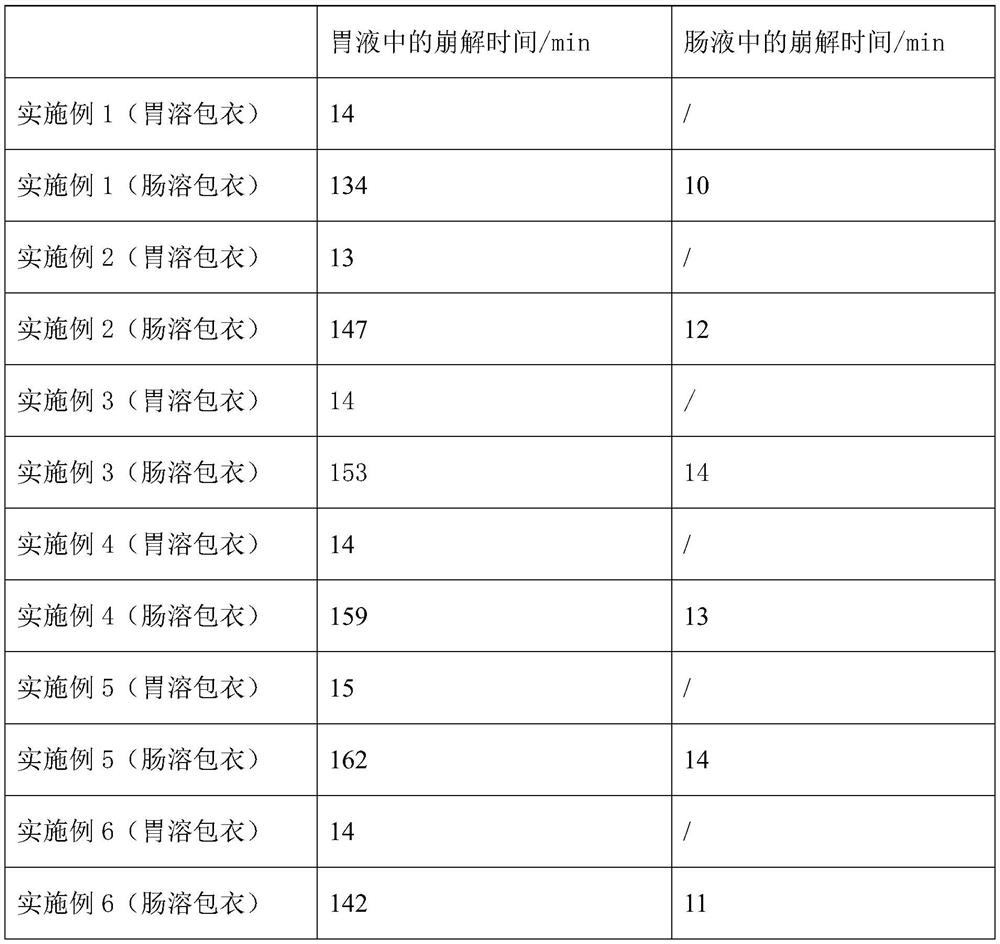
The invention discloses a Remdesivir tablet and a preparation method thereof. The tablet comprises a tablet core and a thin-film coating layer, wherein the tablet core comprises the following ingredients in parts by weight: 50-65% of Remdesivir, 4-7% of disintegrating agent, 4-7% of binding agent, 0.7-1.2% of lubricating agent, 0.1-0.9% of pH conditioning agent and the balance of filling agent, wherein the thin-film coating layer comprises the following ingredients: a coating material of a gastric-dissolved thin-film coating layer or the coating material of an enteric-coated thin-film coating layer, and the pH regulator is an organic acid type, a high-polymerization type or an alkalizer. The prepared Remdesivir tablet has good long-term stability and high blood concentration.
Owner:顾世海
Ultra porous hydrogel complex substance, preparing method and use in pharmaceutics thereof
InactiveCN1253147CEasy to manufactureRapid swellingPeptide/protein ingredientsPharmaceutical delivery mechanismFoaming agentCrosslinked polymers
The invention discloses a superporous hydrogel composite, its preparation method and its application in pharmacy. The superporous hydrogel composite of the present invention contains a crosslinked polymer and carbomer, and has a superporous structure. The polymer is formed by polymerization of at least one unsaturated ethylenic monomer and a polyene crosslinking agent. The superporous hydrogel composite of the present invention is prepared by the following steps: mixing at least one unsaturated ethylenic monomer, a polyene crosslinking agent, carbomer and a foaming agent; Formation of superporous hydrogel composites under the condition of bubbles. The superporous hydrogel complex of the present invention can be used in gastric floating preparations and protein polypeptide oral drug delivery systems. Compared with the prior art, it has bioadhesive properties and protease inhibition.
Owner:FUDAN UNIV
Traditional Chinese composition regulating intestine and stomach functions of infants and preparing method and preparation thereof
InactiveCN105477500AImprove spleen and stomach functionConvenience dietOrganic active ingredientsDigestive systemActive componentSemen
The invention discloses a traditional Chinese composition regulating intestine and stomach functions, which comprises following components in parts by mass: 5-50 parts of fructus alpiniae oxyphyllae, 2-20 parts of folium mori, 2-20 parts of Exocarpium Citri Rubrum, 10-160 parts of Chinese yam, 5-50 parts of semen coicis, 5-35 parts of phaseolus calcaratus, 5-50 parts of malt, 2-20 parts of endothelium corneum gigeriae galli, 3-30 parts of lophatherum gracile, 10-120 parts of poria cocos, 3-60 parts of white hyacinth bean, 3-36 parts of the root of kudzu vine, 1-15 parts of orange peel and 0.5-5 parts of fructo-oligose. Active components of the traditional Chinese composition have homology of medicine and food and are easily absorbed by infants; respective components are synergic in medicine effect, and can obviously promote infant spleen and stomach functions and promote body health.
Owner:郑乐雄
Self-assembling proliposome soft capsule and preparation method thereof
InactiveCN101780056AAvoid safety hazardsImprove complianceOrganic active ingredientsCapsule deliveryOral medicationPolyethylene glycol
The invention relates to the field of pharmaceutical preparations, in particular to a nimodipine self-assembling proliposome soft capsule and a preparation method thereof. The invention is characterized in that self-assembling proliposome liquid medicine which is composed by medicine, phospholipids, dispersion medium and polyethylene glycol modifier is wrapped by a soft capsule. The invention fills the self-assembling proliposome liquid medicine into the soft capsule, then self-assembling proliposome is completely wrapped by a capsule shell, and thereby the soft capsule not only can play the role of isolating oxygen, moisture and the like, but also can cover up bad taste and bring convenience to oral administration. After being disintegrated by gastric juice, the soft capsule can quickly hydrate and self-assemble to form liposome with high entrapment rate and uniform particle size.
Owner:CHINA PHARM UNIV
Butyl phthalide medicinal composite, preparation method and sustained-release preparation thereof
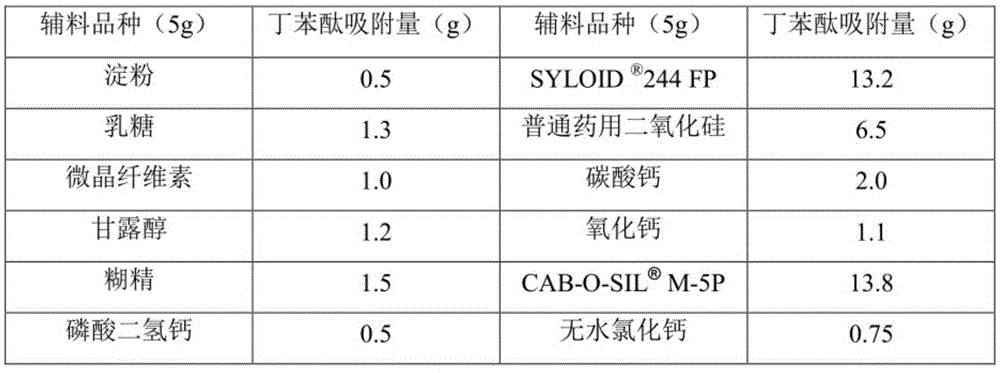
ActiveCN105380908AImprove stabilityOral administration is convenientPowder deliveryOrganic active ingredientsActive componentSilicon dioxide
The invention discloses a butyl phthalide medicinal composite, which comprises butyl phthalide and silicon dioxide according to a mass ratio of 0.1-4:1. The preparation method comprises the following steps: weighing butyl phthalide and silicon dioxide, spraying butyl phthalide into silicon dioxide powder, and evenly mixing to obtain the powdery butyl phthalide medicinal composite. At the same time, the invention also discloses a sustained-release preparation of the butyl phthalide medicinal composite. The sustained-release preparation is characterized in that the butyl phthalide medicinal composite is taken as the active component, which is wrapped by one or more pharmaceutically-acceptable sustained-release coating materials. The preparation method is simple, the preparation time is short, the cost is low, and the preparation method is economic. The prepared medicinal composite has the advantages that the drug volatilization is effectively controlled, the stability is high, the drug loading capacity is large, the volume is small, and the dosage form is convenient for oral taking. The shortages of conventional commercial oral butyl phthalide preparations in clinic are overcome.
Owner:HEBEI UNIVERSITY
Pharmaceutical compositions of lenvatinib
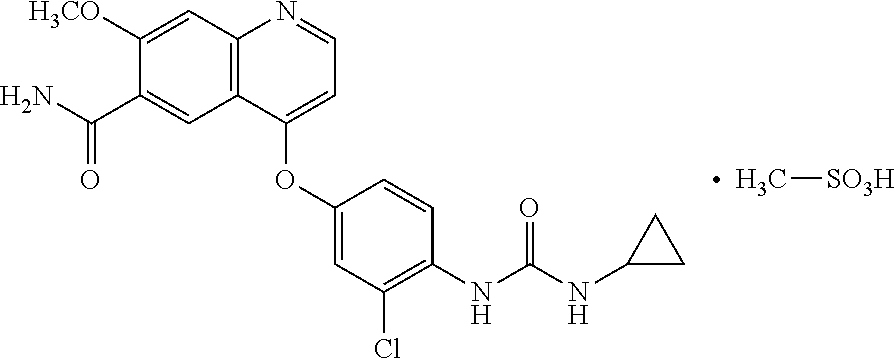
ActiveUS20190275026A1Facilitates oral administrationOral administration is convenientInorganic non-active ingredientsGranular deliveryDrugChemistry
The present invention relates to a pharmaceutical composition comprising lenvatinib mesylate and a stabilizer in an amount of about 10% to about 20% based on the total weight of the composition, wherein the stabilizer is selected from the group consisting of calcium hydroxide and potassium hydroxide; and its process for preparation thereof.
Owner:SHILPA MEDICARE LTD
Method for producing pancreatin pellets
ActiveUS8691282B2Improve propertiesImproved and economical processPowder deliveryPeptide/protein ingredientsMaterials scienceResidual moisture
In order to avoid compromising the pharmacological effect of pancreatin caused by the addition of auxiliary materials or binding agents, a pancreatin pellet having a 100% pancreatin content consists exclusively of pancreatin with a residual moisture content of less than 3% by weight, preferably less than 1% by weight or less than 0.5% by weight.
Owner:NORDMARK PHARMA GMBH
Pharmaceutical compositions of lenvatinib
ActiveUS10583133B2Oral administration is convenientInorganic non-active ingredientsGranular deliveryCalcium hydroxideLenvatinib Mesylate
The present invention relates to a pharmaceutical composition comprising lenvatinib mesylate and a stabilizer in an amount of about 10% to about 20% based on the total weight of the composition, wherein the stabilizer is selected from the group consisting of calcium hydroxide and potassium hydroxide; and its process for preparation thereof.
Owner:SHILPA MEDICARE LTD
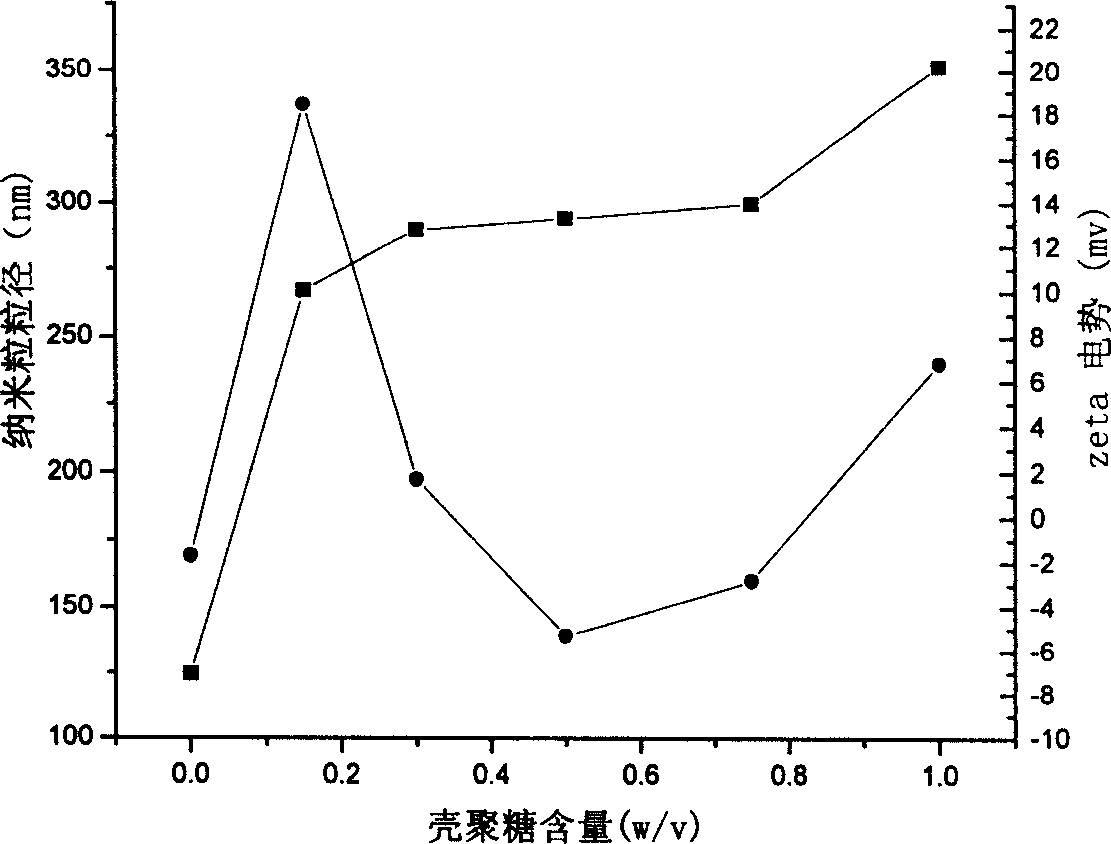
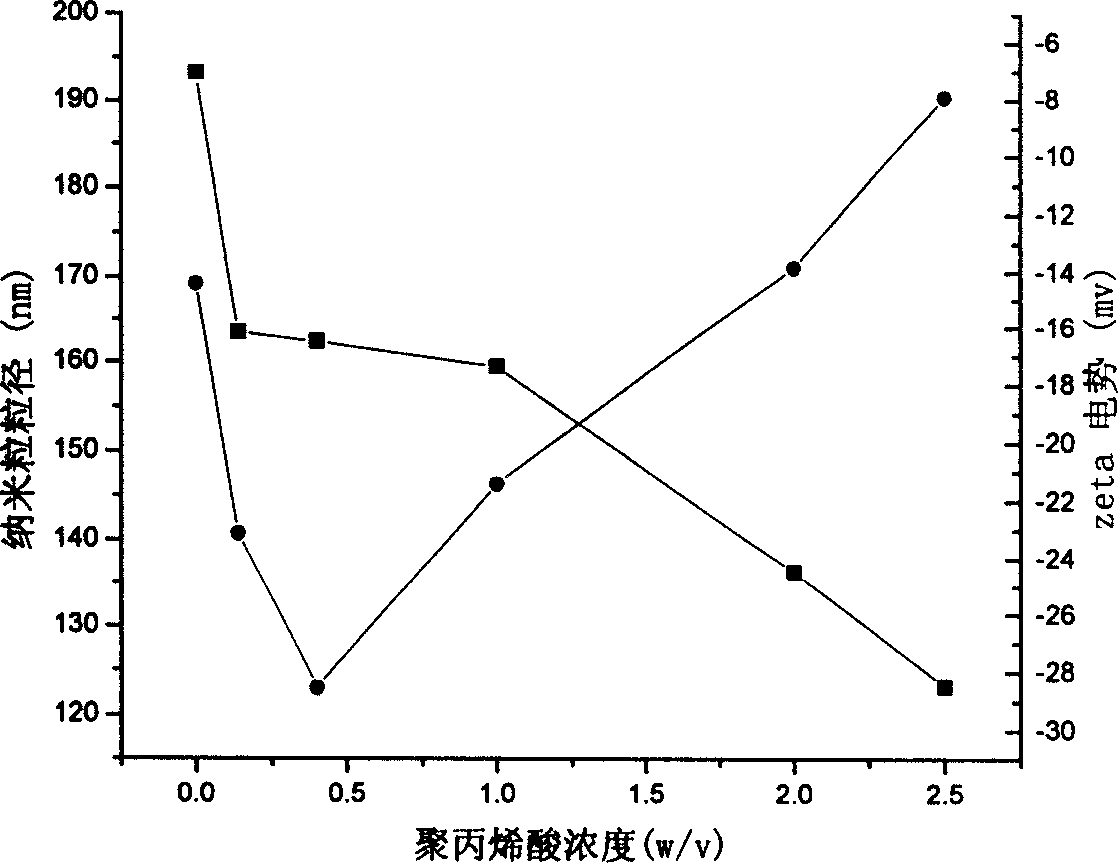
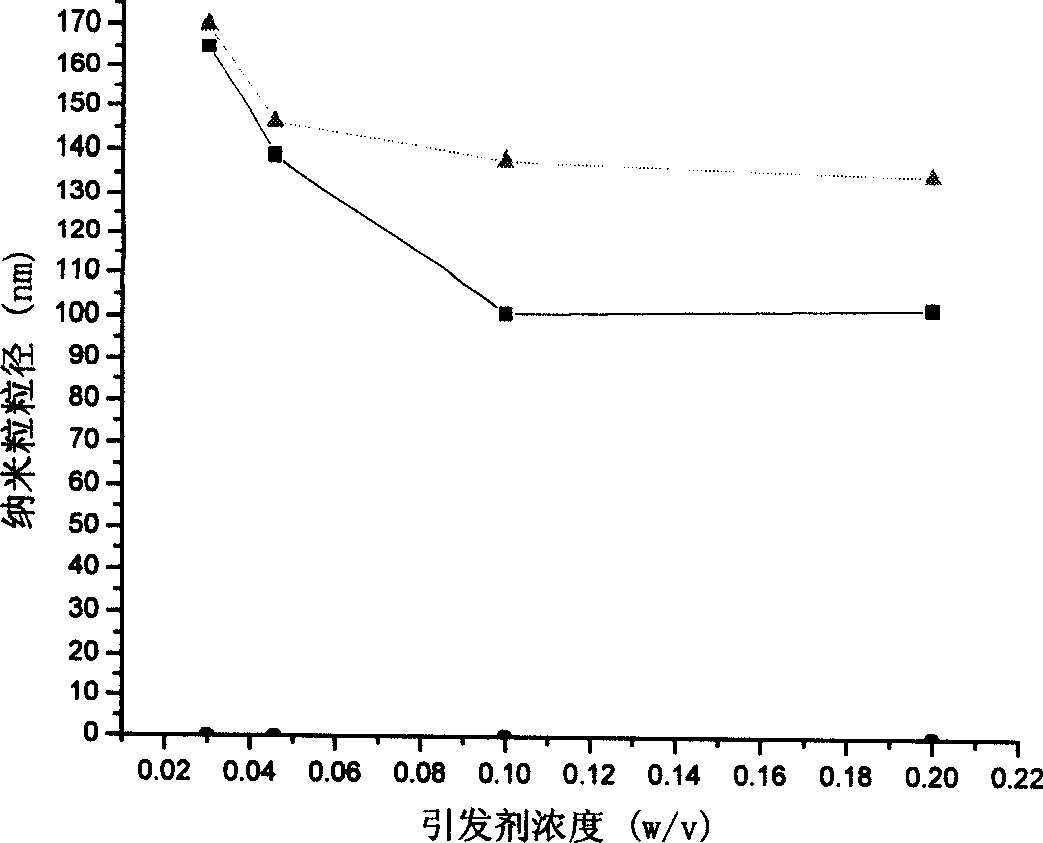
Nano granules adhesive to mucous membrane, preparation method and application
InactiveCN1760223AUniform particle sizeEvenly dispersedCosmetic preparationsBiocidePolymer dissolutionChemistry
A mucosa adhesive nanoparticle for medicine, food and cosmetics is prepared from skeleton (alkyl polyacrylate, alkyl polymethylacrylate, or polycyanoacrylate) and coating (mucosa adhesive polymer) through dissolving the coating, adding skeleton and trigger, heating while reaction, continuous reaction and purifying.
Owner:FUDAN UNIV
Metal organic framework nanoparticles for oral protein administration and preparation method of metal organic framework nanoparticles
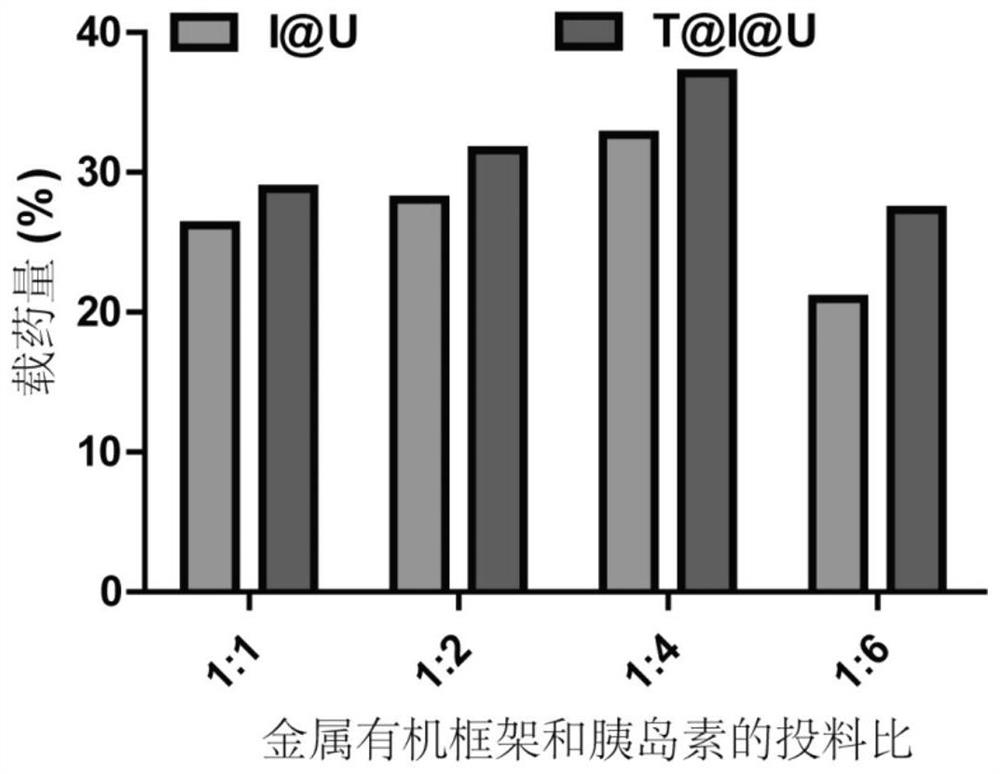
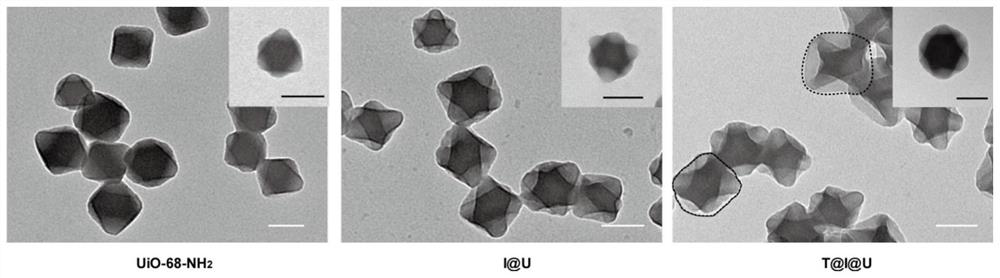
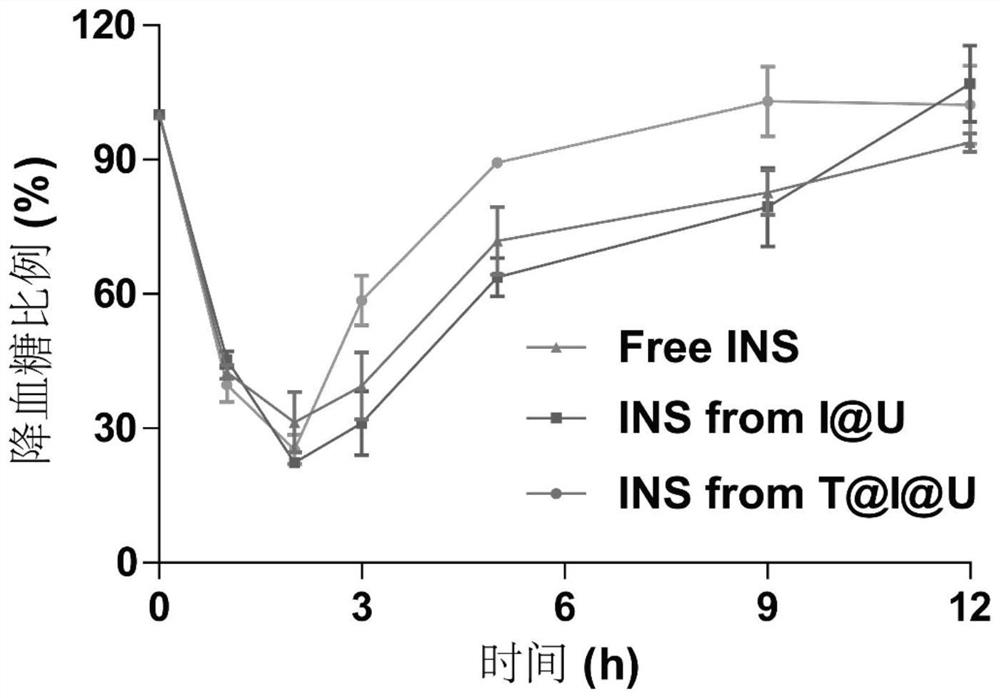
PendingCN114344484AImprove drug loading capacityStrong protectionPeptide/protein ingredientsPharmaceutical delivery mechanismProtein proteinMolecular biology
The invention discloses metal organic framework nanoparticles for oral protein administration and a preparation method of the metal organic framework nanoparticles, and belongs to the technical field of pharmaceutics. The metal organic framework nanoparticles provided by the invention can promote oral absorption of protein / polypeptide with the molecular weight of at most 10000 Daltons. The metal organic framework nano-particle is prepared by loading a nano-scale acid-resistant metal organic framework with a small molecule protein / polypeptide drug through hydrophobic interaction and modifying a targeting molecule on the surface of the nano-scale acid-resistant metal organic framework. The porous acid-resistant metal organic framework shows high drug loading capacity and strong protectiveness to protein, and shows good biocompatibility and sustained and controlled release kinetics in vivo. The targeting molecule can target a receptor on an intestinal epithelial cell membrane, the problem that the permeation efficiency of protein in an intestinal epithelial cell layer is extremely low is solved, and the oral bioavailability of the protein is improved. The preparation method is simple and convenient, the production cost is low, and painless, controlled-release and convenient protein oral administration is expected to be realized.
Owner:WUHAN UNIV
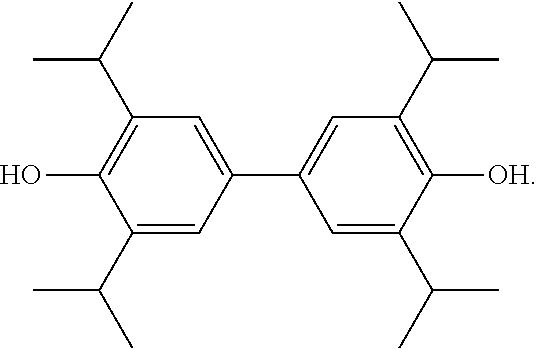
2,2',6,6'-tetraisopropyl-4,4'-2-biphenol soft capsule and method for preparing same
Disclosed is a 2,2′,6,6′-tetraisopropyl-4,4′-biphenol soft capsule composed of a capsule shell and the contents in the capsule, wherein the contents in the capsule include 2,2′,6,6′-tetraisopropyl-4,4′-biphenol, a solvent, and an antioxidant, among others.
Owner:XIAN LIBANG PHARMA
2,2′,6,6′-tetraisopropyl-4,4′-2-biphenol soft capsule and method for preparing same
ActiveUS9693963B2Low water solubilityOral administration is convenientNervous disorderHydroxy compound active ingredientsAntioxidantSolvent
Disclosed is a 2,2′,6,6′-tetraisopropyl-4,4′-biphenol soft capsule composed of a capsule shell and the contents in the capsule, wherein the contents in the capsule include 2,2′,6,6′-tetraisopropyl-4,4′-biphenol, a solvent, and an antioxidant, among others.
Owner:XIAN LIBANG PHARMA
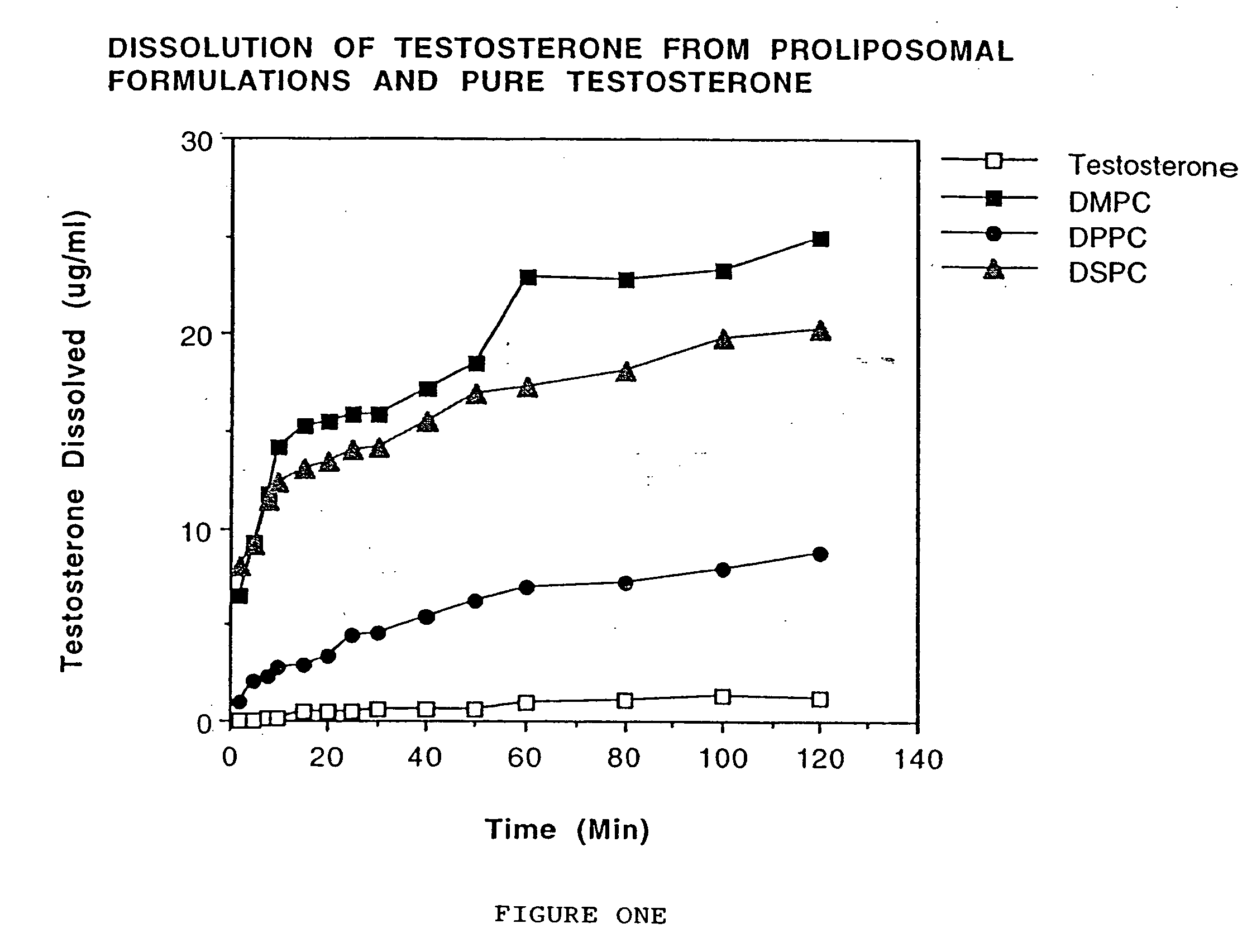
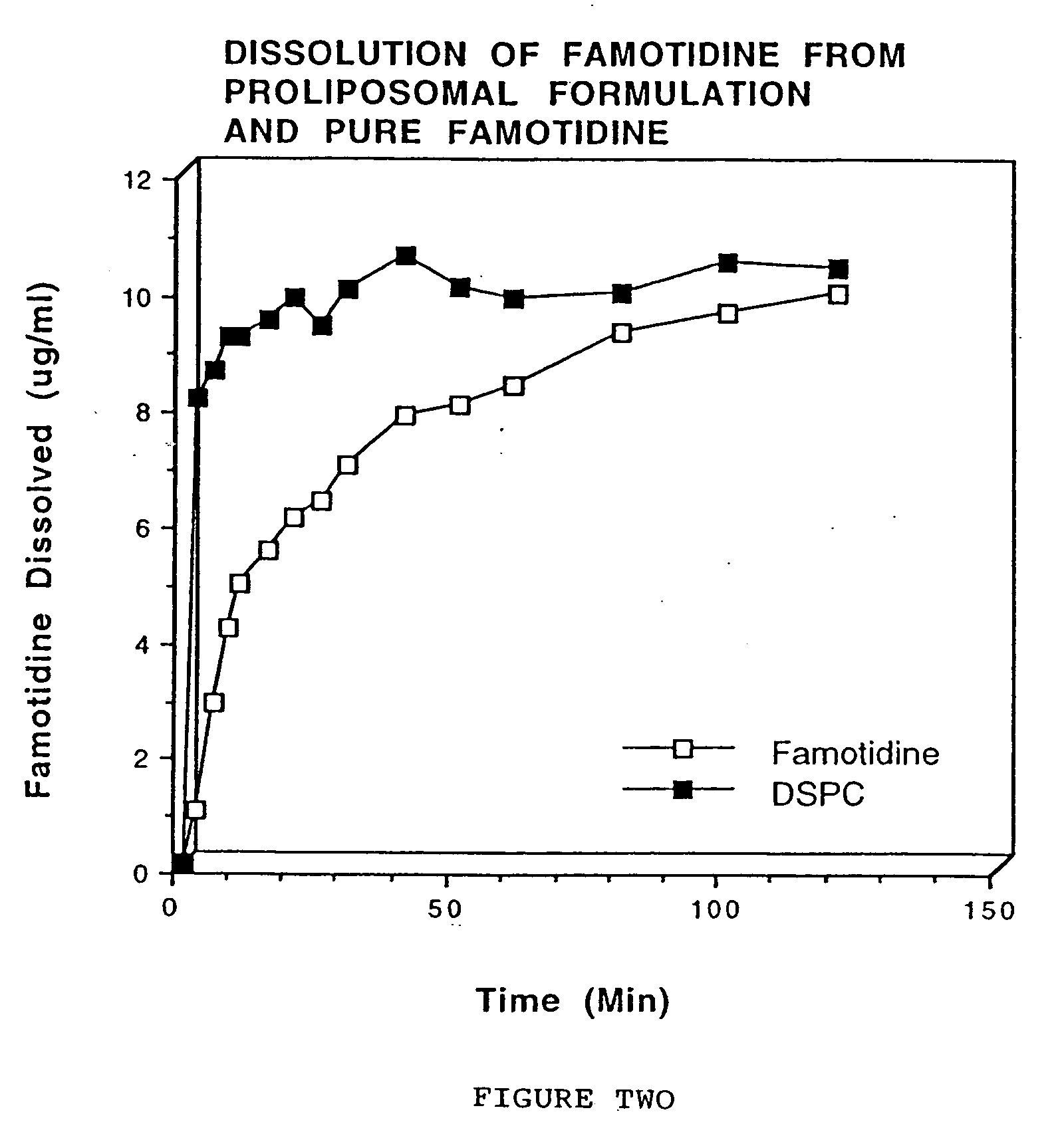
Enteric-coated proliposomal formulations for poorly water soluble drugs
InactiveUS20050031687A1Enhances stability and bioavailabilitySimple and inexpensiveOrganic active ingredientsAnthropod material medical ingredientsWater solublePhospholipid
This invention relates to enteric-coated proliposomal formulations for oral medicaments. In particular, it relates to an enteric-coated proliposomal oral drug delivery system for poorly water soluble drugs and methods for making the same. The drug delivery system comprises a pharmaceutical agent, a phospholipid and an enteric coating material. The present invention provides enhanced stability and bioavailability for orally administered drugs.
Owner:BETAGERI GURU V
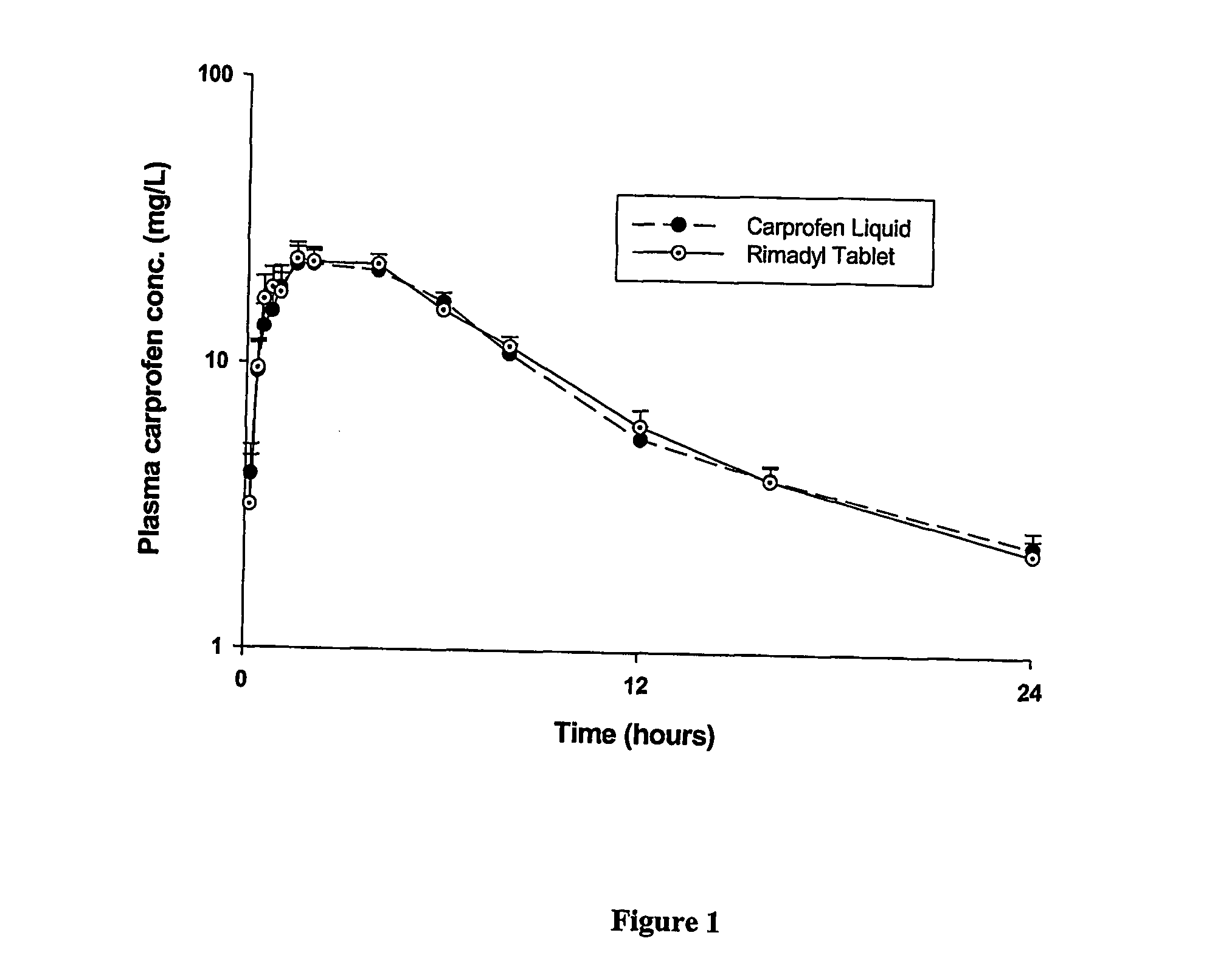
Stable carprofen composition
InactiveUS20070042006A1Suitable for oralOral administration is convenientBiocideDispersion deliveryCo solventWarm-blooded
A stable solvent-based composition is described which is particularly useful in warm blooded animals such as dogs. The composition comprises a therapeutically effective amount of carprofen, one or more polyols, one or more stabilising agents and optionally, one or more co-solvents.
Owner:JUROX
Oral amphetamine composition
ActiveUS20180311187A1Oral administration is convenientRapid onsetOrganic active ingredientsPharmaceutical non-active ingredientsAmphetamine SulfateMedicine
In various embodiments, the present invention is directed to oral pharmaceutical compositions. For example, in some embodiments, the present invention is directed to taste-masked compositions. In some embodiments, the taste masked compositions comprise a highly water soluble drug such as amphetamine, e.g., in the form of a salt such as amphetamine sulfate. In various embodiments, the present invention is directed to taste-masked, orally disintegrating compositions.
Owner:ADARE PHARM INC
Synthetic micromolecule compound capable of conveying bioactive substances and application thereof
ActiveCN101962319AImprove stabilityImprove absorption rateOrganic chemistryPharmaceutical non-active ingredientsTetrazoleMedicinal chemistry
The invention relates to a synthetic micromolecule compound capable of conveying bioactive substances and application thereof. The general formula of the micromolecule compound or any medicinal salt thereof is disclosed in the specification, wherein R4 is an aromatic ring of C6-C10 or a heteroaromatic ring of C2-C9; R5 is a -(CH2)n-, a naphthenic group of C3-C5, an aromatic ring of -(CH2)n-C6-C10or a heteroaromatic ring of C2-C9; and R6 is a carboxyl group (COOH), -CONHOH, tetrazole, OH and -SO3H.
Owner:SANSURE (SHANGHAI) GENE TECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com