Nano granules adhesive to mucous membrane, preparation method and application
A nanoparticle and adhesive technology, applied in the fields of botanical equipment and methods, applications, chemical instruments and methods, etc., can solve the problems that cannot improve the oral bioavailability of protein and polypeptide drugs, inactivation of protein and polypeptide drugs, and inactivation of nanoparticles. Stability and other issues, to achieve the effect of convenient preparation, uniform particle size, and increased penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: N, N, the synthesis of N-trimethyl chitosan quaternary ammonium salt
[0052] Disperse 2g of chitosan (30,000, 100,000-300,000, greater than 300,000) in 50ml of dimethyl sulfoxide in an eggplant-shaped bottle, soak and stir at room temperature for 24 hours, then add 4.8g of potassium iodide, 2g of sodium hydroxide and A solution made of 6ml of double distilled water was stirred slowly at 36°C for 16h. Then use four times the volume of the mixed solution of absolute ethanol and absolute ether to precipitate the crude product of the reaction, i.e. iodide N, N, N-trimethyl chitosan quaternary ammonium salt. Due to the poor stability of the iodized salt, it was dissolved in water, converted into N, N, N-trimethyl chitosan quaternary ammonium chloride by ion exchange, and then precipitated with absolute ethanol and anhydrous ether, and then After filtration and vacuum drying, the pure product was obtained, which was used for the following synthesis of mucoadhe...
Embodiment 2
[0053] Embodiment 2: chitosan coated nanoparticles
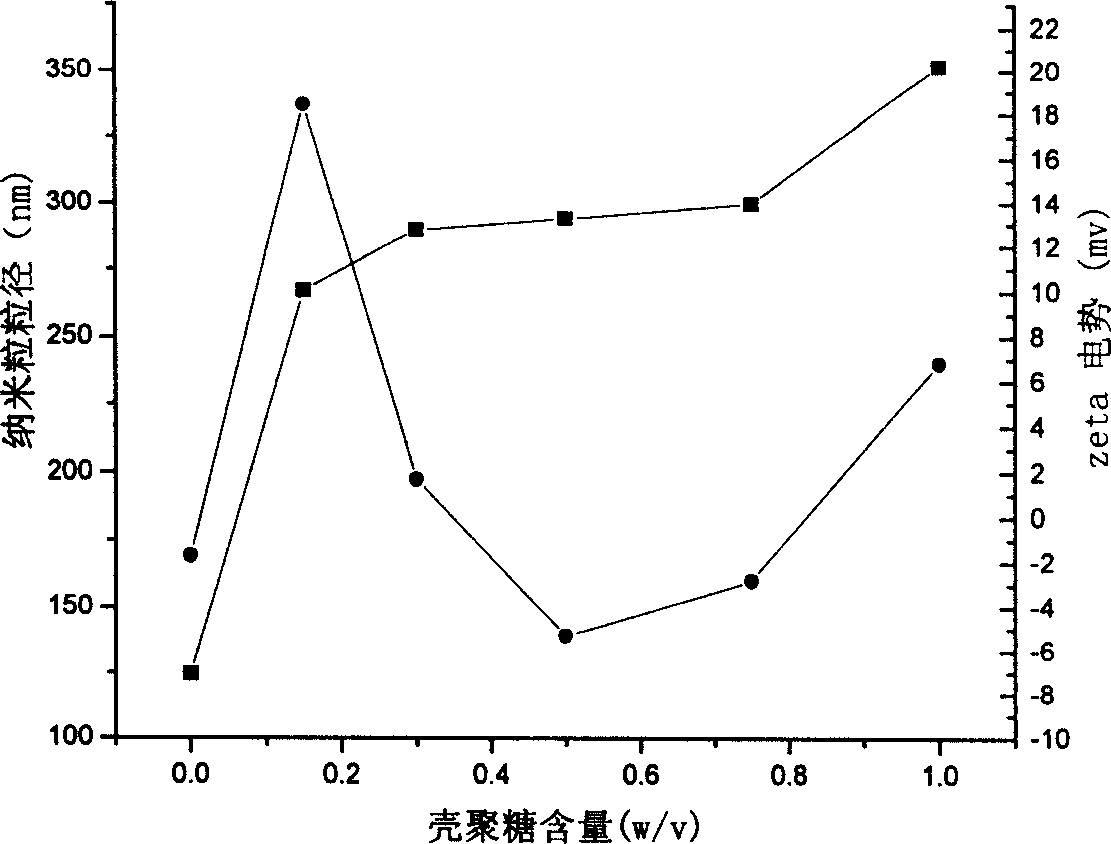
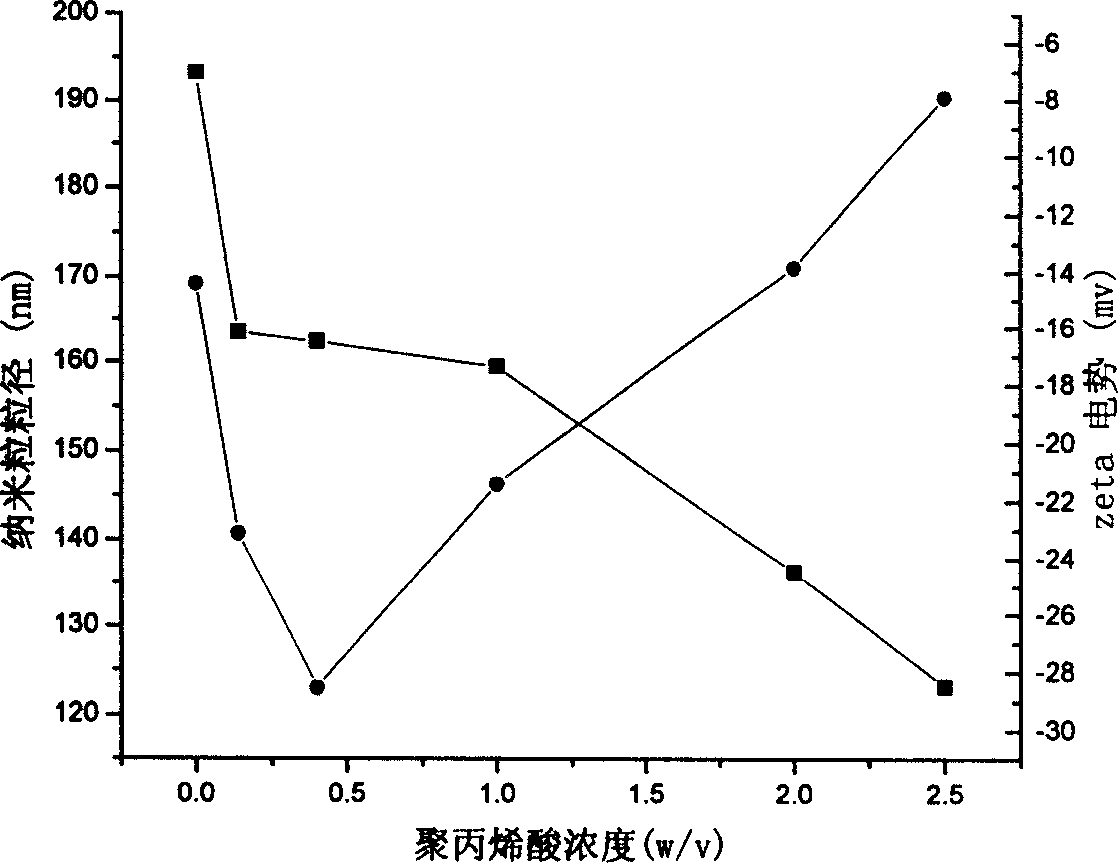
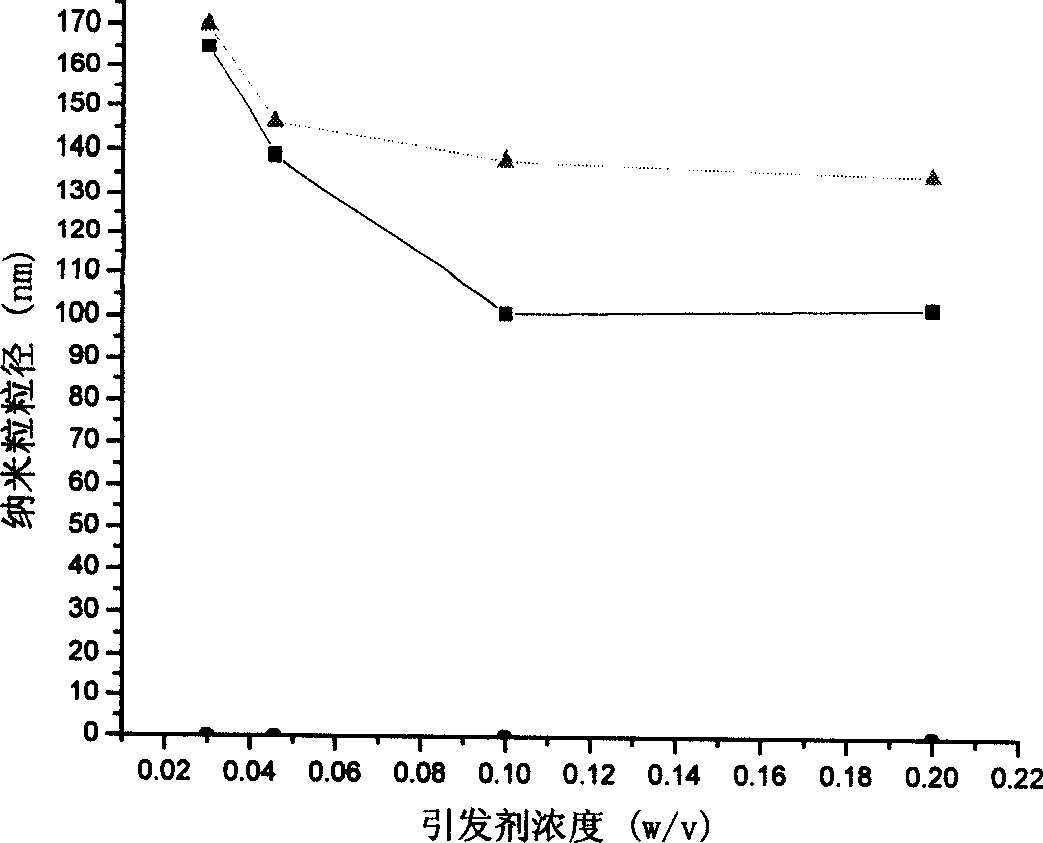
[0054] Weigh 1g of chitosan (greater than 300,000), place it in an Erlenmeyer flask, add 98ml of 1% acetic acid solution, stir, heat to 25°C, dissolve, then add 1.6ml of methyl methacrylate to it, after 20min Ammonium persulfate was added, the temperature rose to 75°C, and the reaction was continued for 12 hours. The resulting suspension was dialyzed against a semipermeable membrane with a molecular size of 12,000 Daltons. After the suspension was diluted 100 times with water, the particle size and Zeta potential under different conditions were measured with a particle size analyzer (Nicomp 380 / ZLS, Santa Barbara, Calif., USA), see accompanying drawings 1 and 3.
Embodiment 3
[0055] Embodiment 3: chitosan coated nanoparticles
[0056] Weigh 0.5g of chitosan (greater than 300,000), place it in a Erlenmeyer flask, add 98ml of 1% acetic acid solution, stir, heat to 40°C, dissolve, then add 1.1ml of methyl methacrylate to it for 10min Then ammonium persulfate was added, the temperature rose to 80°C, and the reaction was continued for 14 hours. The resulting suspension was dialyzed against a semipermeable membrane with a molecular size of 12,000 Daltons. After the suspension was diluted 100 times with water, the particle size and Zeta potential under different conditions were measured with a particle size analyzer (Nicomp 380 / ZLS, Santa Barbara, Calif., USA), see accompanying drawings 1 and 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com