Self-assembling proliposome soft capsule and preparation method thereof
A proliposome and self-assembly technology, which is applied in capsule delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of patients' blood vessel stimulation, inconvenient clinical application, poor stability, etc., achieve low cost and solve complex production process , the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The composition of the prescription is as follows: Nimodipine 8g
[0042] Egg phospholipids 200g
[0043] Macrogol 400 220g
[0044] Poloxamer 188 (trade name F-68) 80g
[0045] Cholesterol 16g
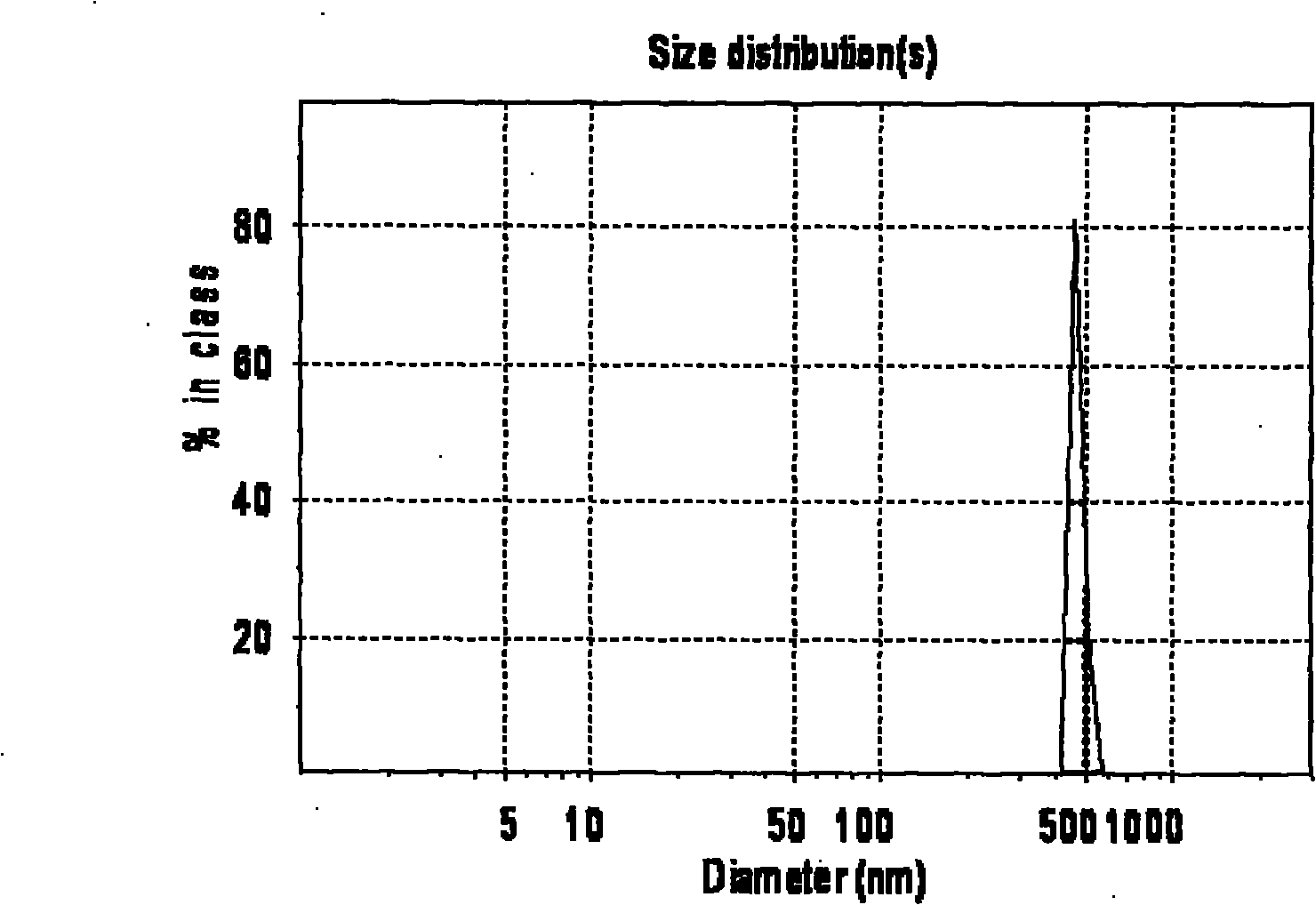
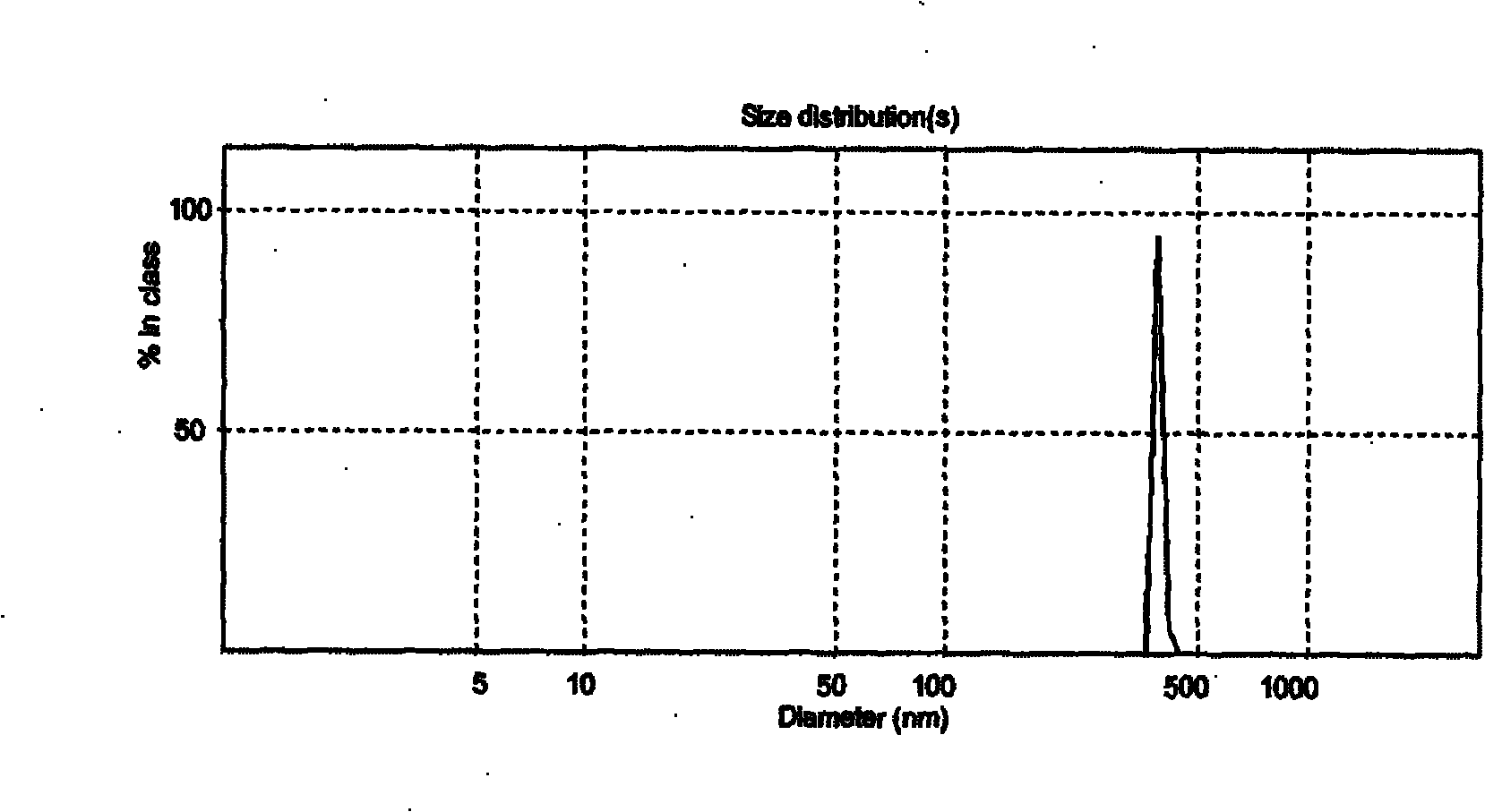
[0046] A total of 1000 soft capsules were made, and the average particle diameters of liposomes formed after disintegration and hydration in artificial gastric juice and water were 468.2nm and 447.8nm respectively; the encapsulation efficiency was 81.77%
[0047] Preparation process: Put the prescribed amount of drugs, phospholipids, and PEG into a vial, add an appropriate amount of absolute ethanol, and dissolve it by ultrasonication, then add cholesterol and poloxamer, and dissolve it by ultrasonication, and then prepare a self-assembled proliposome. Quantitative filling is then filled in the soft capsule, and the nimodipine self-assembled proliposome soft capsule is formed.
Embodiment 2
[0049] The composition of the prescription is as follows: Nimodipine 8g
[0050] Soy Lecithin 180g
[0051] Macrogol 400 200g
[0052] Polyoxyethylene castor oil 80g
[0053] Sodium deoxycholate 8g
[0054] A total of 1000 soft capsules were made, and the average particle diameters of the liposomes formed after disintegration and hydration in artificial gastric juice and water were 516.5nm and 503.4nm respectively; the encapsulation efficiency was 80.2%
[0055] Preparation method: with embodiment 1.
Embodiment 3
[0057] The composition of the prescription is as follows: Nimodipine 6g
[0058] Hydrogenated Phospholipids 100g
[0059] Macrogol 600 200g
[0060] Tween 80 150g
[0061] Sodium deoxycholate 10g
[0062] Anhydrous ethanol amount
[0063] A total of 1,000 soft capsules were made, and the average particle diameters of liposomes formed after disintegration and hydration in artificial gastric juice and water were 537.3nm and 521.8nm respectively; the encapsulation efficiency was 80.43%
[0064] Preparation method: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com