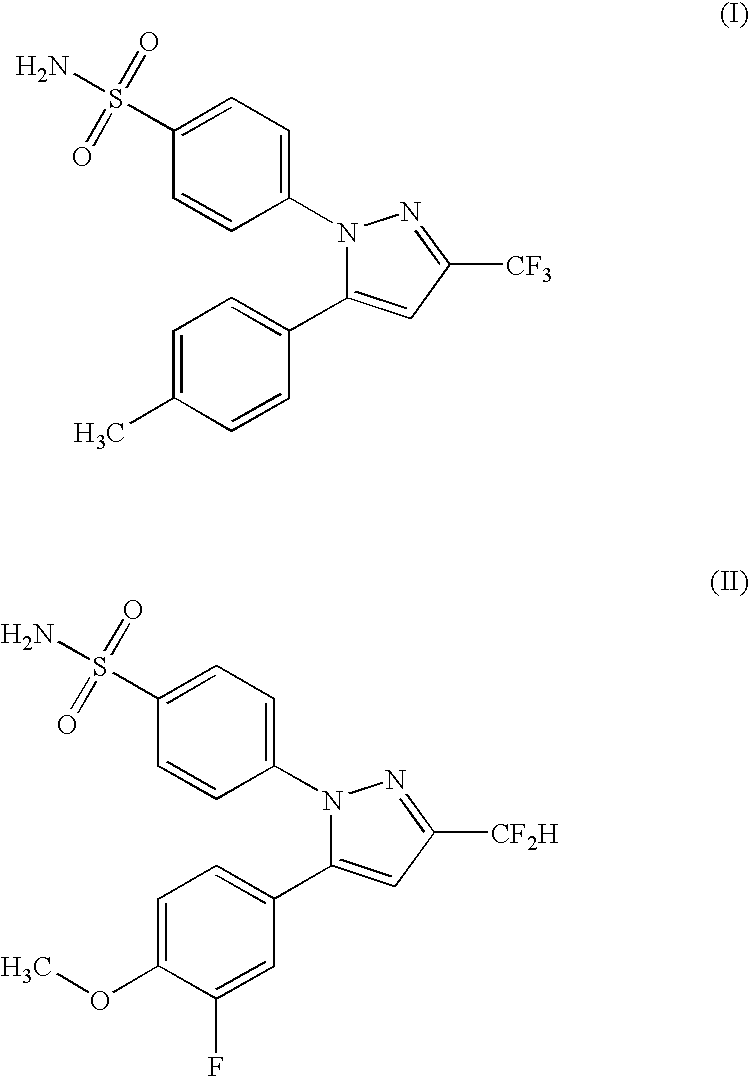
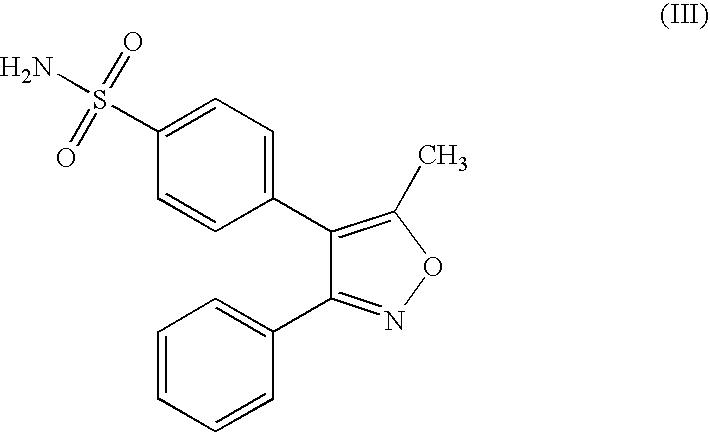
Stabilized oral pharmaceutical composition
a pharmaceutical composition and stability technology, applied in the field of orally deliverable pharmaceutical compositions, can solve the problems of limiting factors such as the size of the capsule or solution required to provide a therapeutic dose, the size of the solution is a practical problem, and the dose requirement is relatively high, so as to achieve infinite dose flexibility, ease of swallowing and dose flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0192] Six celecoxib solution formulations SF-1 to SF-6 were prepared having components as shown in Table 1. In each case the solvent liquid consisted of PEG-400, either alone (SF-1) or together with at least one free radical-scavenging antioxidant (SF-2 to SF-6). Celecoxib was present in solution at a concentration of 50 mg / g in all formulations. Antioxidant amounts are shown as % weight / weight.
TABLE 1Composition of celecoxib solution formulations SF-1 to SF-6FormulationComponentsSF-1Celecoxib, PEG-400SF-2Celecoxib, PEG-400, 0.1% vitamin ESF-3Celecoxib, PEG-400, 0.1% BHASF-4Celecoxib, PEG-400, 0.1% BHTSF-5Celecoxib, PEG-400, 0.1% propyl gallateSF-6Celecoxib, PEG-400, 0.05% BHA, 0.05% BHT
example 2
[0193] A gradient HPLC assay was used to determine impurities in celecoxib solution formulations SF-1 to SF-6 of Example 1 after storage at various temperatures for different periods of time. Solution formulation samples were drawn and were dissolved in methanol to obtain a celecoxib concentration of about 0.4 to about 0.5 mg / ml prior to injection. Chromatographic conditions were as follows: (a) flow rate: 1 ml / min.; (b) detection: UV 254 nm; (c) injection volume: 10 μl; (d) column: 5 μm Supercosil, LC-DP, 250×4.6 mm; (e) column temperature: 40° C.; (f) mobile phase A: 10 mM NH4AC or KH2PO4, pH 3; (g) mobile phase B: 100% acetonitrile; (h) running time: 45 minutes. Data are shown in Tables 2 and 3.
TABLE 2Impurity level (%) in formulations SF-1 to SF-5 following storagedays stored at 70° C.Formulation914162028333590SF-12.93.77.612.6SF-20.020.020.022.8SF-30.020.020.020.09SF-40.030.040.060.30SF-5NDNDND0.15
ND = None detected
[0194]
TABLE 3Impurity level (%) in formulations SF-1, SF-2, ...
example 3
[0196] Solution formulation SF-1 of Example 1 was bubbled with ethylene oxide, a putative source of free radicals, for 15 minutes, and was then stored at 70° C. for 10 days. After storage, the formulation was analyzed for the presence of impurities. Addition compounds detected therein were isolated by reversed-phase, semi-preparative HPLC. A 20×250 mm Kromasil C18 column was employed with either an isocratic or a gradient, acetonitrile-aqueous trifluoroacetic acid mobile phase. Detection was accomplished at 254 nm. Pooled fractions containing individual addition compounds, herein referred to as Peak 1, Peak 2 and Peak 3 addition compounds, were concentrated, desalted and reduced in chemical noise-causing components by trapping on a 7×300 mm Hamilton PRP-1 column. The eluent from the trapping column containing the individual addition compounds was freeze-dried to yield the final isolates. Peak 1 addition compound was 99% pure and Peak 2 addition compound was >99% pure by analytical H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com