Patents
Literature
219 results about "Stage melanoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
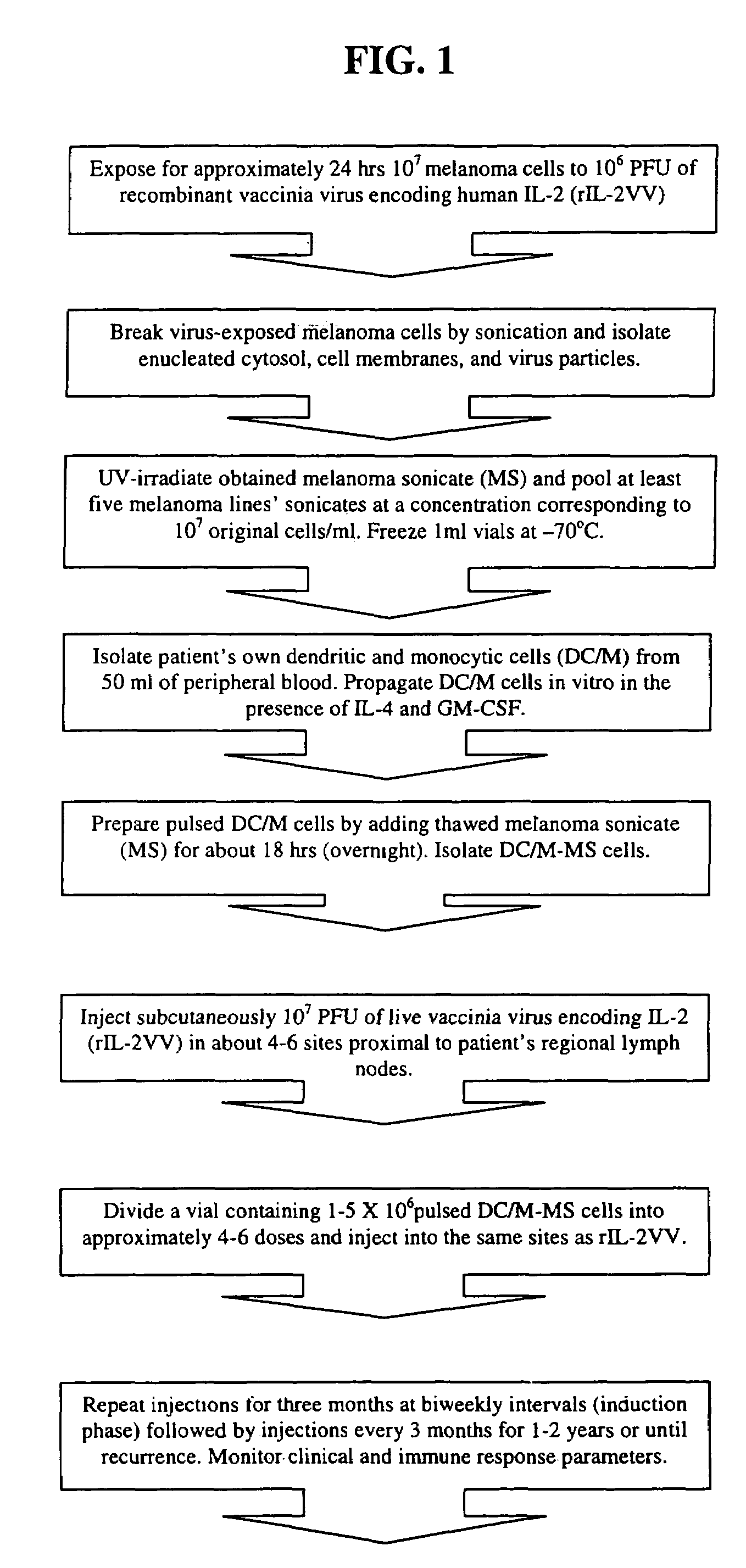
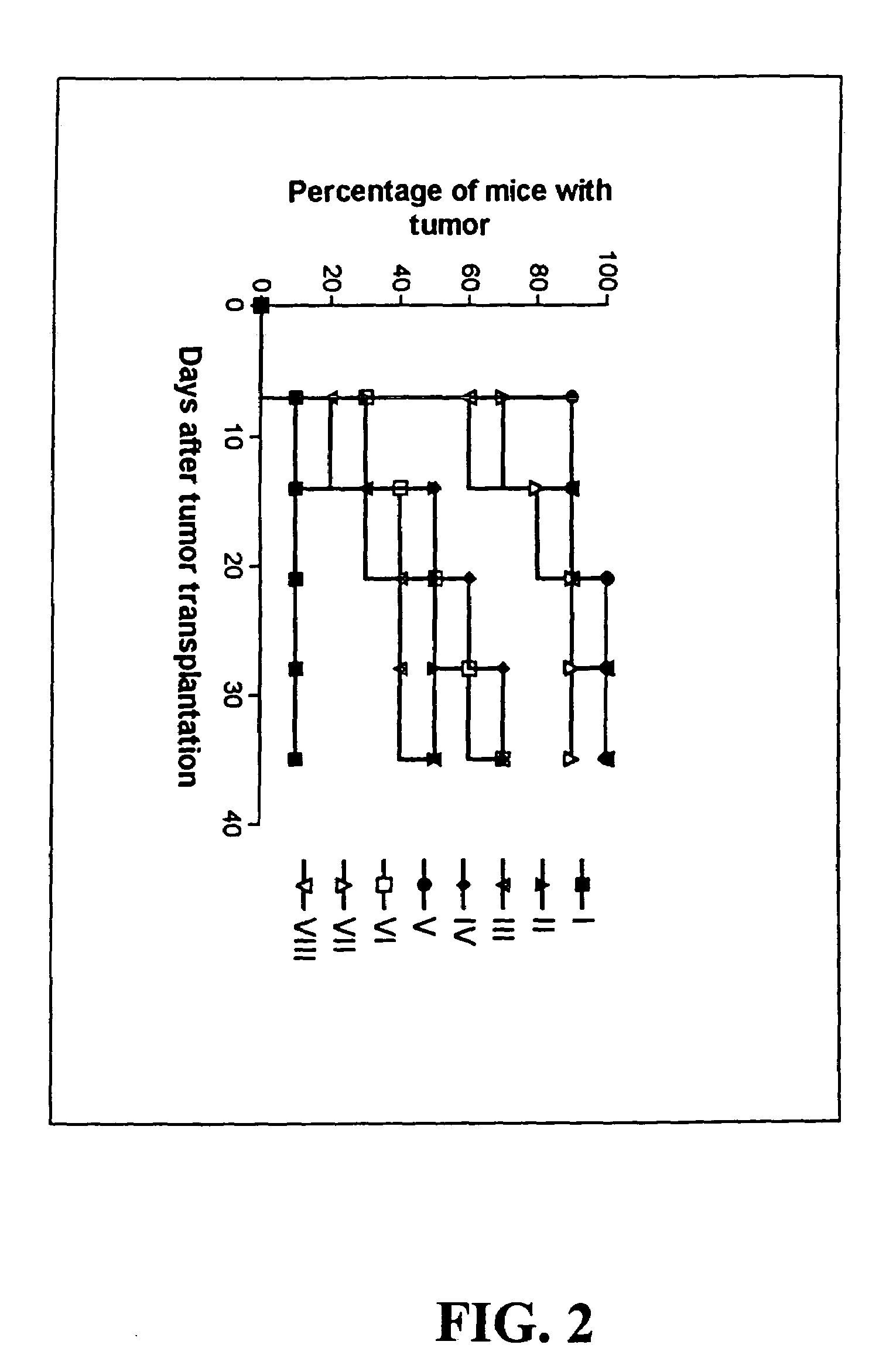
Vectors for the diagnosis and treatment of solid tumors including melanoma
The present invention is directed to the isolation and use of super-infective, tumor-specific vectors that are strains of parasites including, but not limited to bacteria, fungi and protists. In certain embodiments the parasites include, but are not limited to, the bacterium Salmonella spp., such as Salmonella typhimurium, the bacterium Mycobacterium avium and the protozoan Leishmania amazonensis. In other embodiments, the present invention is concerned with the isolation of super-infective, tumor-specific, suicide gene-containing strains of parasites for use in treatment of solid tumors.
Owner:YALE UNIV
Methods and Antibody Compositions for Tumor Treatment
ActiveUS20150266966A1Decreased killing of T-cellsReduce the burden onImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCD20
The present invention provides bispecific antibodies that bind to CD3 and tumor antigens and methods of using the same. According to certain embodiments, the bispecific antibodies of the invention exhibit reduced effector functions and have a unique binding profile with regard to Fcγ receptors. The bispecific antibodies are engineered to efficiently induce T cell-mediated killing of tumor cells. According to certain embodiments, the present invention provides bispecific antigen-binding molecules comprising a first antigen-binding domain that specifically binds human CD3, a second antigen-binding molecule that specifically binds human CD20, and an Fc domain that binds Fcγ receptors with a specific binding pattern. In certain embodiments, the bispecific antigen-binding molecules of the present invention are capable of inhibiting the growth of B-cell or melanoma tumors expressing CD20. The bispecific antibodies of the invention are useful for the treatment of various cancers as well as other CD20-related diseases and disorders.
Owner:REGENERON PHARM INC
Composition and vaccine for treating lung cancer
InactiveUS20160168227A1High in proteinEffectively stimulating the (adaptive) immune systemOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
The present invention relates to a composition comprising at least one mRNA encoding a combination of antigens capable of eliciting an (adaptive) immune response in a mammal, wherein the antigens are selected from the group consisting of 5T4 (Trophoblast glycoprotein, TPBG), Survivin (Baculoviral TAP repeat-containing protein 5; BIRC5), NY-ESO-1 (New York esophageal squamous cell carcinoma 1, CTAG1B), MAGE-C1 (Melanoma antigen family C1), MAGE-C2 (Melanoma antigen family C2), and MUC1 (Mucin 1). The invention furthermore relates to a vaccine comprising at least one mRNA encoding such a combination of antigens, and to the use of said composition (for the preparation of a vaccine) and / or of the vaccine for eliciting an (adaptive) immune response for the treatment of lung cancer, preferably of non-small cell lung cancer (NSCLC), and diseases or disorders related thereto. Finally, the invention relates to kits, particularly to kits of parts, containing the composition and / or the vaccine.
Owner:CUREVAC AG
Methods for treating melanomas
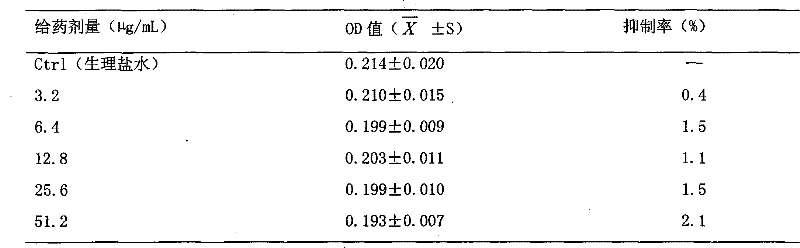
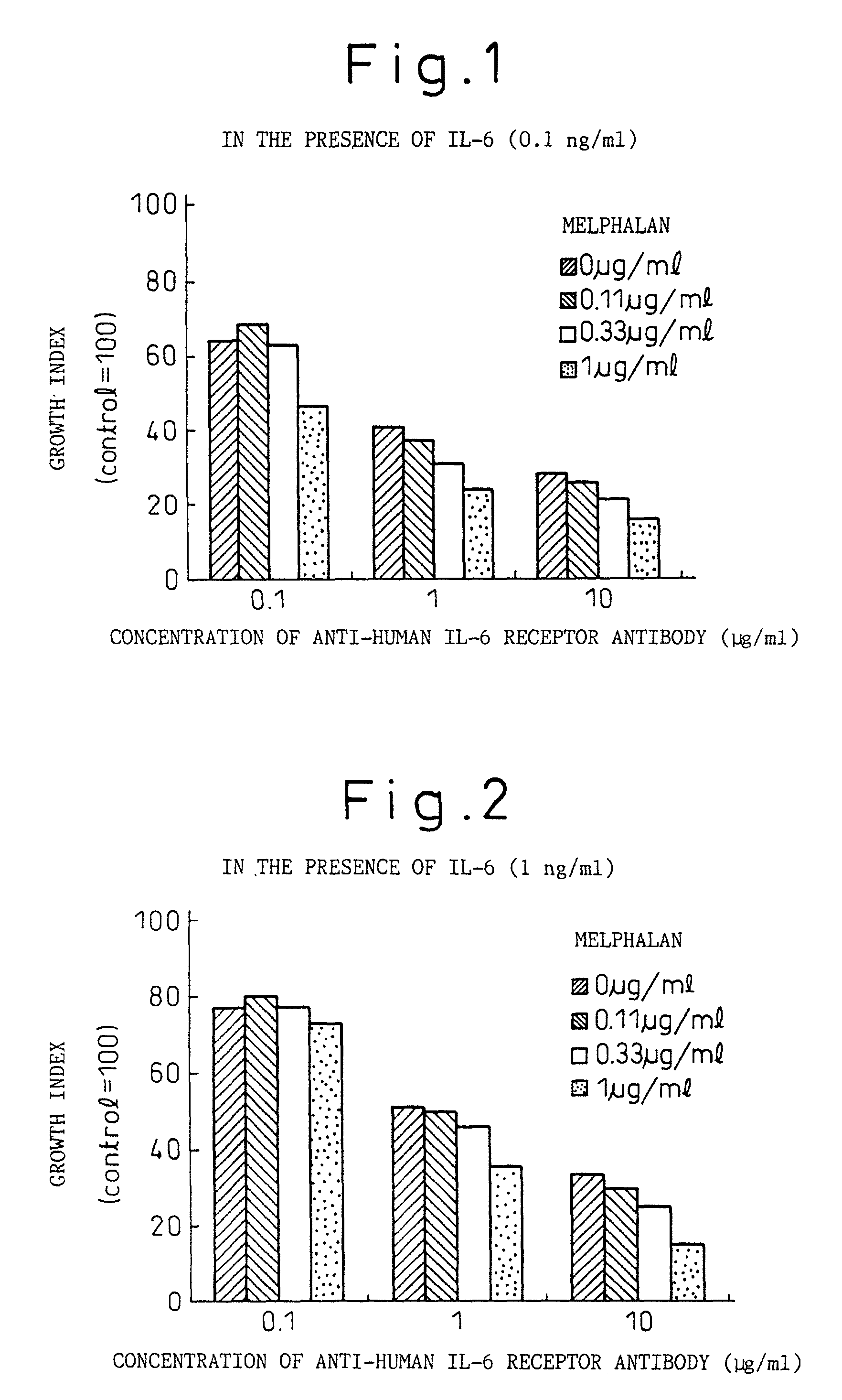
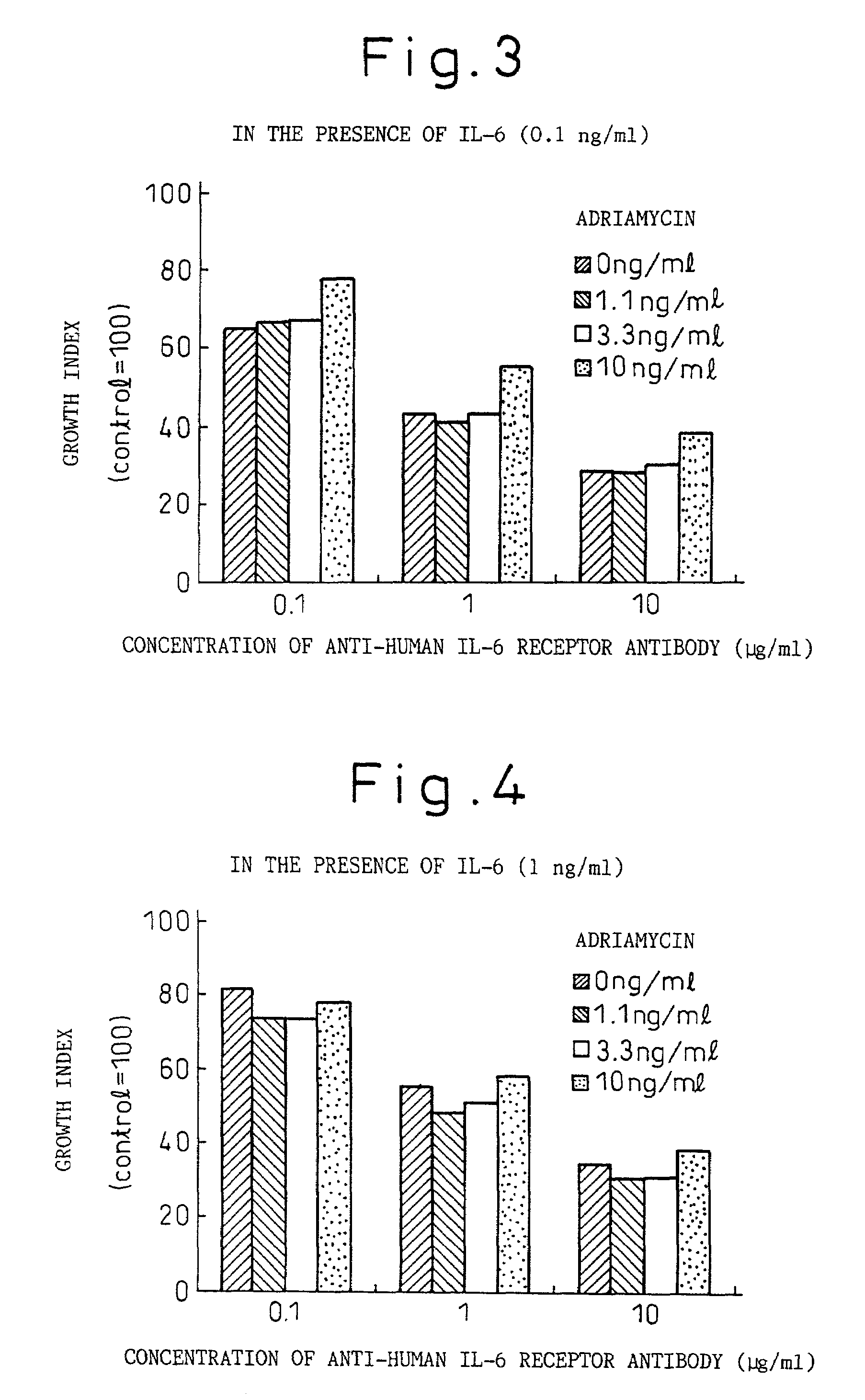
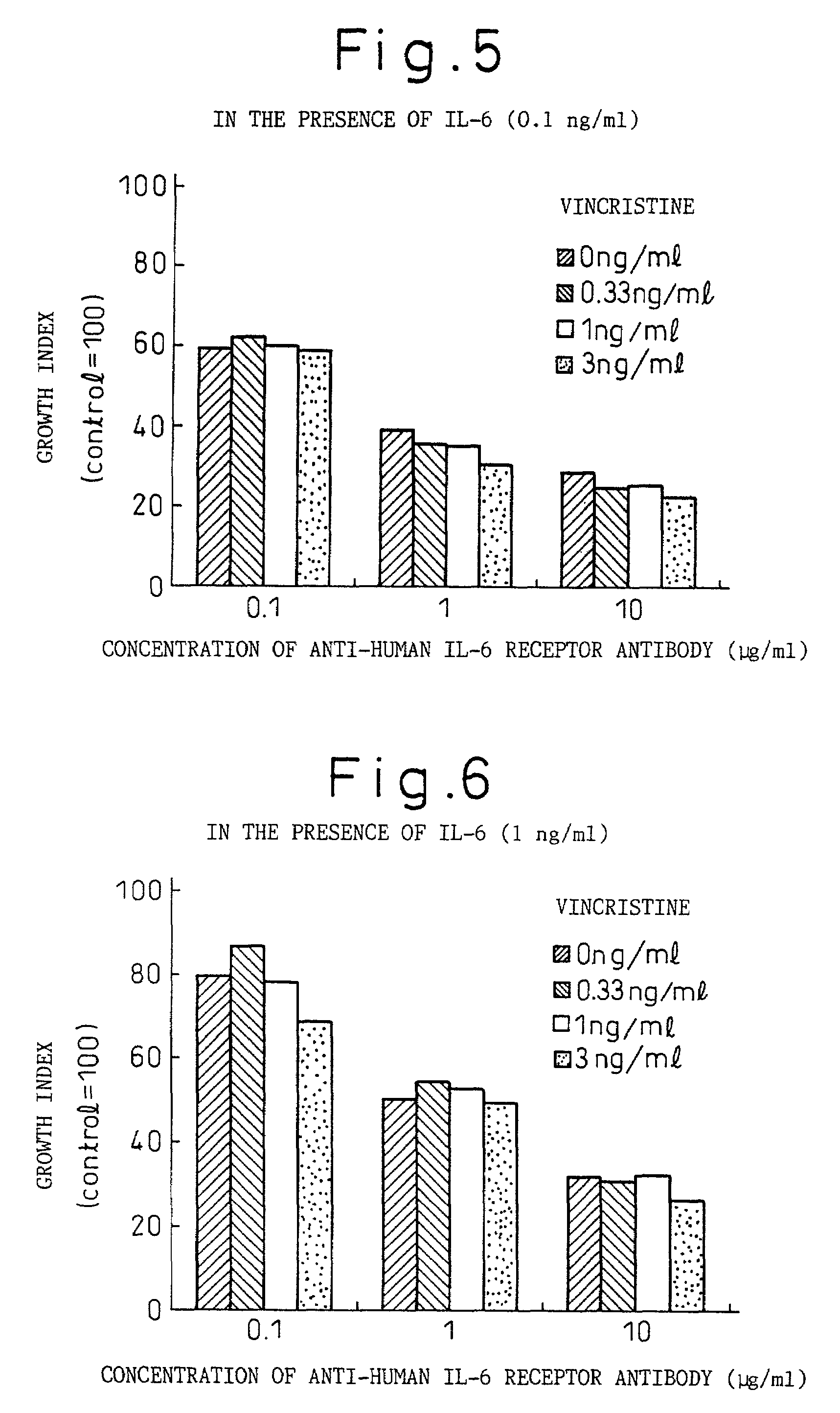
A therapeutic agent for myeloma comprising a combined use of a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody. Thus, a therapeutic agent for myeloma comprising anti-IL-6 receptor antibody for use in combination with a nitrogen mustard anticancer agent; a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent for use in combination with anti-IL-6 receptor antibody; and a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD
Melanoma vaccine and methods of making and using same
Owner:WALLACK MARC K
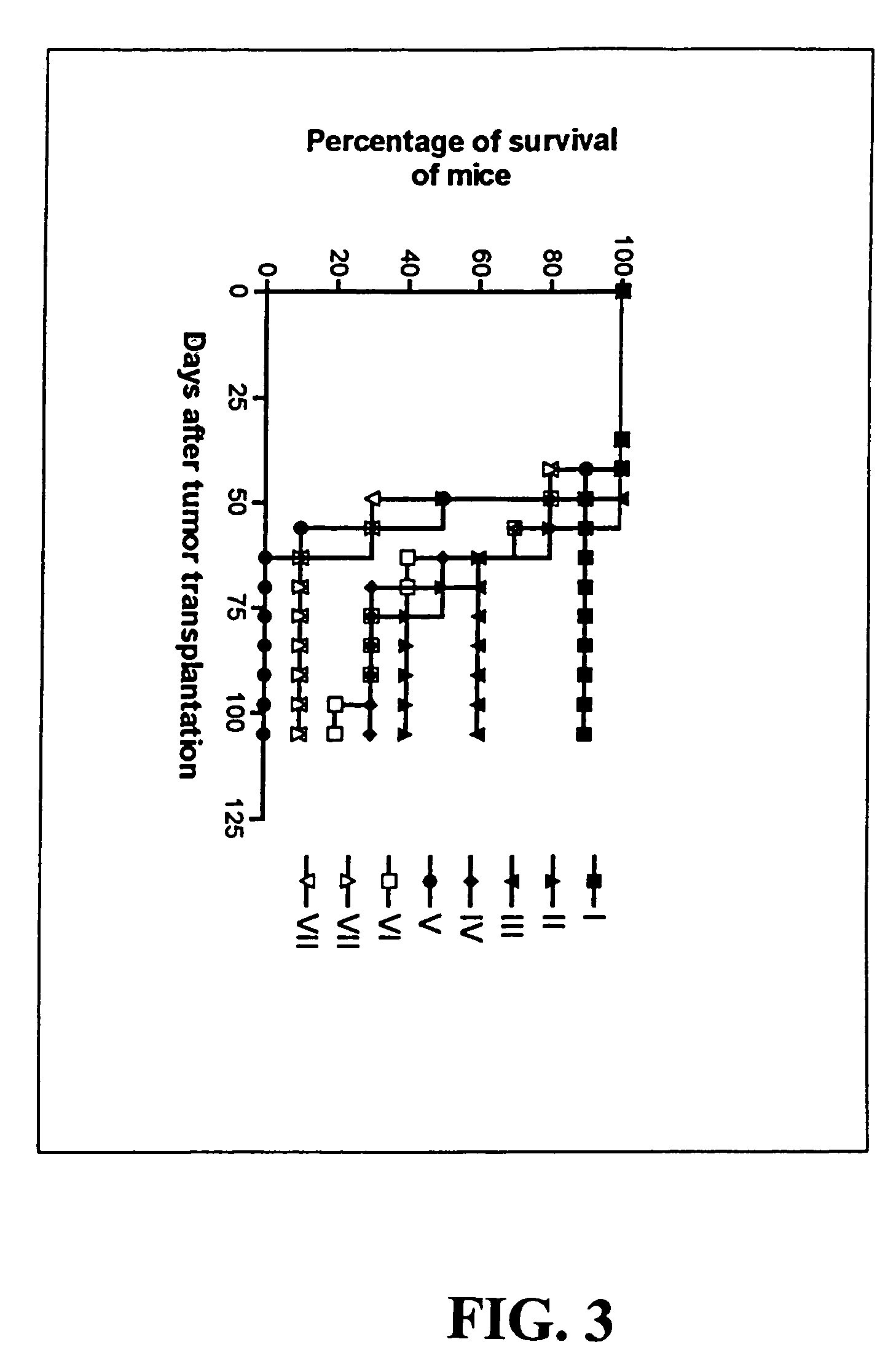
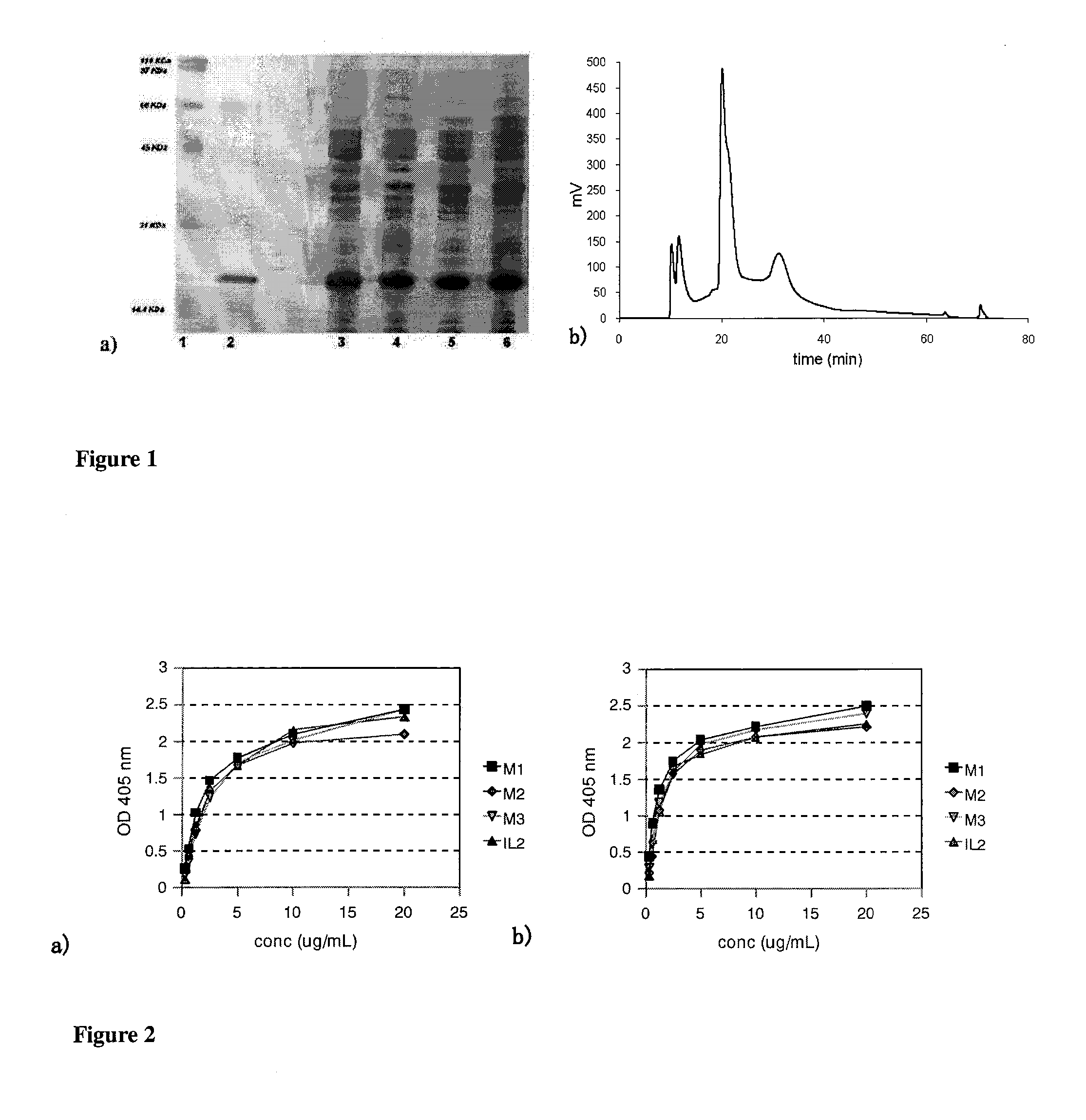
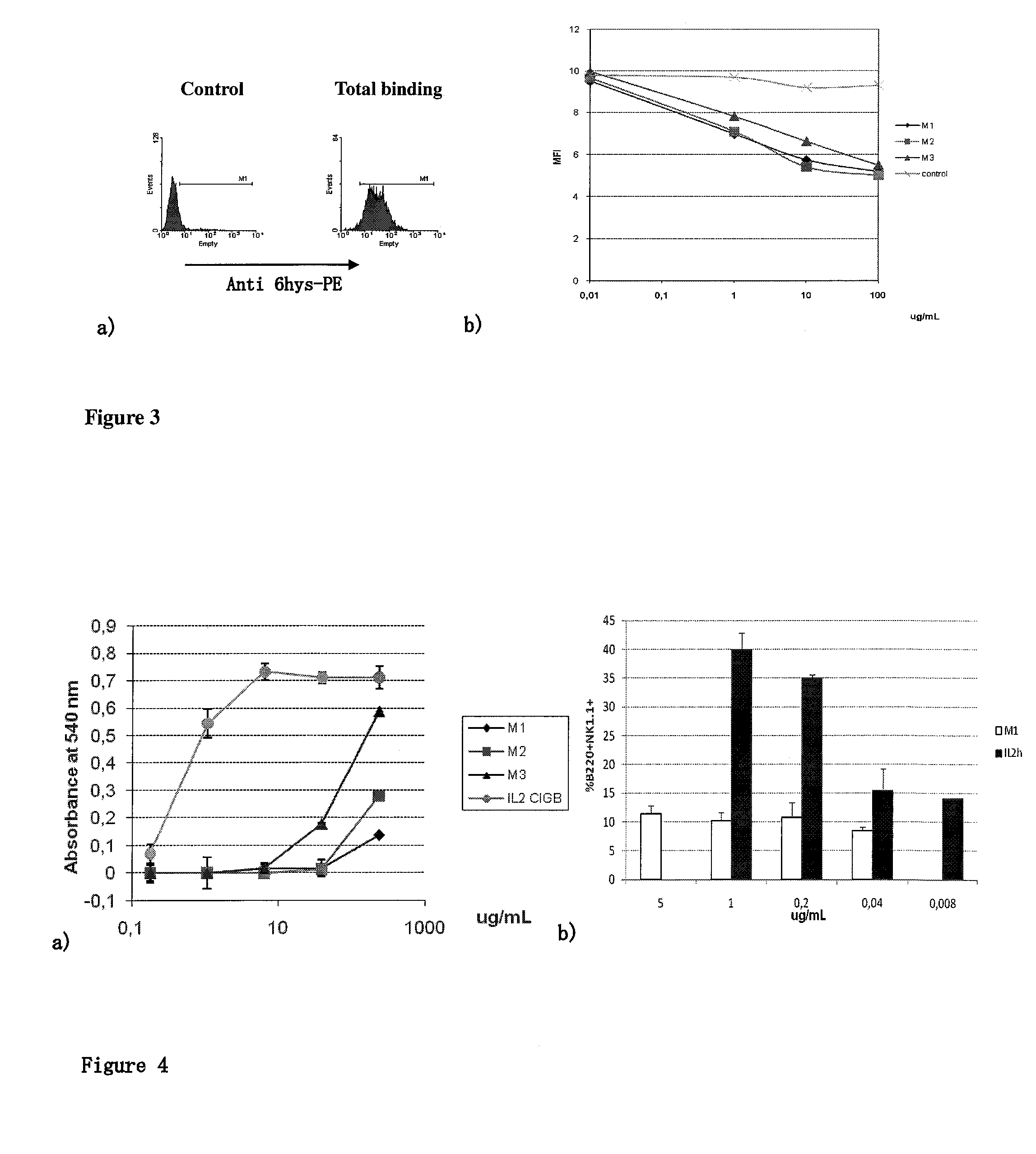
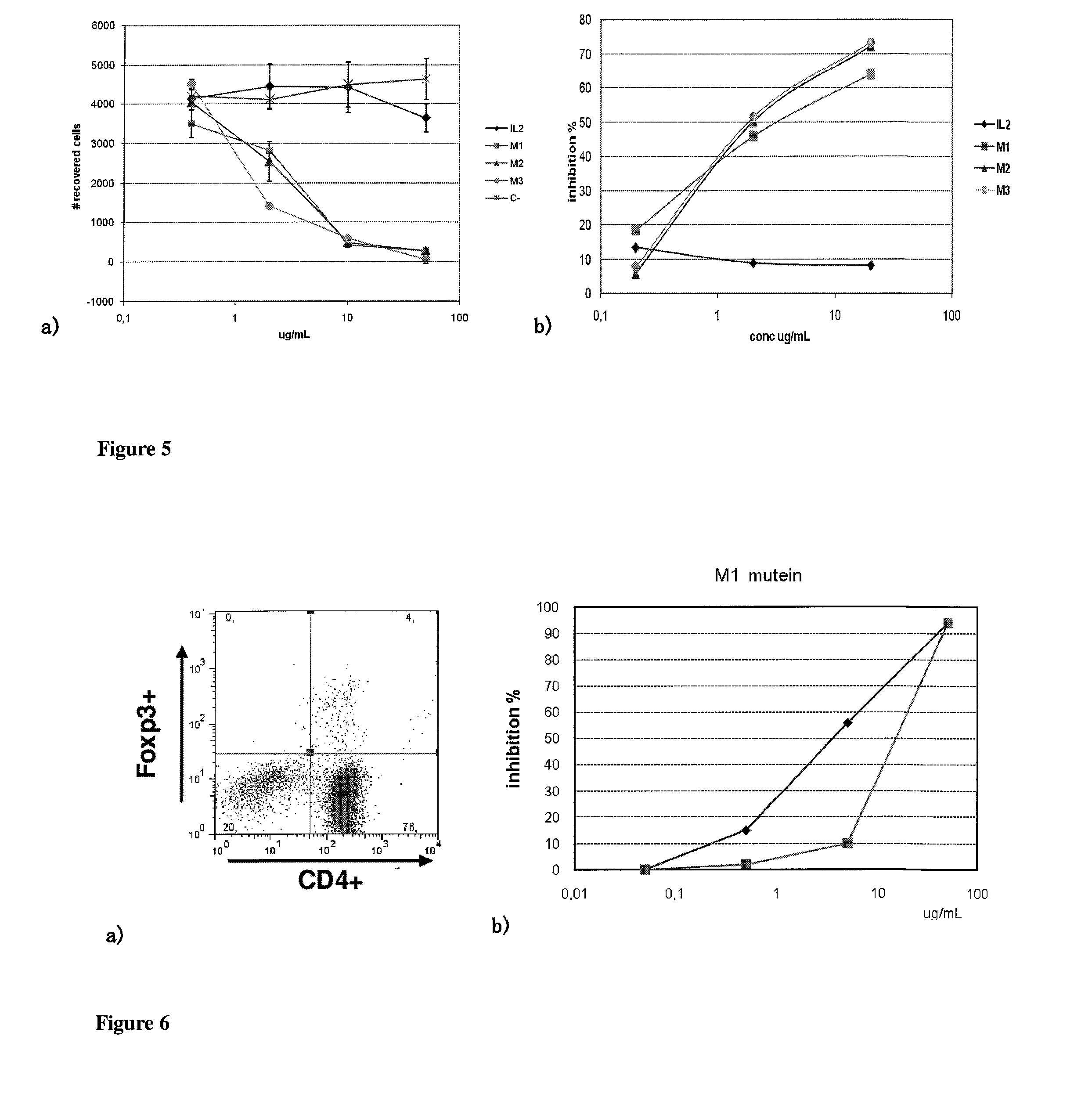
Immunomodulatory interleukin-2 polypeptides and methods of treating melanoma
The present invention relates generally to polypeptides whose primary sequence has high sequence homology with human interleukin 2 (IL-2) with some punctual mutations in the sequence of native IL-2. The polypeptides of the present invention have an immunomodulatory effect on the immune system, which is selective / preferential on regulatory T cells. The present invention also relates to specific polypeptides whose amino acid sequence is disclosed herein. In another aspect the present invention relates to pharmaceutical compositions comprising as active ingredient the polypeptides disclosed. Finally, the present invention relates to the therapeutic use of the polypeptides and pharmaceutical compositions disclosed due to their immune modulating effect on diseases such as cancer and chronic infectious diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
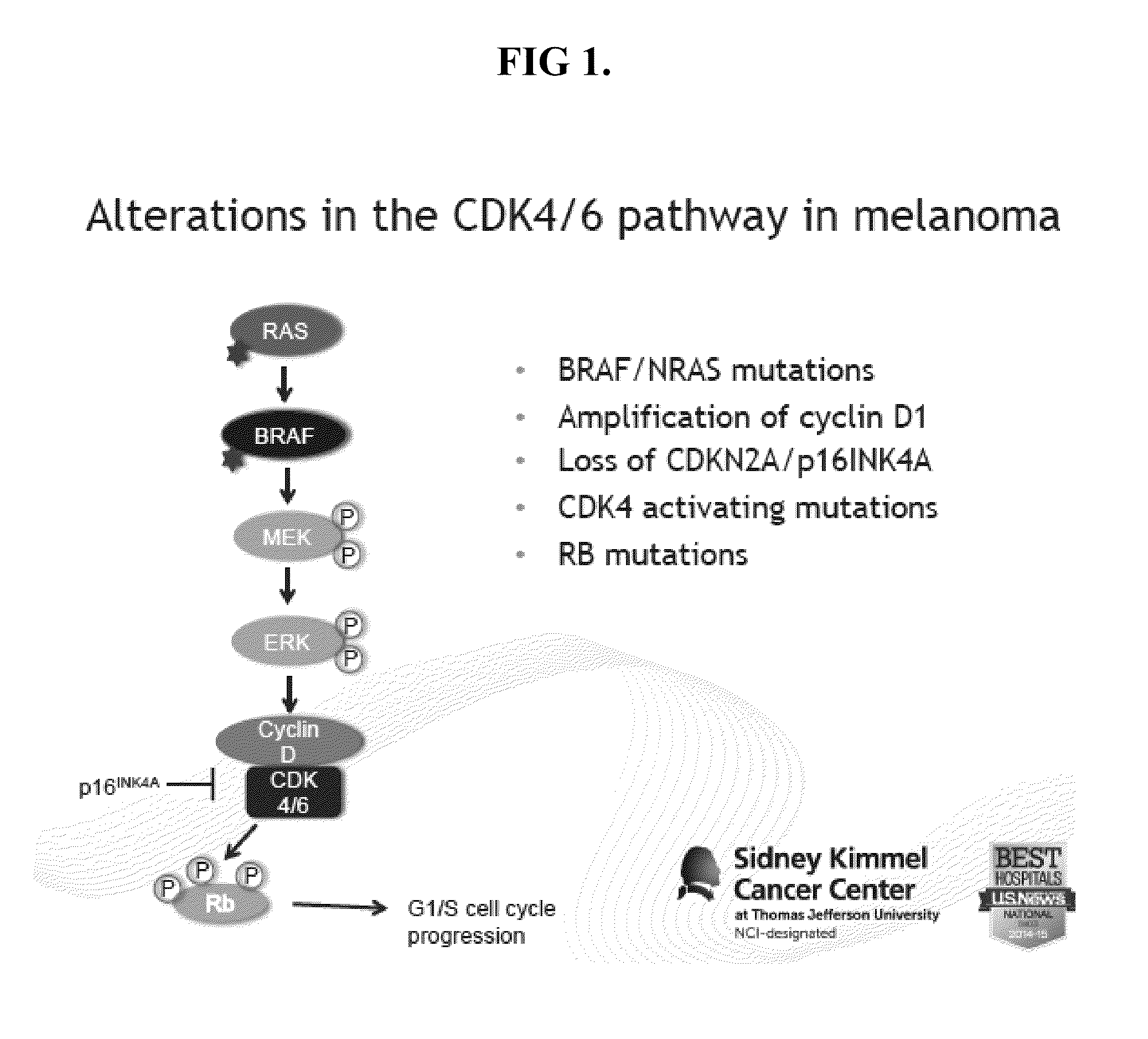
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
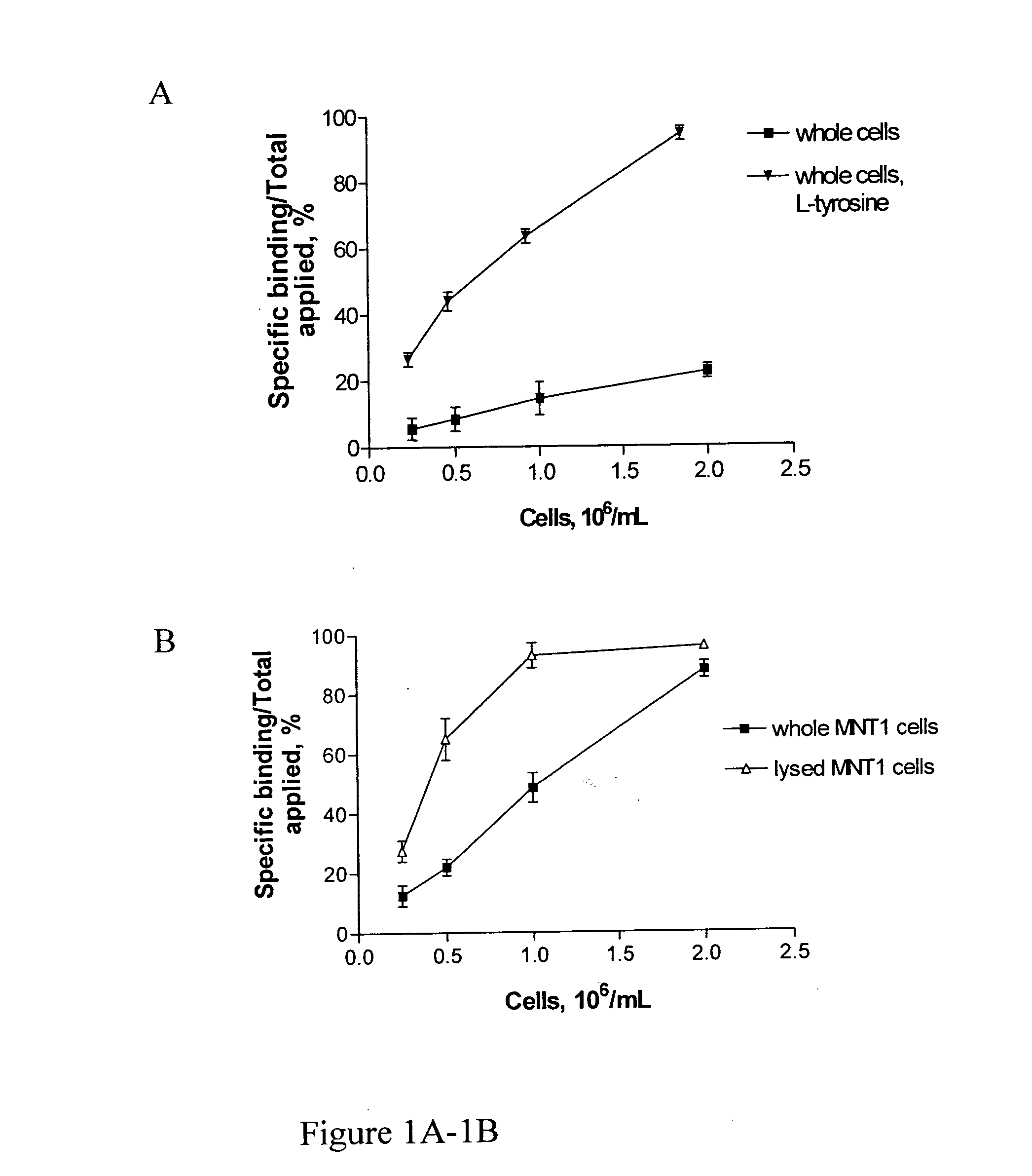
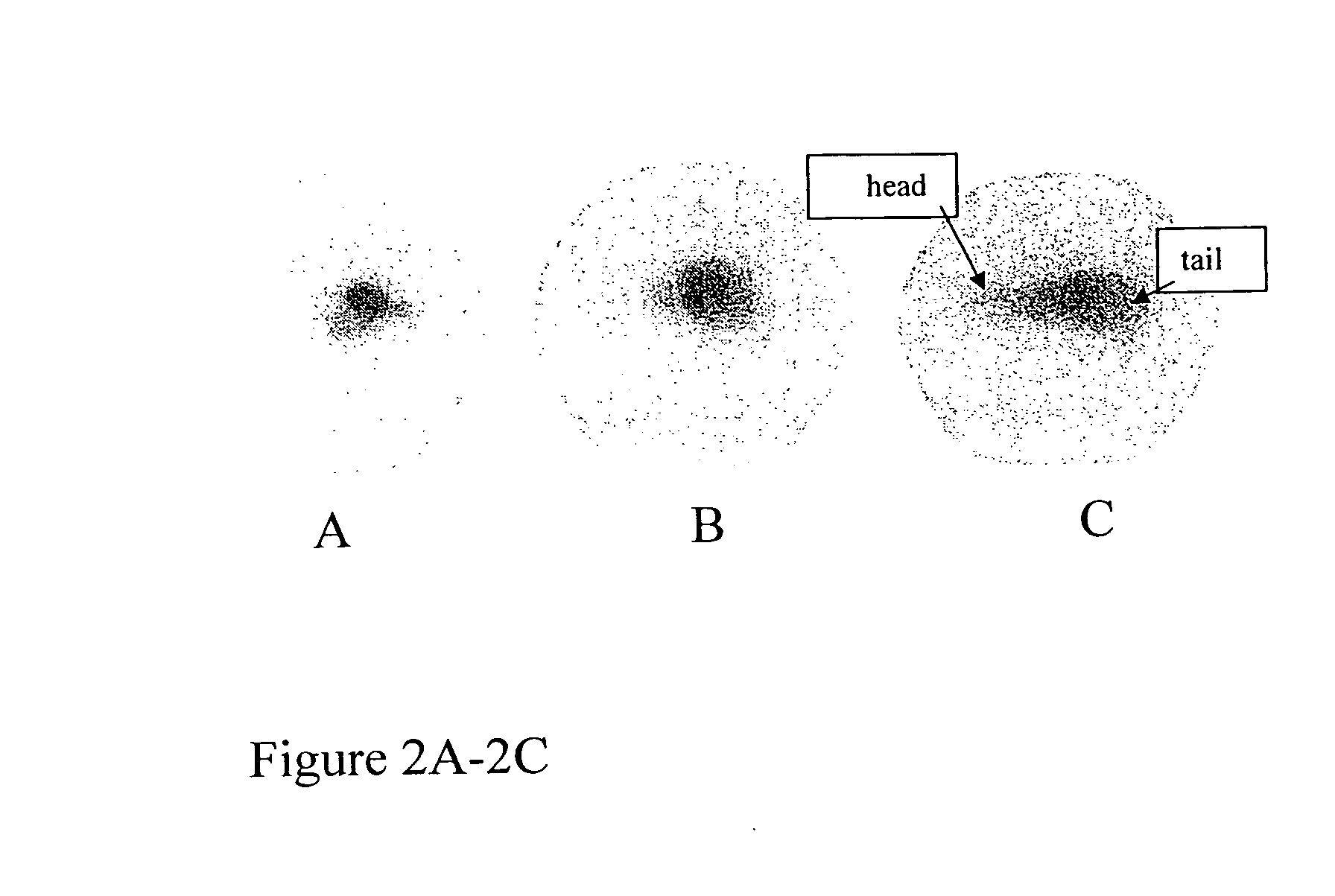
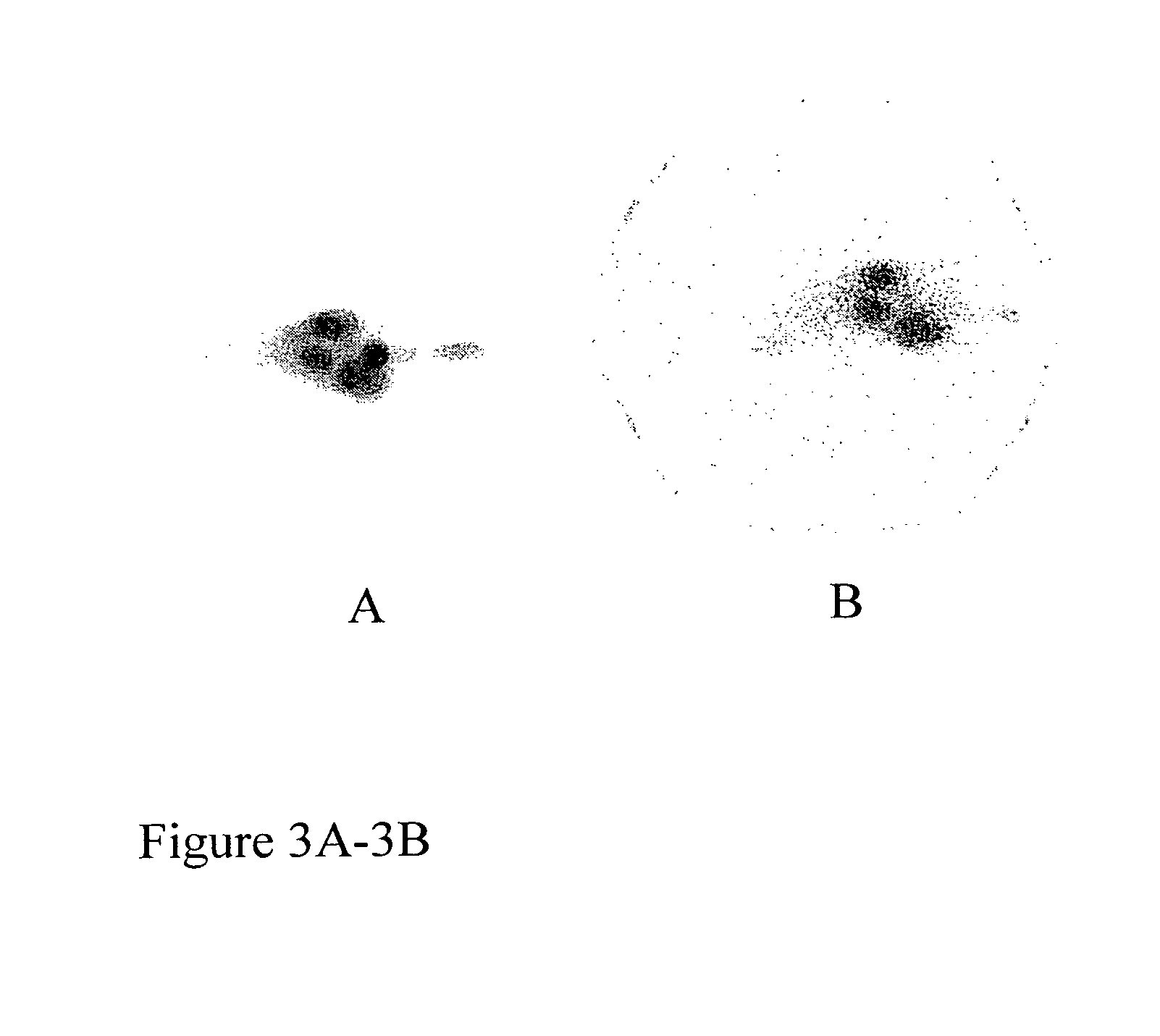
Radiolabeled antibodies and peptides for treatment of tumors
InactiveUS20060039858A1Effective treatmentMicrobiological testing/measurementRadioactive preparation carriersAbnormal tissue growthCellular component
This invention provides methods for imaging and / or treating a tumor in a subject which comprise administering to the subject an amount of a radiolabeled antibody and / or peptide effective to image and / or treat the tumor, where the radiolabeled antibody and / or peptide binds to a cellular component released by dying tumor cells. This invention also provides methods for imaging and / or treating melanin-containing melanomas or other melanin-containing tumors in a subject which comprise administering to the subject an amount of a radiolabeled anti-melanin antibody and / or peptide effective to image and / or treat the melanoma or tumor. The invention also provides compositions and methods of making compositions comprising radiolabeled antibodies and / or peptides for imaging and treating tumors, including melanin-containing melanomas.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
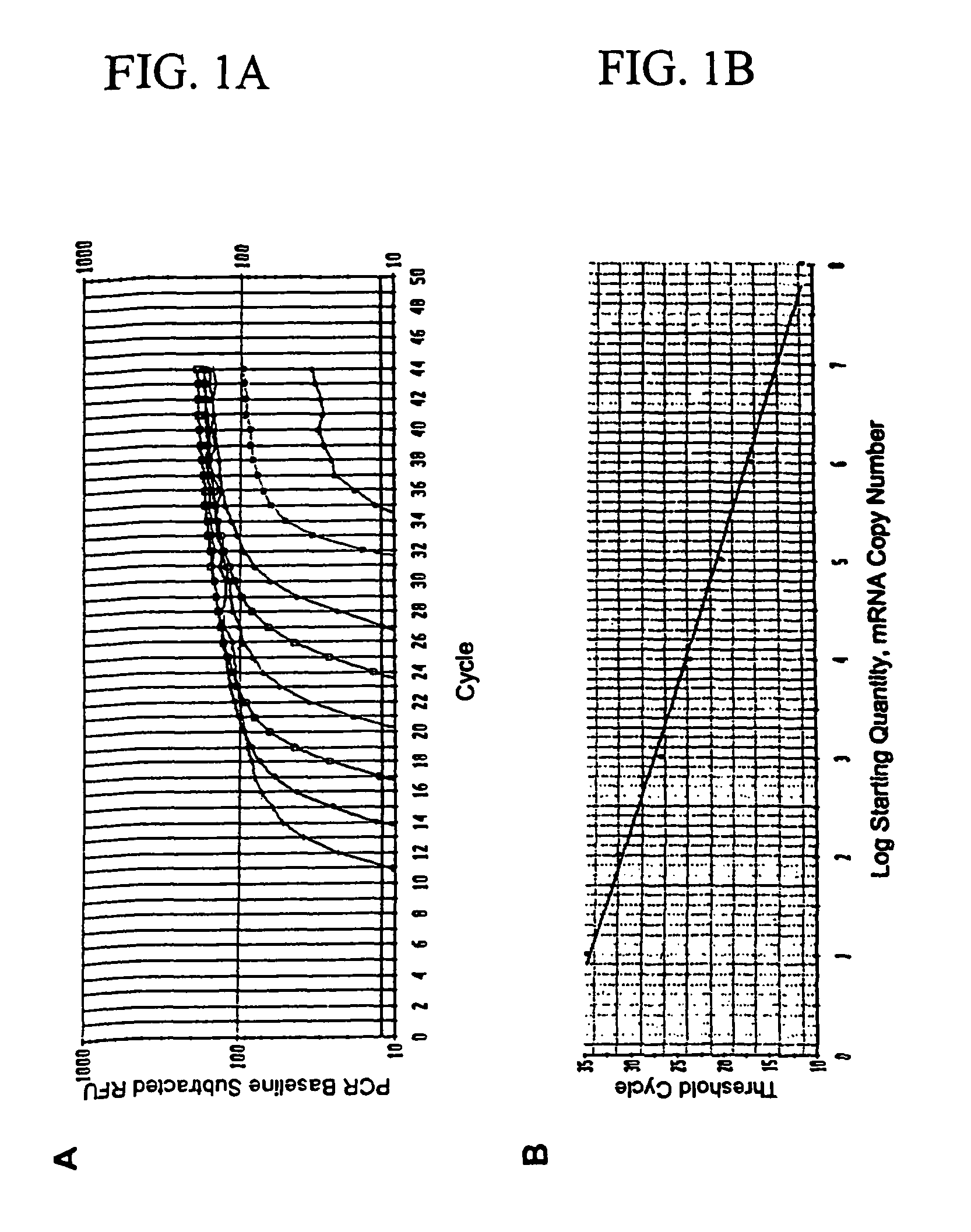
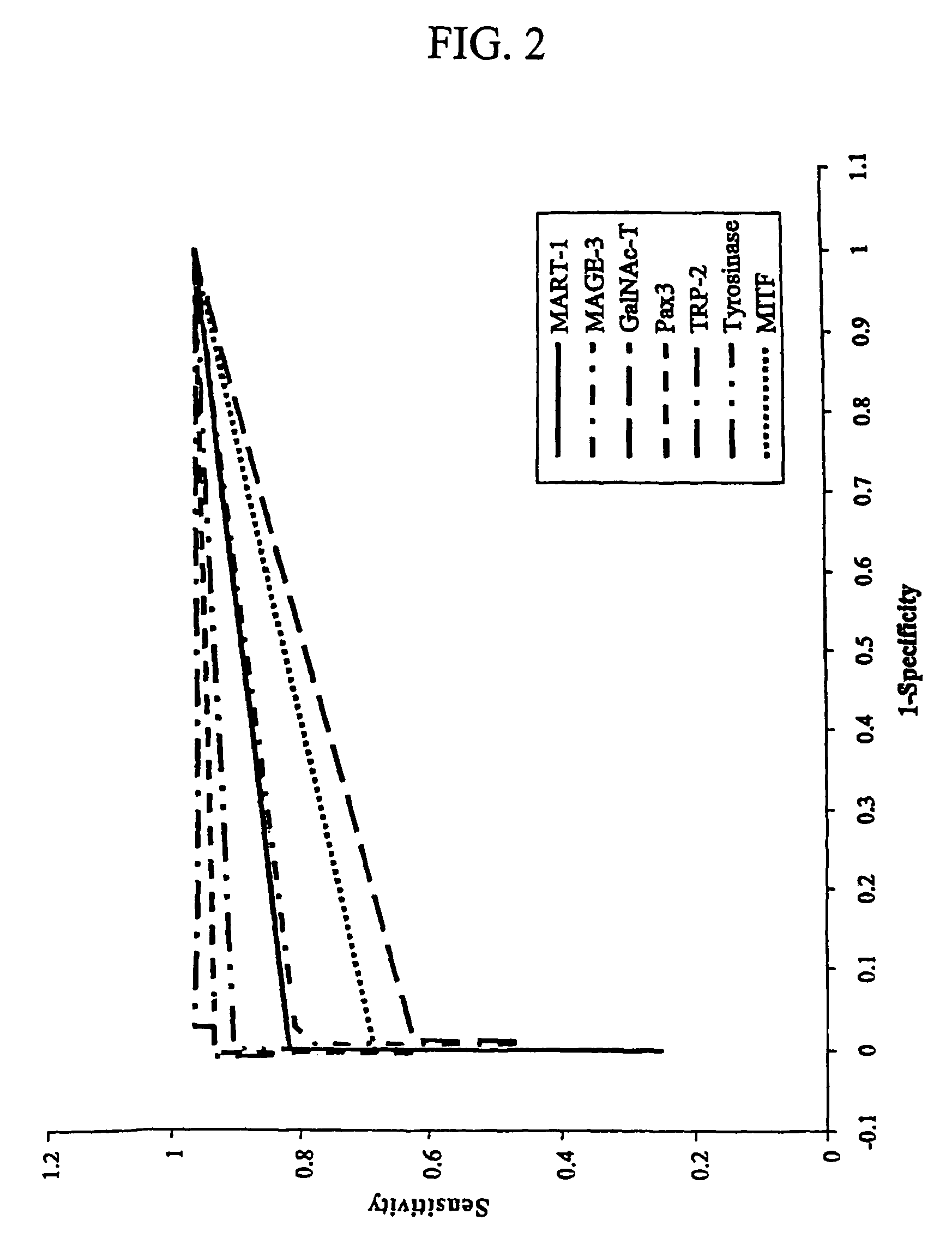
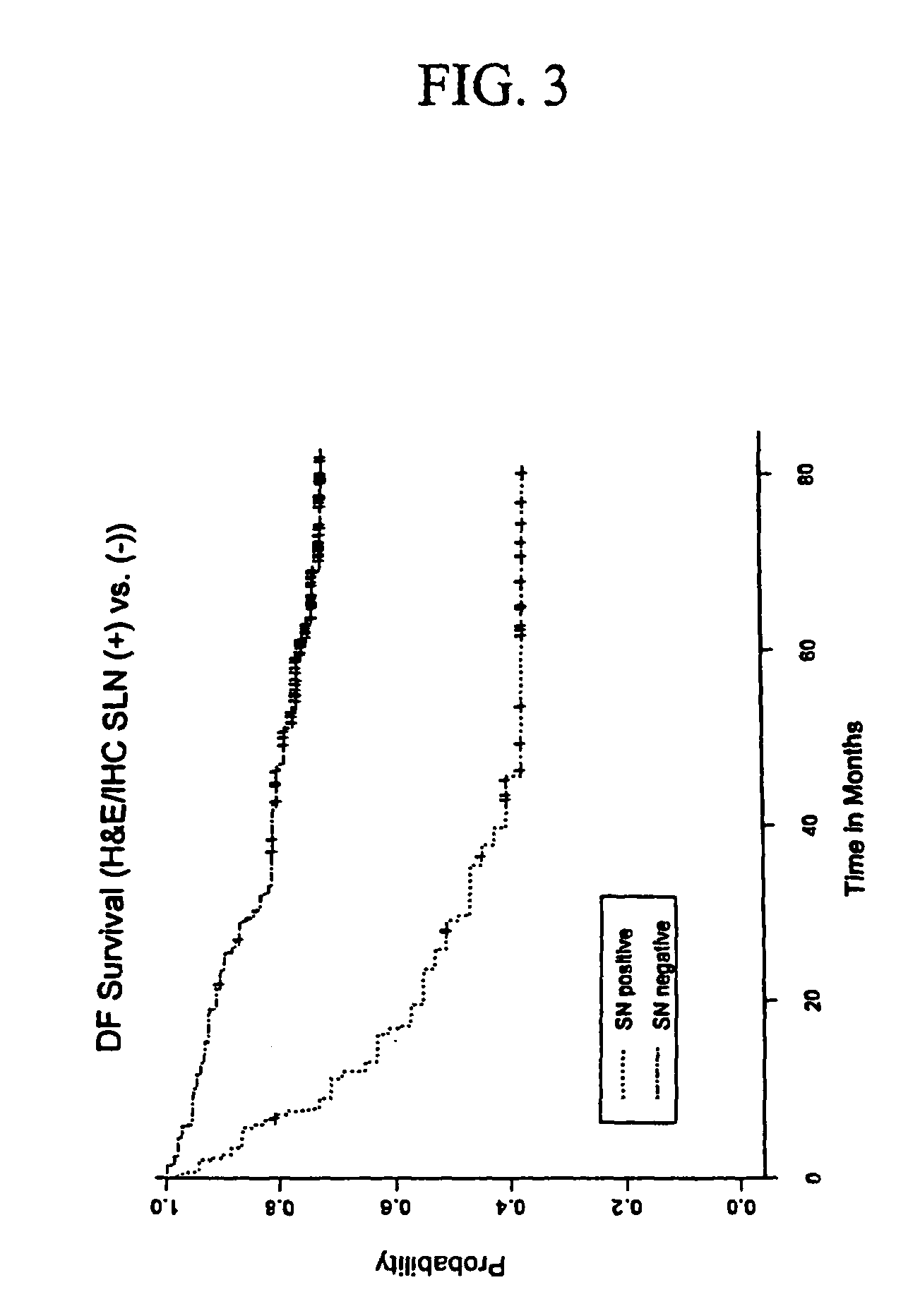
Detection of micro metastasis of melanoma and breast cancer in paraffin-embedded tumor draining lymph nodes by multimarker quantitative RT-PCR
InactiveUS7910295B2High sensitivityStrong specificityMicrobiological testing/measurementBiological testingAbnormal tissue growthMetastatic melanoma
The invention provides a quantitative realtime RT-PCR assay for detection of metastatic breast, gastric, pancreas or colon cancer cells or metastatic melanoma. The assay allows to predict disease recurrence and survival in patients with AJCC stage I and II, and III disease using multimarker panels. The method for detecting metastatic melanoma cells utilizes panels of markers selected from a group consisting of MAGE-A3, GalNAcT, MART-1, PAX3, Mitf, TRP-2, and Tyrosinase. The method for detecting metastatic breast, gastric, pancreas or colon cancer cells in paraffin-embedded samples utilizes panels of markers selected from a group consisting of C-Met, MAGE-A3, Stanniocalcin-1, mammoglobin, HSP27, GalNAcT, CK20, and β-HCG.
Owner:JOHN WAYNE CANCER INST
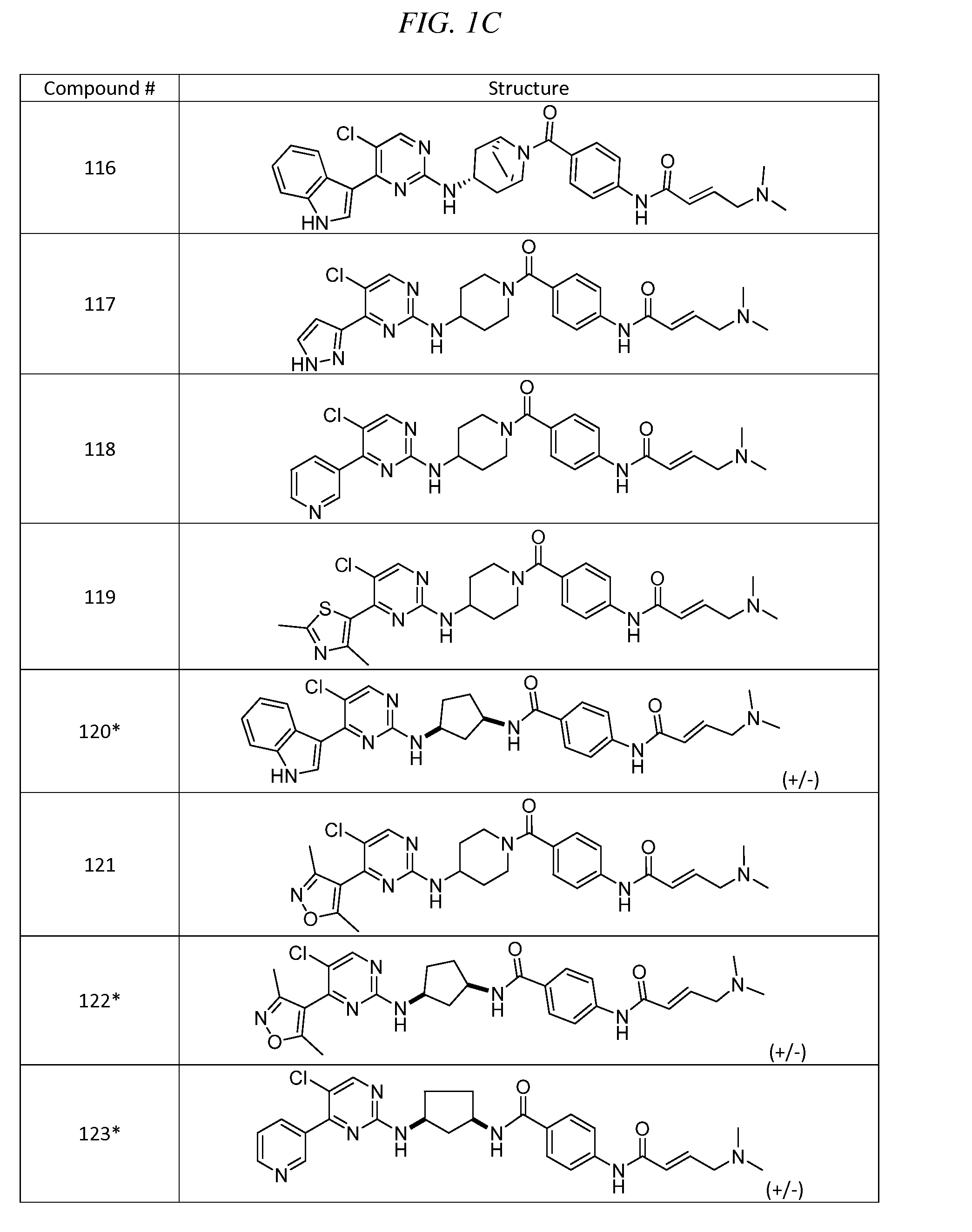
Heteromaromatic compounds useful for the treatment of prolferative diseases
InactiveUS20160264552A1Improve efficacyPrevent relapseOrganic active ingredientsOrganic chemistryAutoimmune conditionStage melanoma
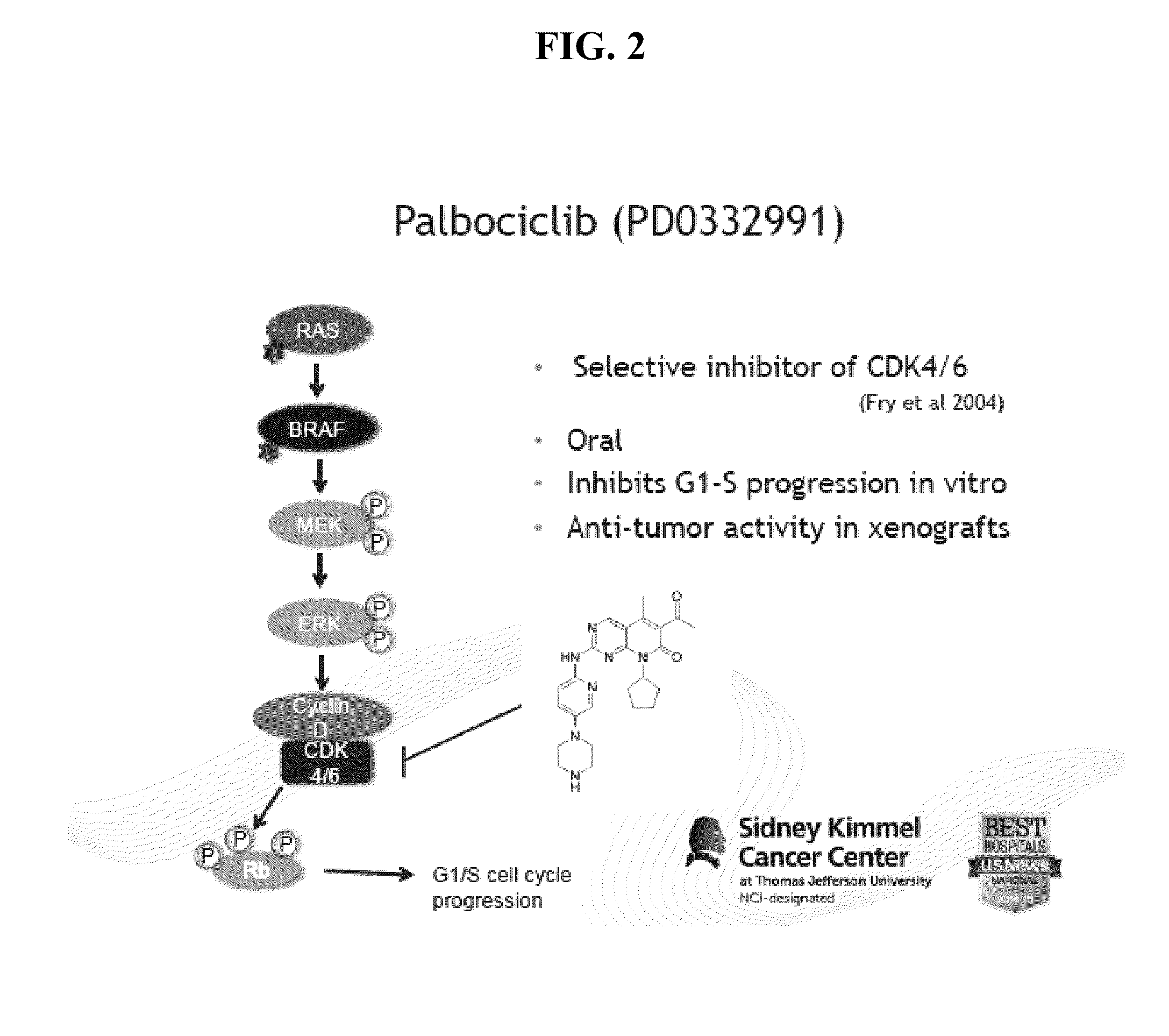
The present invention provides novel compounds of Formula (I) and Formula (II), and pharmaceutically acceptable salts, solvates, hydrates, tautomers, stereoisomers, isotopically labeled derivatives, and compositions thereof. Also provided are methods and kits involving the compounds or compositions for treating or preventing proliferative diseases (e.g., cancers (e.g., leukemia, melanoma, multiple myeloma), benign neoplasms, angiogenesis, inflammatory diseases, autoinflammatory diseases, and autoimmune diseases) in a subject. Treatment of a subject with a proliferative disease using a compound or composition of the invention may inhibit the aberrant activity of a kinase, such as a cyclin-dependent kinase (CDK) (e.g., cyclin-dependent kinase 7 (CDK7)), and therefore, induce cellular apoptosis and / or inhibit transcription in the subject. (I)
Owner:SYROS PHARMACEUTICALIS INC
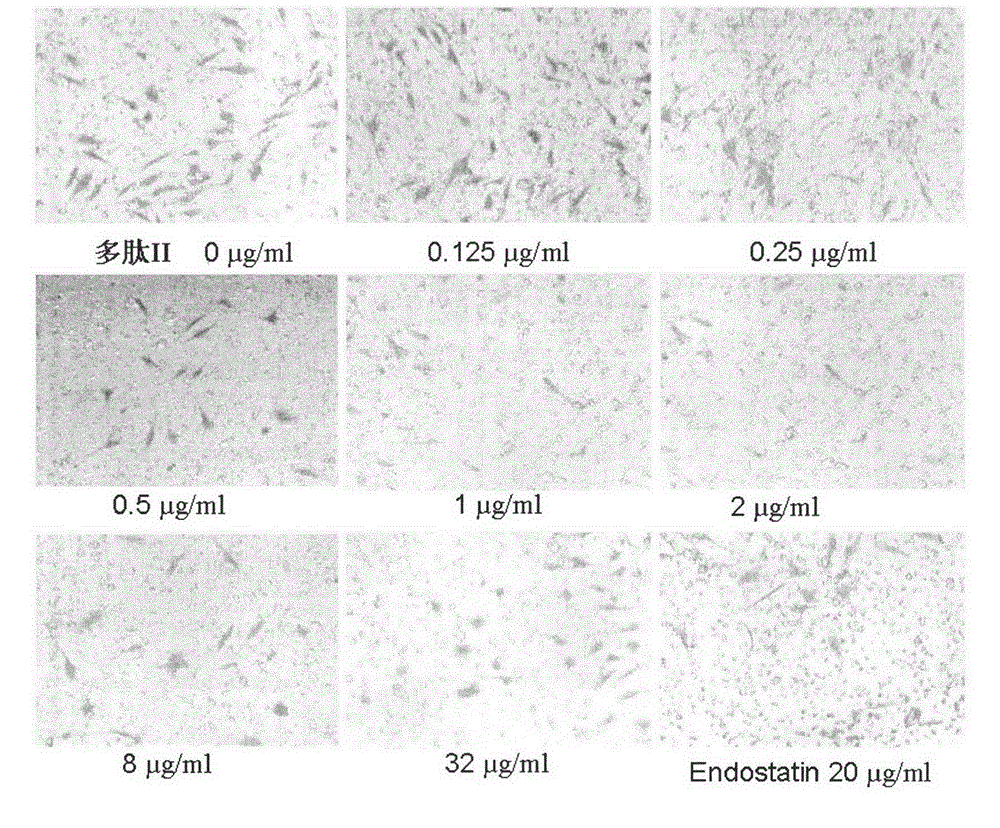
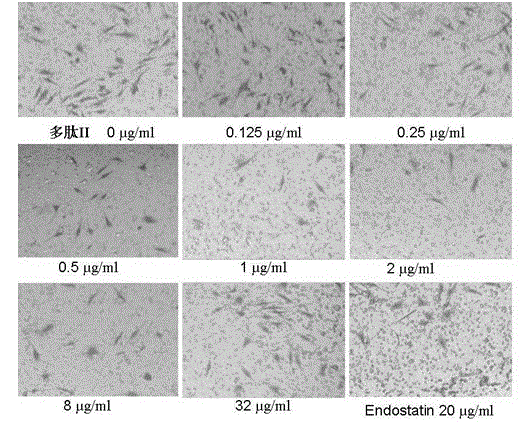
Application of angiogenesis inhibitor polypeptide to preparation of medicine for treating tumor and rheumatoid arthritis
InactiveCN102746380ASuppress acute inflammationGrowth inhibitionPeptide/protein ingredientsAntipyreticCervixKidney
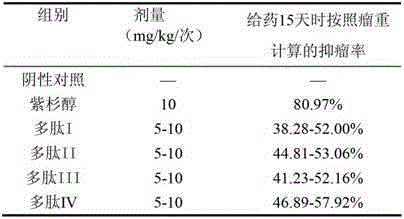
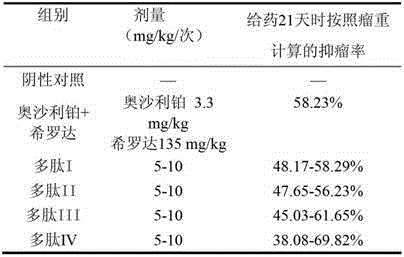
The invention relates to the field of medicines, in particular to an angiogenesis inhibitor for inhibiting several matrix metal protein enzyme and inhibiting the transfer of the vascular endothelial cell, which can be applied to preparation and treatment of entity tumor and rheumatoid arthritis. The polypeptide comprises a polypeptide I, polypeptide II, polypeptide III and polypeptide IV (Seq NO.1, Seq NO.2, Seq NO.3, Seq NO.4); The polypeptide can be used for treating entity tumor and rheumatoid arthritis. The four angiogenesis inhibitors can be applied to preparation of medicine for treating tumor, and is characterized in that: the tumor is derived from primary of neck, brain, thyroid gland, esophagus, pancreas, lung, liver, stomach, mammary gland, kidney, colon or rectum, ovary, cervix, uterus, prostate, bladder and testis or secondary cancer, melanoma, hemangioma and sarcoma.
Owner:CHINA PHARM UNIV
Matrix metallo-proteinase inhibitor polypeptide and application thereof
ActiveCN103145855ASuppress acute inflammationPeptide/protein ingredientsAntipyreticKidneyCervix uterus
The invention relates to the field of medicines, and in particular relates to an inhibitor of angiogenesis capable of inhibiting matrix metallo-proteinase and migration of vascular endothelial cells. The inhibitor can be used for preventing and treating solid tumor and rheumatoid arthritis. The polypeptide specifically means polypeptide I, polypeptide II, polypeptide III and polypeptide IV (with reference to SeqNO.1, SeqNO.2, SeqNO.3 and SeqNO.4). The polypeptide can be used for treating solid tumor and rheumatoid arthritis. The four inhibitors of angiogenesis can be applied to preparing medicines for treating tumor. The polypeptide is characterized in that tumor is originated from essential or subsequent cancers, melanoma, hemangioma and sarcoma of heat and neck, brain, thyroid, esophagus, pancreas, lung, liver, stomach, breast, kidney, gall bladder, colon or rectum, ovary, cervix uterus, uterus, prostate, bladder and testis.
Owner:NANJING ANJI BIOLOGICAL TECH CO LTD
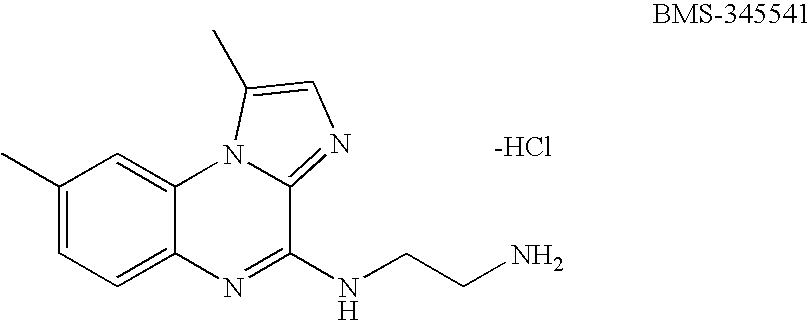
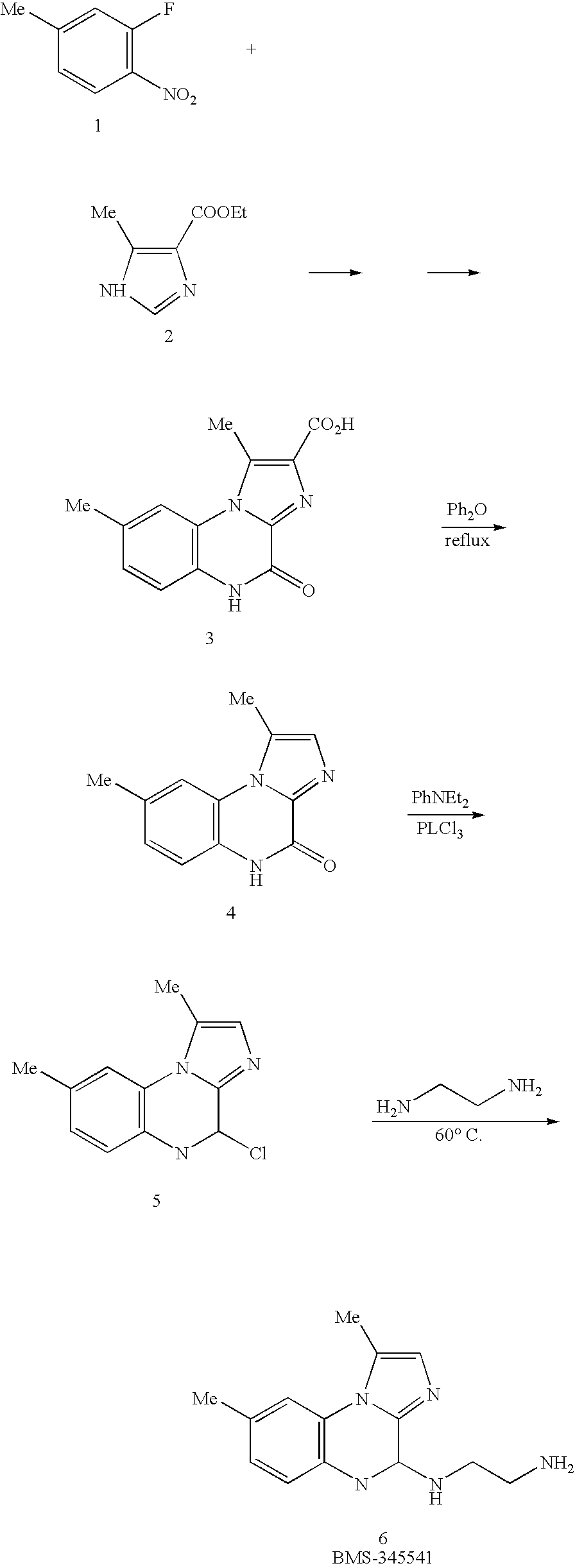
Imidazoquinoxaline compound for the treatment of melanoma
InactiveUS20060025419A1Inhibit growthMinimal effectBiocideAnimal repellantsBMS-345541Cancer research
The present invention concerns a (4(2′-aminoethyl)amino-1,8-dimethylimididazo(1,2-a)quinoxaline)-4,5-dihydro-1,8-dimethylimidazo(1,2-a)quinoxalin-4-one-2-carboxylic acid (BMS-345541) or an analog thereof for the treatment of melanoma cancer. This compound inhibits NFκB activity and expression, and induces apoptosis in melanoma cancer cells. The present invention also provides a method for assaying for the inhibition of melanoma cancer cell growth.
Owner:VANDERBILT UNIV
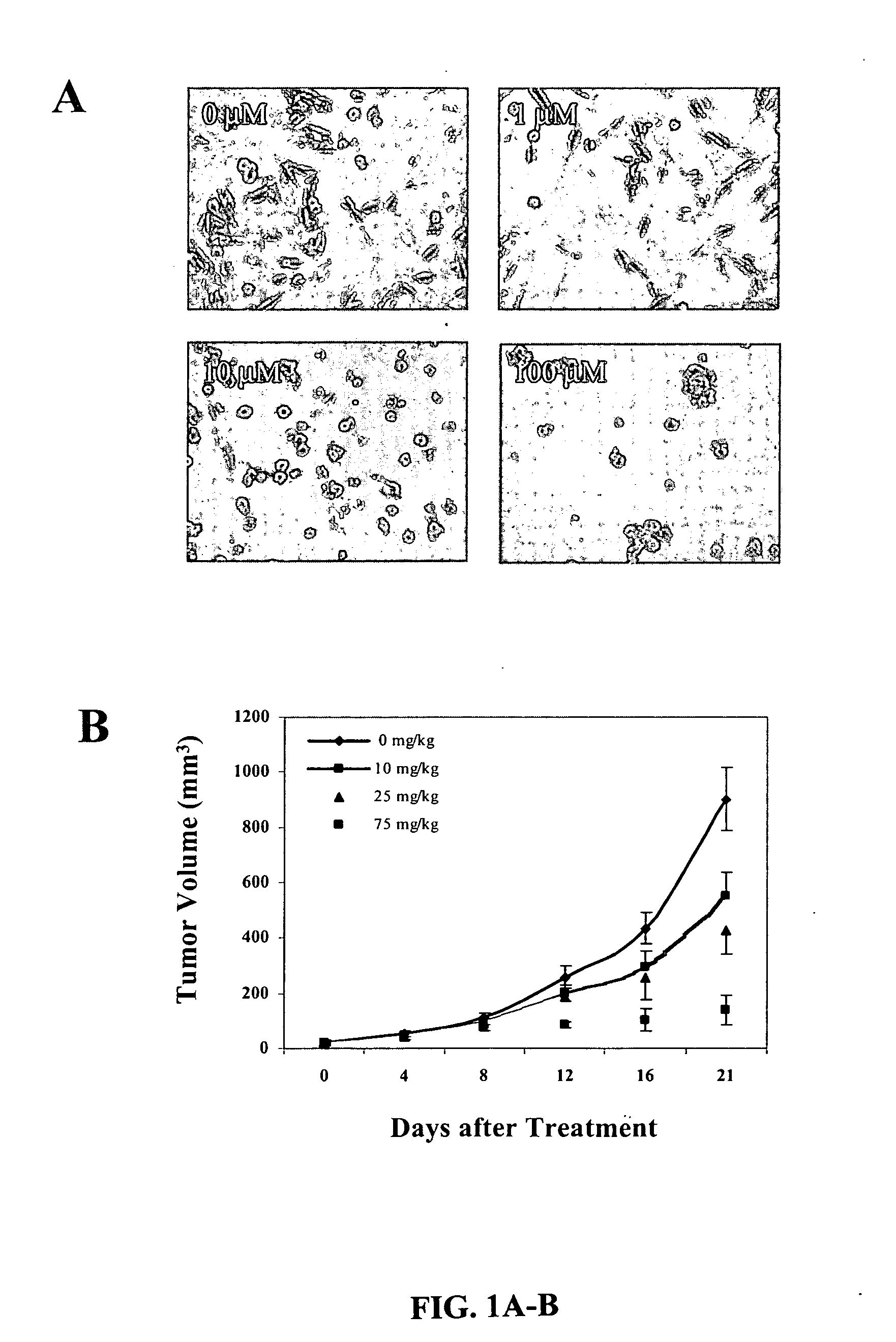
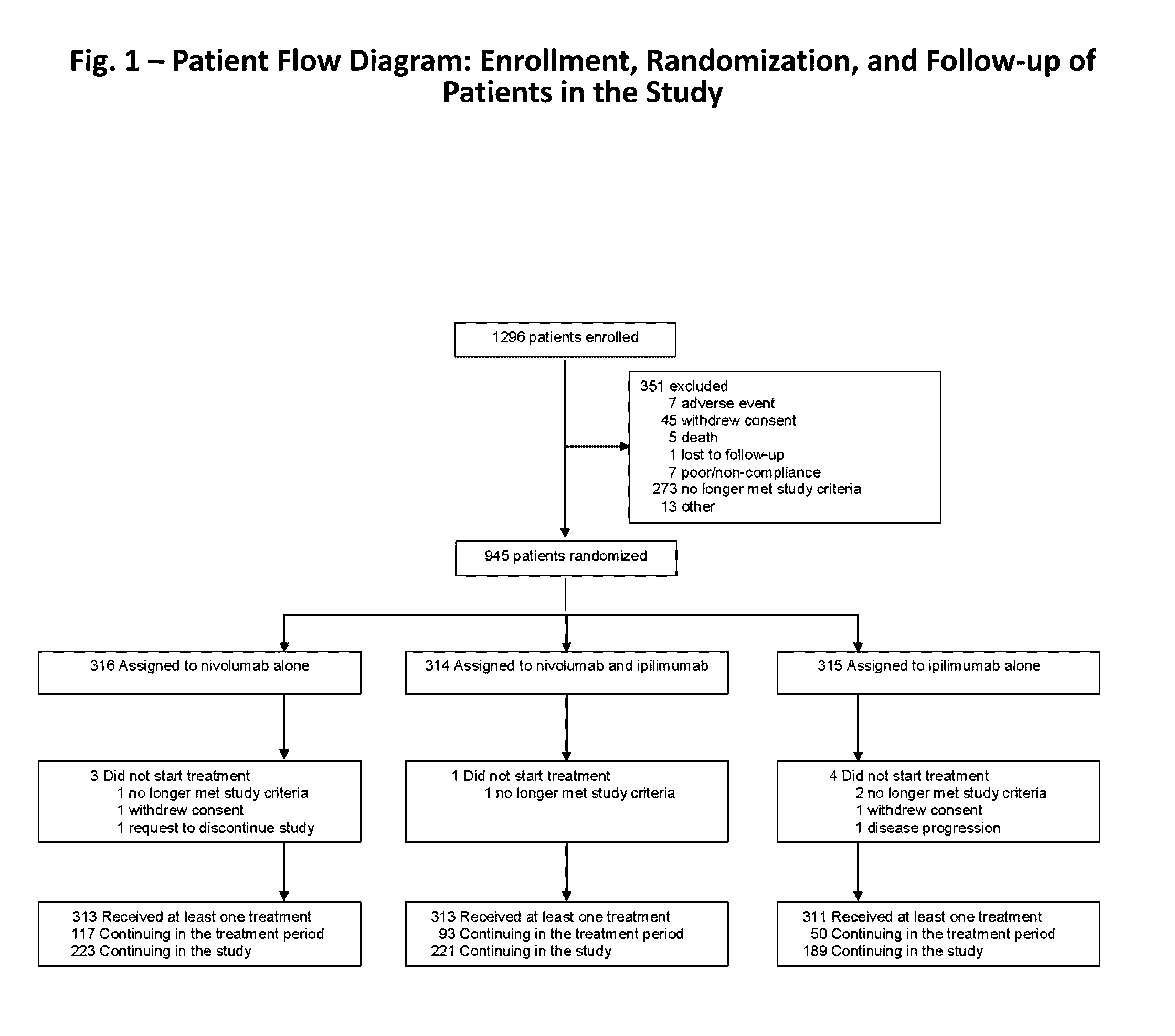
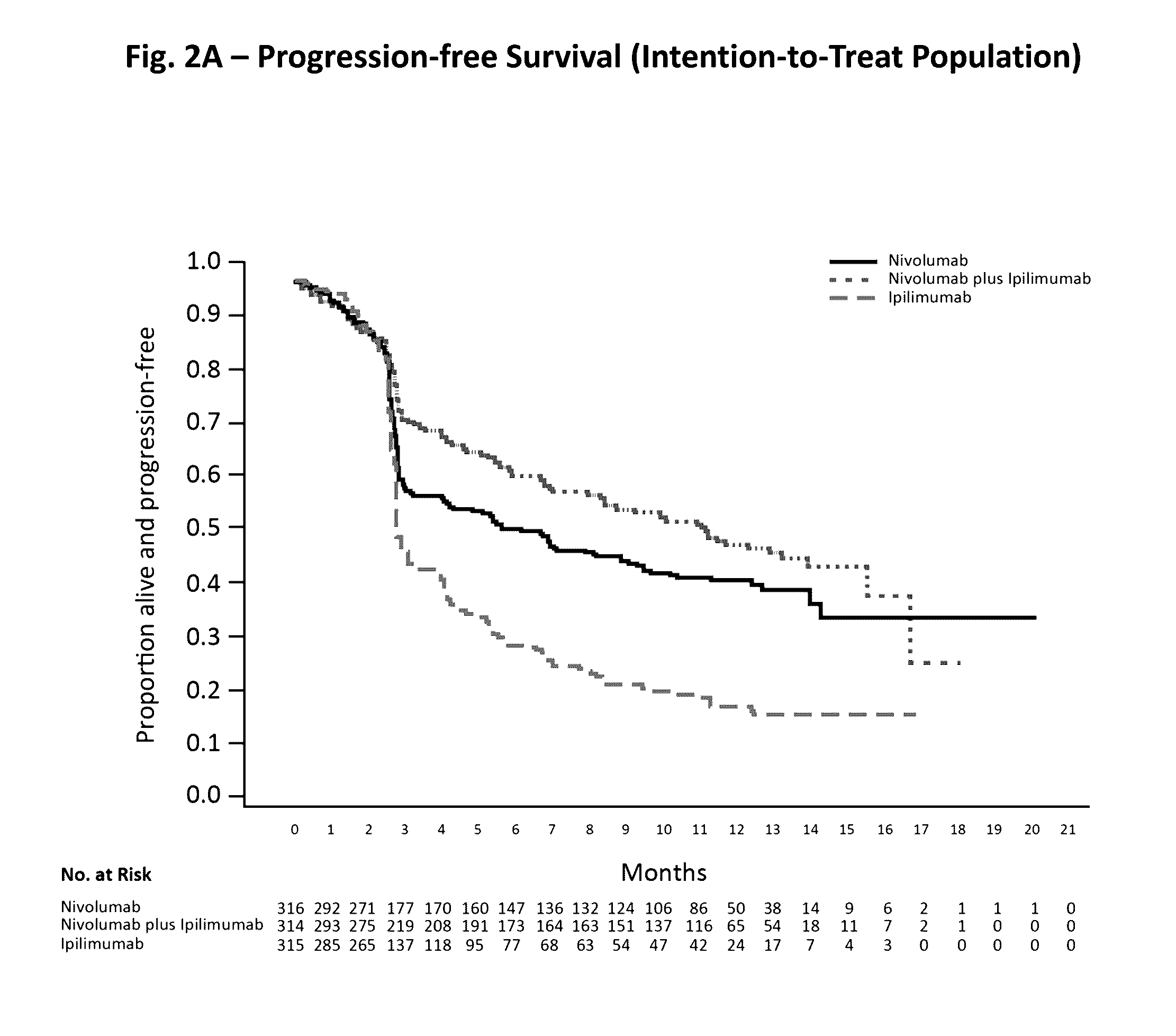
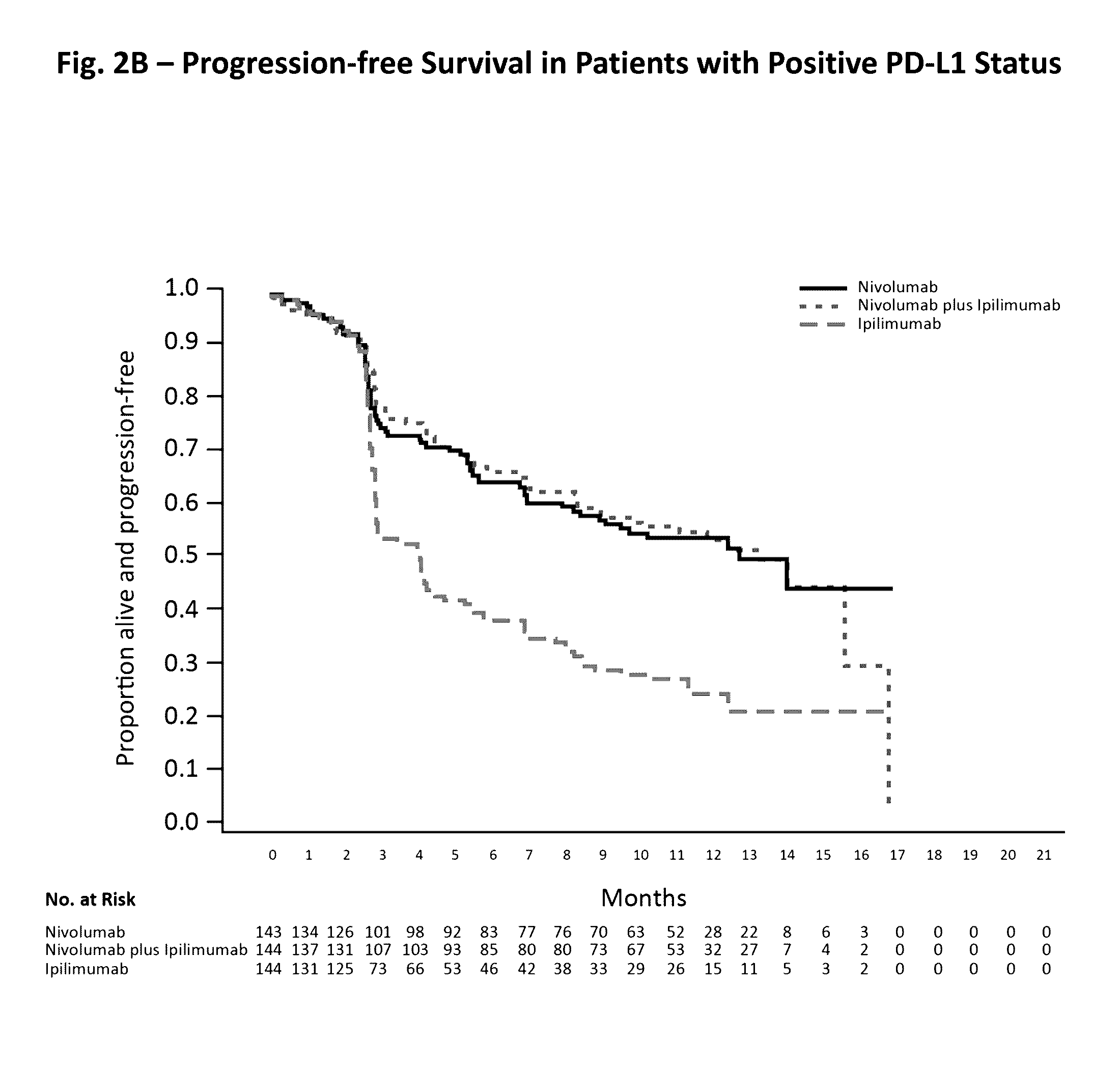
Treatment of PD-L1-Negative Melanoma Using an Anti-PD-1 Antibody and an Anti-CTLA-4 Antibody
ActiveUS20160340428A1Shrink tumorIncreasing objective response rateBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsProgression-free survivalMelanoma
The invention provides a method of treating a melanoma comprising (i) identifying a patient having a PD-L1-negative melanoma and (ii) administering to the patient a combination of an anti-PD-1 antibody or an antigen-binding portion thereof and an anti-CTLA-4 antibody or an antigen-binding portion thereof. The methods of the invention can extend progression-free survival for over 8 months and / or reduces the tumor size at least about 10%, about 20%, about 30%, about 40%, or about 50% compared to the tumor size prior to the administration.
Owner:BRISTOL MYERS SQUIBB CO
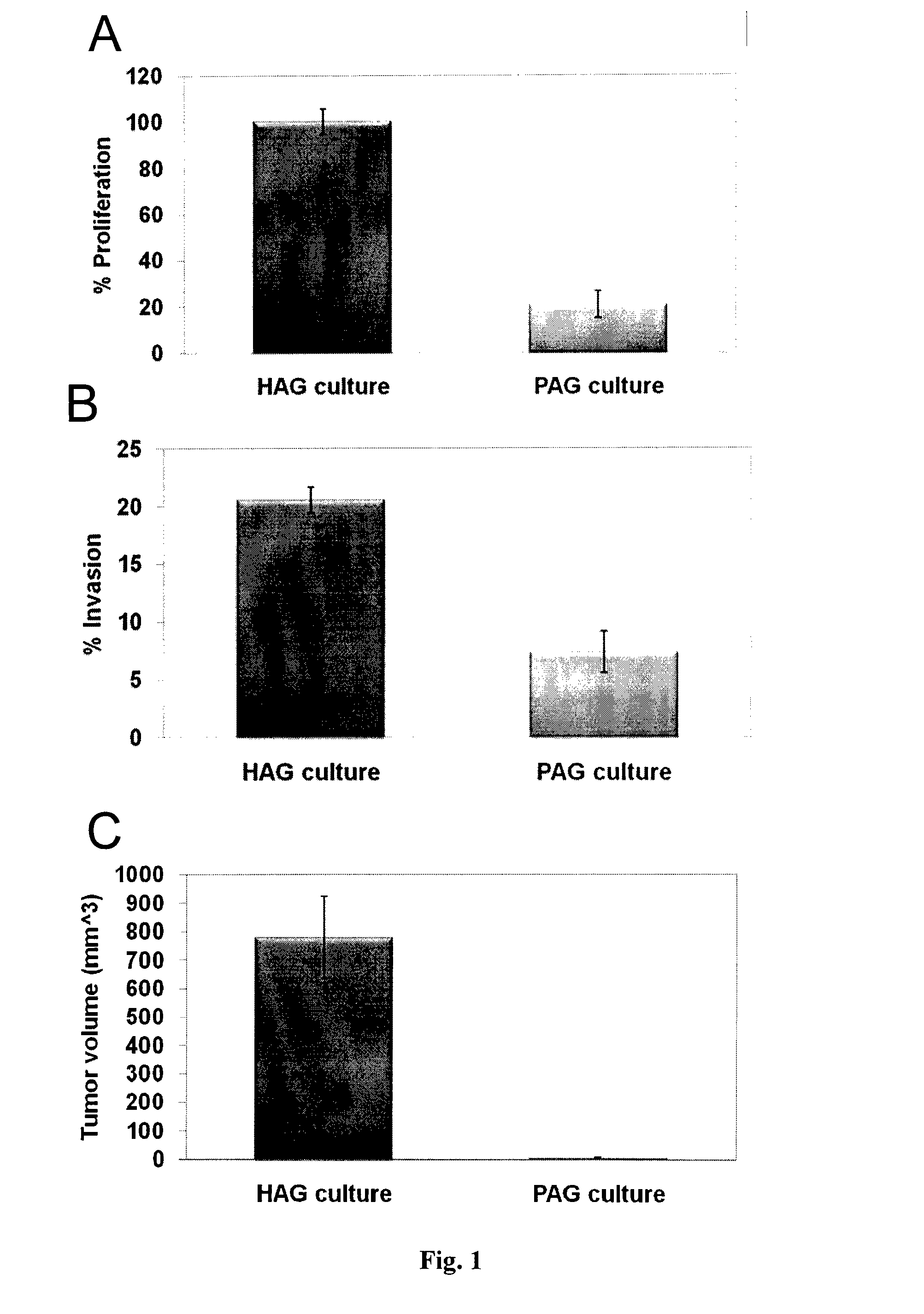
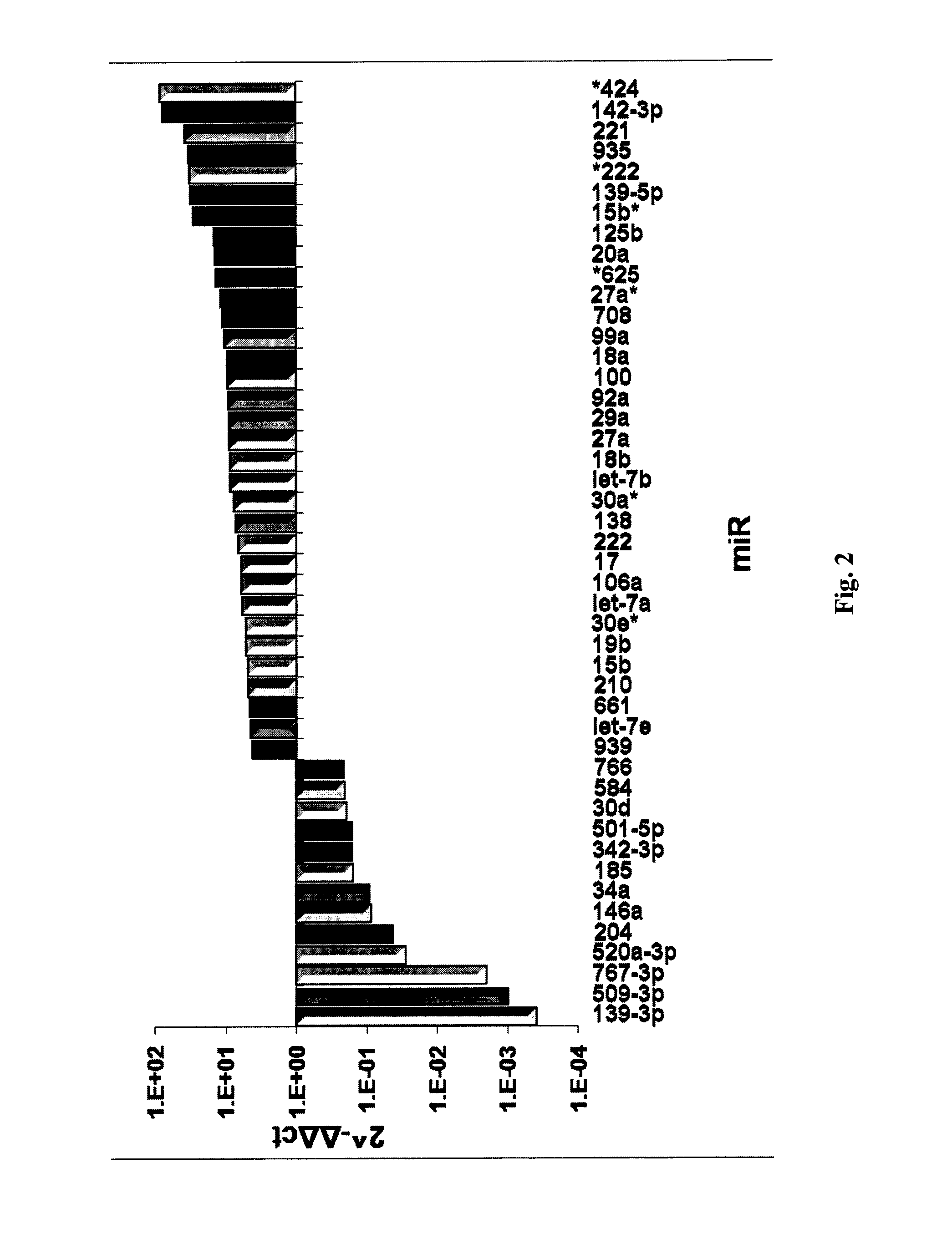
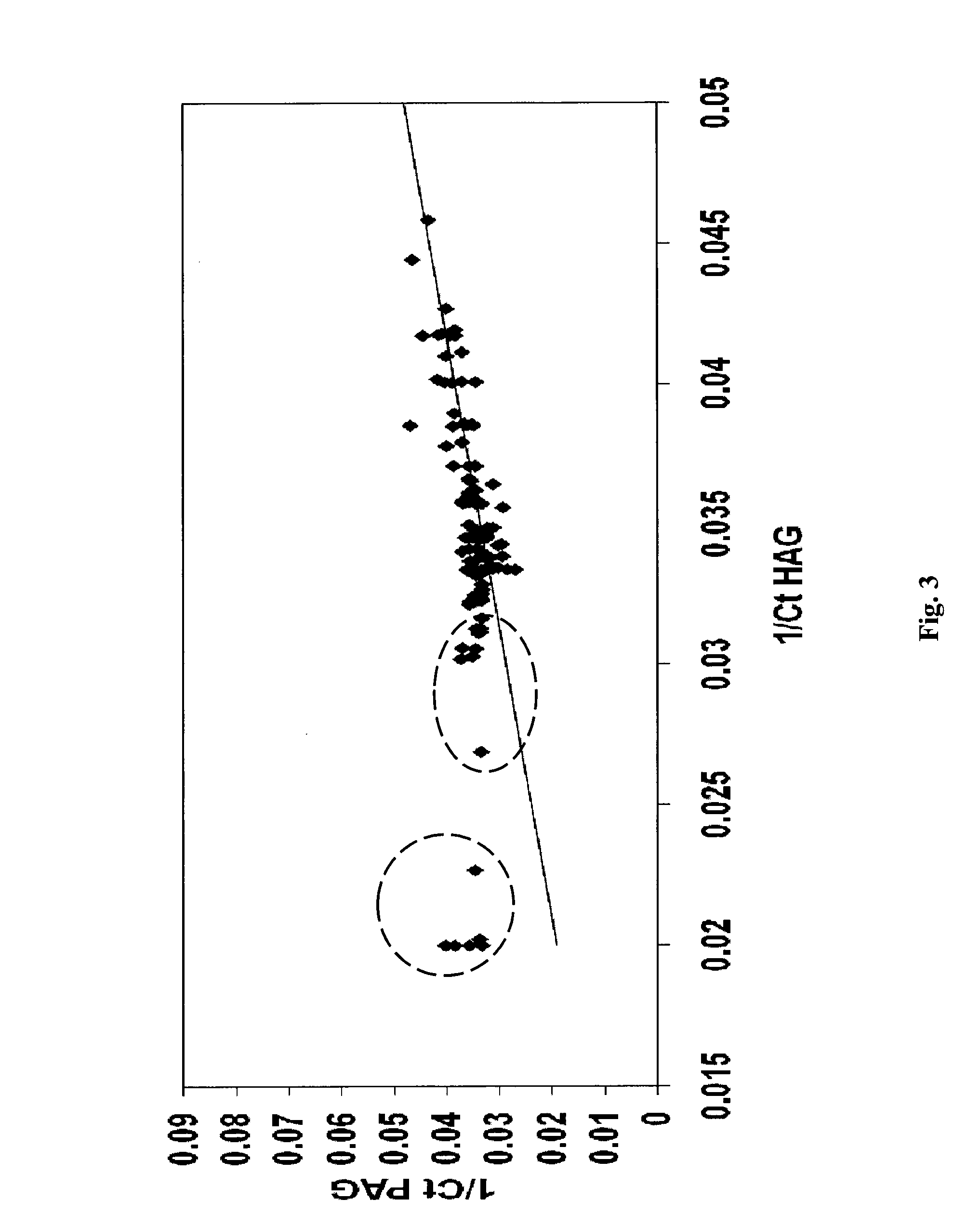
Microrna patterns for the diagnosis, prognosis and treatment of melanoma
InactiveUS20130197060A1Easy to manageReduce exposureOrganic active ingredientsMicrobiological testing/measurementStage melanomaMicroRNA
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES +1
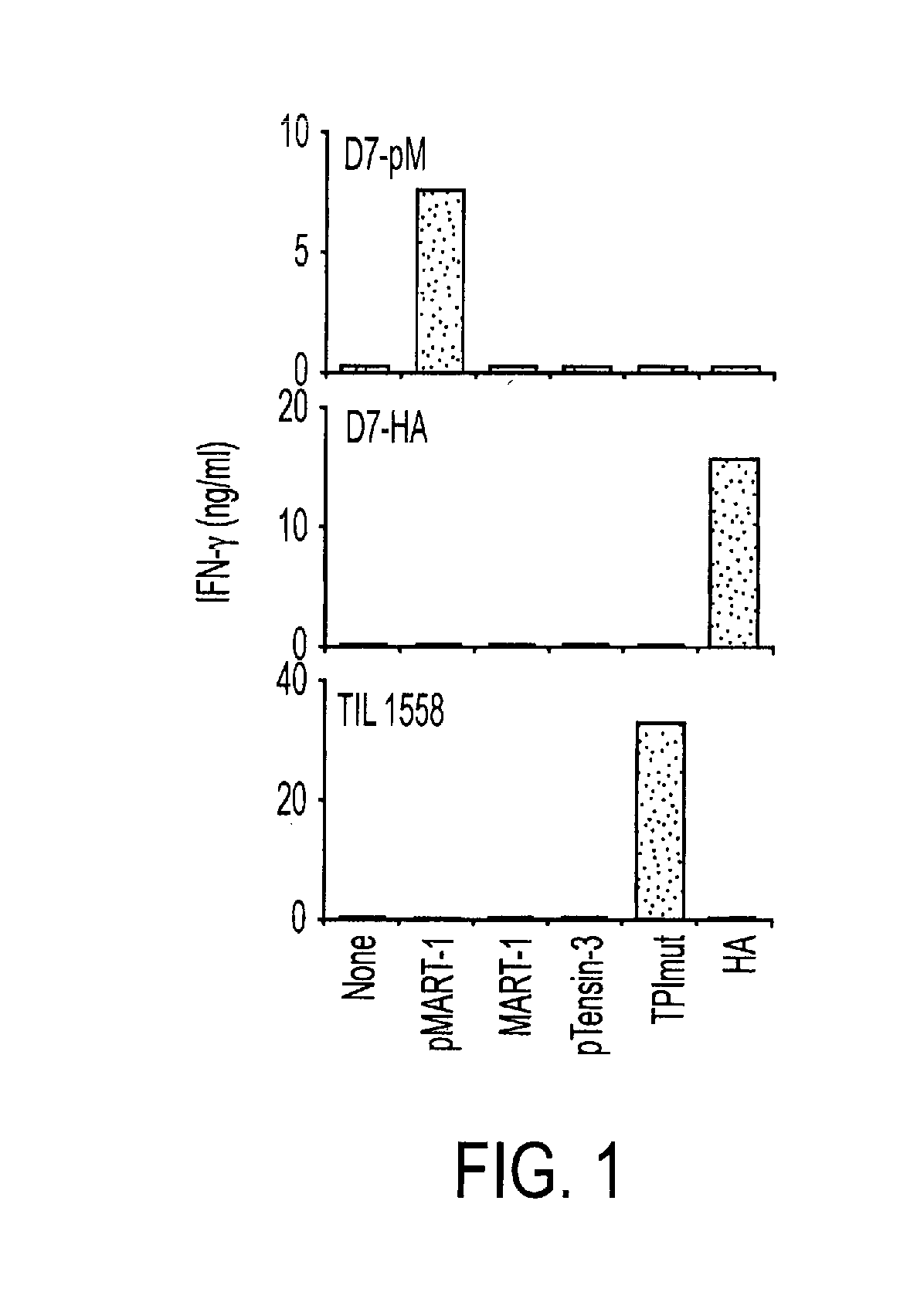
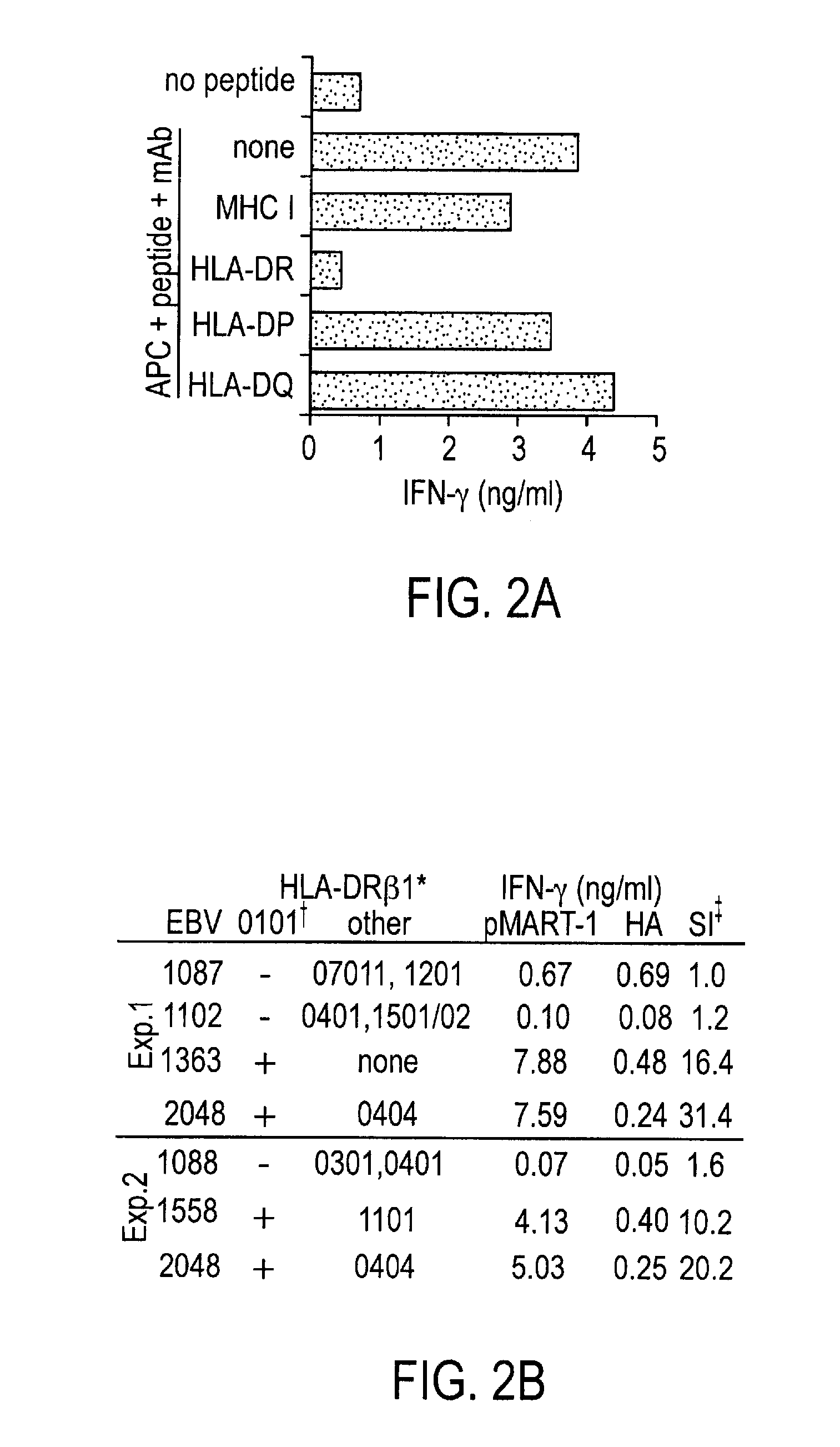
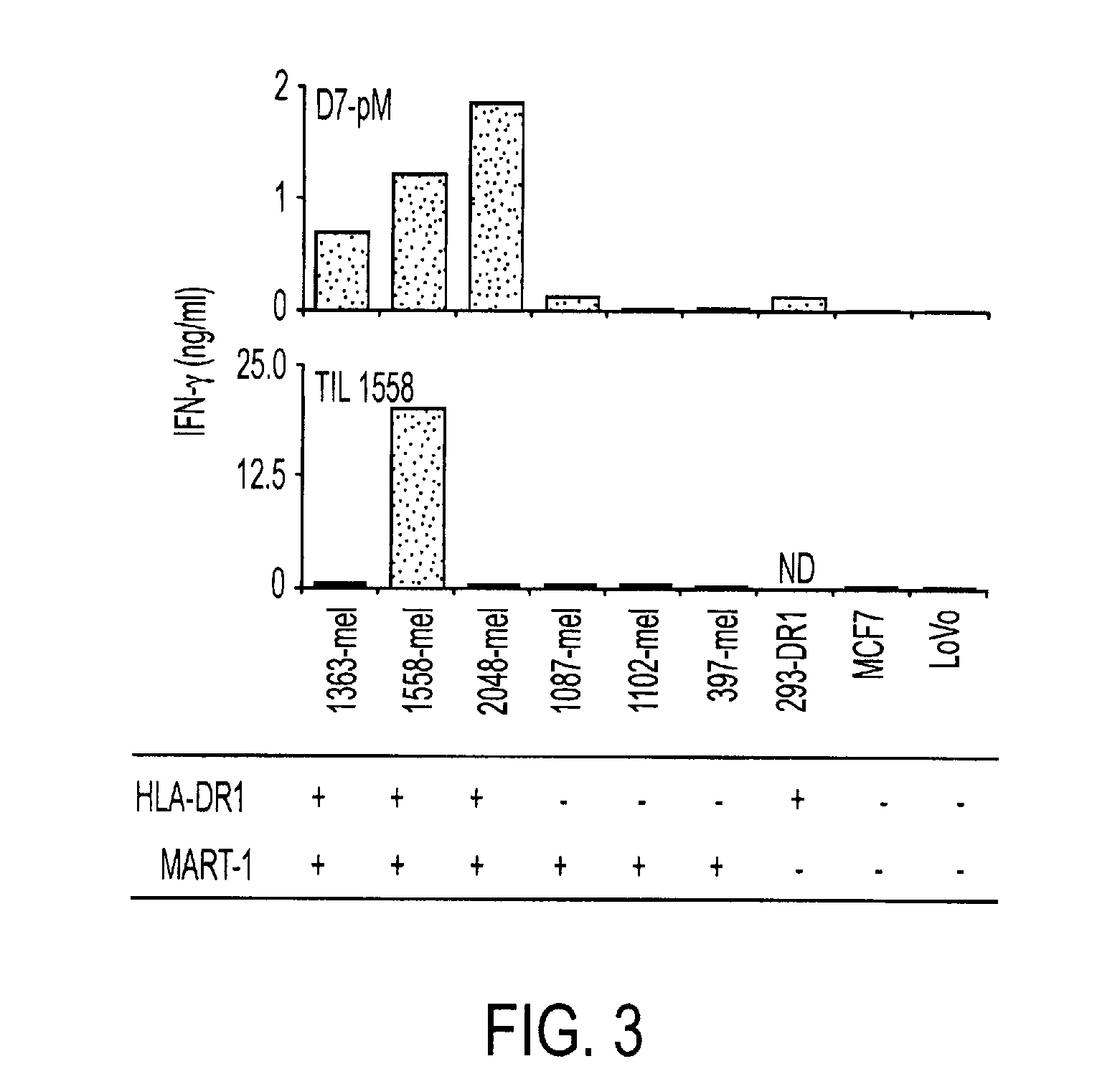
Phosphopeptides as melanoma vaccines
ActiveUS20120177669A1Reduce riskTumor rejection antigen precursorsPeptide/protein ingredientsAntigenLymphatic Spread
We characterized a total of 175 HLA-DR-associated phosphopeptides using sequential affinity isolation, biochemical enrichment, mass spectrometric sequencing and comparative analysis. Many were derived from source proteins which may have roles in cancer development, growth and metastasis. Most were expressed exclusively by either melanomas or transformed B cells, suggesting the potential to define cell type-specific phosphatome “fingerprints”. We generated HLA-DRβ1*0101-restricted CD4+ T cells specific for a phospho-MART-1 peptide identified in two melanoma cell lines. These T cells showed specificity for phosphopeptide-pulsed antigen presenting cells as well as for intact melanoma cells. MHC II-restricted phosphopeptides recognizable by human CD4+ T cells are potential targets for cancer immunotherapy.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
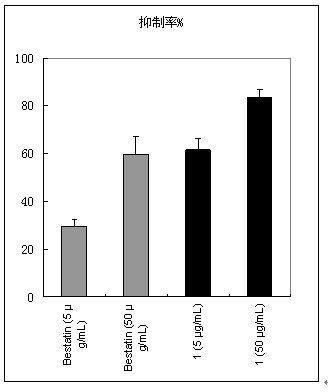
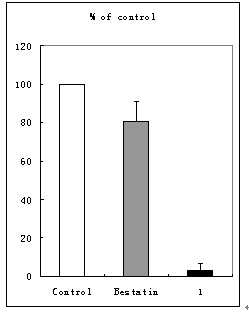
Aminopeptidase N inhibitor, preparation method and application
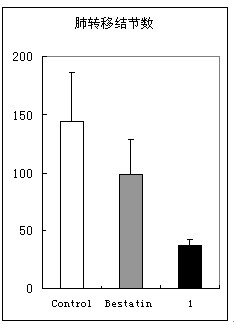
The invention belongs to the technical field of drugs, and particularly relates to aminopeptidase N inhibitor, a preparation method and application to tumor resistance. The technical scheme includes that the aminopeptidase N inhibitor is chemically named as (S)-4-methyl-2-(3-naphthyl-1-ylmethyl-ureido)-valeryl group hydroxylamine. The constitutional formula (I) of the aminopeptidase N inhibitor is shown here. The pharmacodynamic effect of the aminopeptidase N inhibitor is evaluated by in-vitro APN (adiponectin) inhibitory experiments, in-vitro resistance to tumor cell invasion, antiangiogenesis and mouse in-vivo resistance to melanoma manual transfer and antiangiogenesis experiments.
Owner:WEIFANG BOCHUANG INT ACAD OF BIOTECH & MEDICINE
Inhibitors of cyclin-dependent kinase 7 (CDK7)
ActiveUS20170183355A1Improve efficacyPrevent relapseOrganic active ingredientsOrganic chemistryAutoimmune conditionStage melanoma
The present invention provides novel compounds of Formula (I) and Formula (II) and pharmaceutically acceptable salts, solvates, hydrates, tautomers, stereoisomers, isotopically labeled derivatives, and compositions thereof. Also provided are methods and kits involving the compounds or compositions for treating or preventing proliferative diseases (e.g., cancers (e.g., leukemia, melanoma, multiple myeloma), benign neoplasms, angiogenesis, inflammatory diseases, autoinflammatory diseases, and autoimmune diseases) in a subject. Treatment of a subject with a proliferative disease using a compound or composition of the invention may inhibit the aberrant activity of cyclin-dependent kinase 7 (CDK7), and therefore, induce cellular apoptosis and / or inhibit transcription in the subject.
Owner:SYROS PHARMACEUTICALIS INC
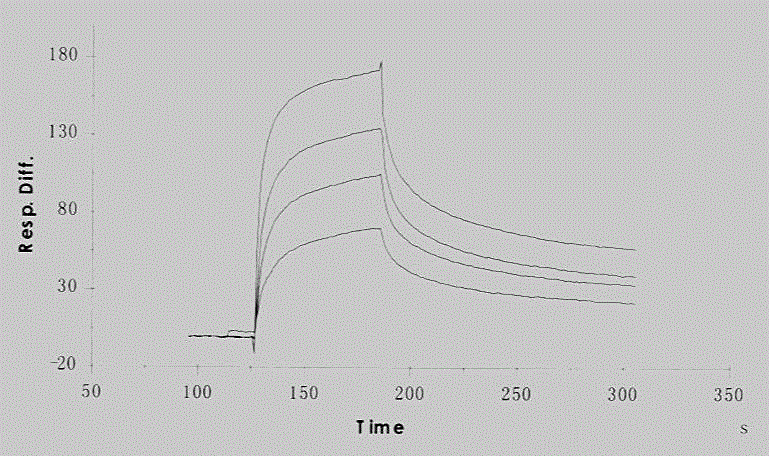
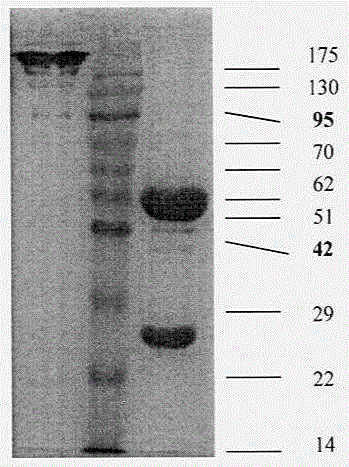
Anti-phosphatidylinositol proteoglycan 3 complete humanized antibody
InactiveCN105037540AImmunoglobulins against animals/humansAntibody ingredientsPhage antibodiesAntigen
The invention discloses a complete humanized antibody which is selected from humanized high-capacity phage antibody library and is high-affinity-combined with phosphatidylinositol proteoglycan 3. The invention includes a selection method of the antibody, an antibody coding sequence and corresponding amino acid residue sequence, and especially includes three CDR-zone sequences respectively at a heavy chain and a light chain, combination characters of an antigen and the construction method of the complete antibody. The antibody is a complete humanized antibody, is used for treatment in human body, is low in immunogenicity, is less in toxic and side effects, and has a potential value of treating liver cancer and melanin tumor.
Owner:BEIJING BIYANG BIOTECH
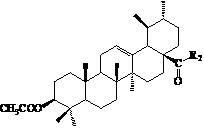
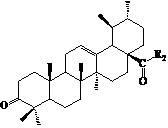
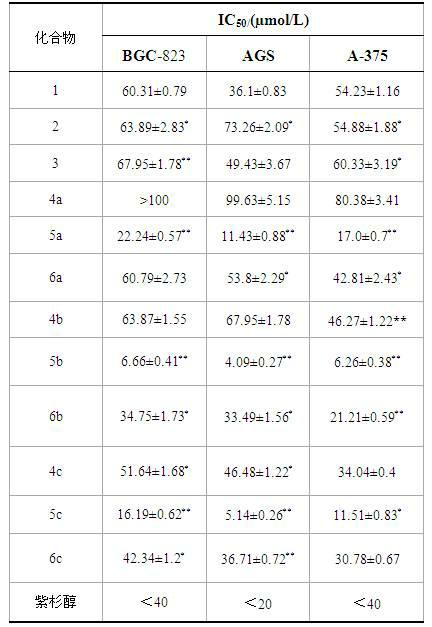
Ursolic acid nitrogen heterocyclic ring structure modifiers with antitumor activity and preparation method for ursolic acid nitrogen heterocyclic ring structure modifiers
InactiveCN102675406AGood antitumor effect in vitroProliferative Inhibitory Effect in VitroOrganic active ingredientsSteroidsCancer cellStage melanoma
The invention relates to the transformation of a chemical structure of a natural medicinal plant, namely ursolic acid, in particular to ursolic acid nitrogen heterocyclic ring structure modifiers with antitumor activity and a preparation method for the ursolic acid nitrogen heterocyclic ring structure modifiers. The ursolic acid nitrogen heterocyclic ring structure modifiers are ursolic acid nitrogen heterocyclic ring derivatives shown as a general formula (I). The preparation method comprises the following steps of: oxidizing or acetylating a C-3 hydroxyl group of the ursolic acid, performing condensation reaction of a C-17 carboxyl group and piperazine or a derivative of the piperazine so as to achieve the structural transformation of the ursolic acid, and thus obtaining a series of ursolic acid nitrogen heterocyclic ring structure modifiers. The in-vitro inhibitory activities of the ursolic acid nitrogen heterocyclic ring structure modifiers on human malignant melanoma A-375 cells, human gastric carcinoma AGS and BGC-823 cells are determined; and methyl thiazolyl tetrazolium (MTT) data show that the modifiers have a certain effect of inhibiting the proliferation of three kinds of cancer cells, and the inhibitory activity of partial compounds is even superior to that of paclitaxel.
Owner:FUZHOU UNIV
Proteolysis targeting chimeric body, prodrug molecule for improving oral bioavailability of protein hydrolysis targeting chimeric body and application thereof
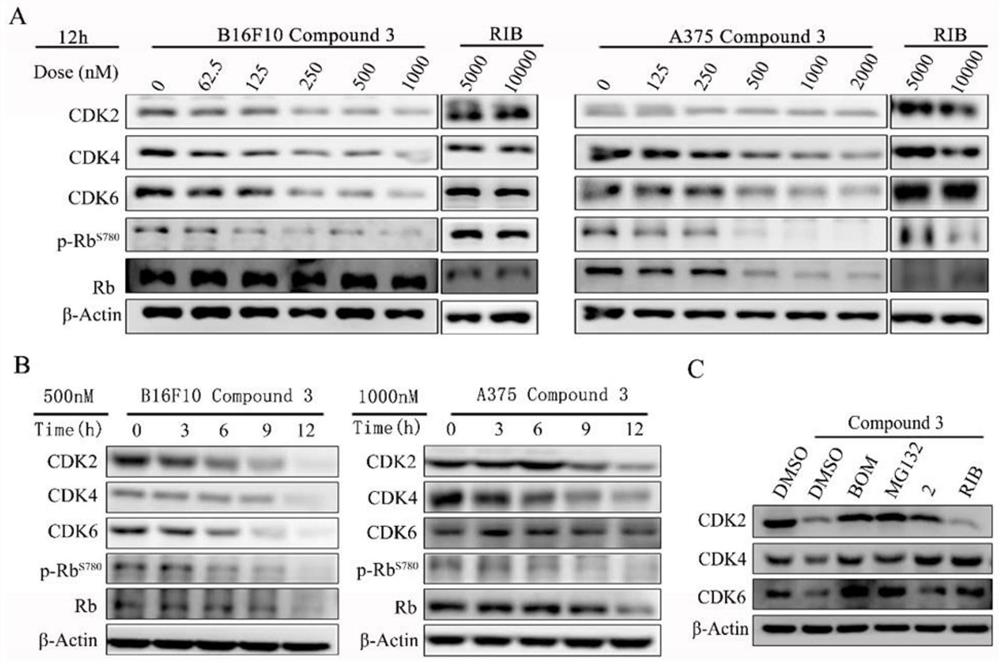
ActiveCN111909155ASignificant induction periodSignificantly induce apoptosisOrganic chemistryAntineoplastic agentsStage melanomaCancer cell
The invention provides a proteolytic targeting chimeric body, a prodrug molecule for improving oral bioavailability of the proteolytic targeting chimeric body and application thereof. According to thetechnical scheme, a novel PROTAC degradation agent compound is developed on the basis of a ribociclib derivative and a CRBN ligand. Small molecules can effectively degrade CDK2 / 4 / 6 and compounds thereof in malignant melanoma at the same time; the cell cycle can be quickly reset, and apoptosis of various cancer cells, especially melanoma cells, can be induced. A mechanism should be explained as that CDK 2 / 4 / 6 deficiency may lead to synthetic lethal effects of malignant melanoma in the presence of a compound. The results show that the combination of CDK2 / 4 / 6 is expected to become a kinase target for treating solid tumors. In addition, the invention also develops a prodrug with high oral bioavailability for the first time, and is convenient for oral administration in animal tests. A universal solution is provided for oral administration of PROTAC molecules with CRBN ligands.
Owner:DONGGUAN UNIV OF TECH
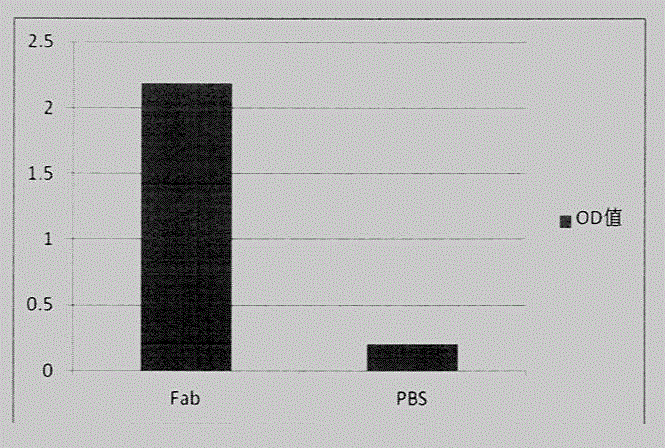
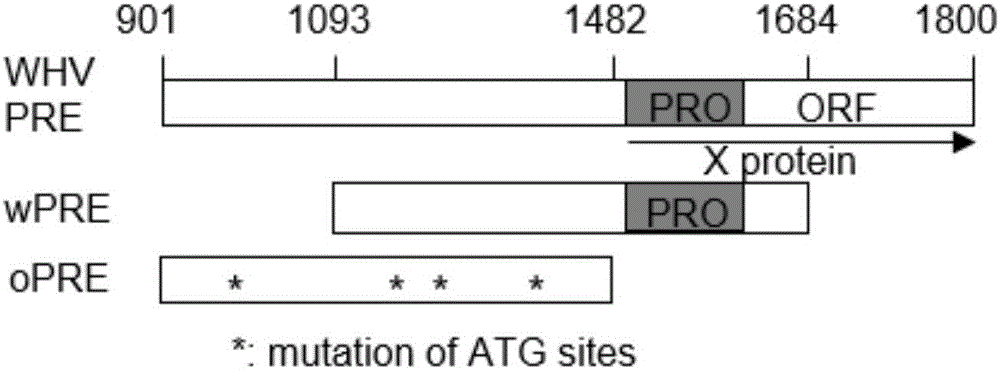
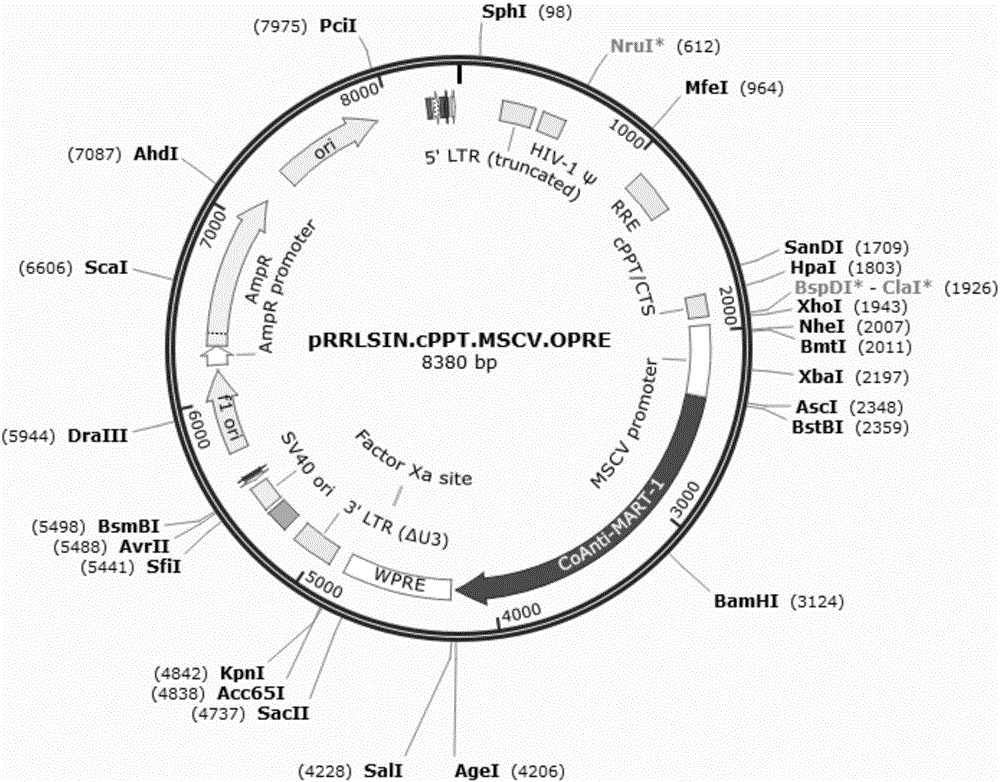
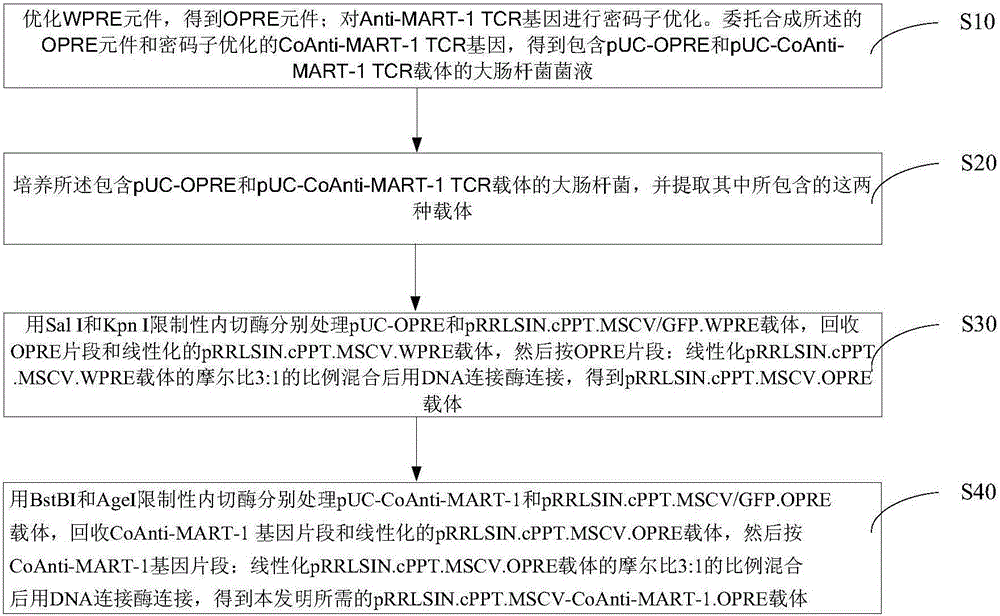
Preparation and application of safety-improved lentiviral vector for expressing codon-optimized Anti-MART-1 TCR gene
The invention discloses the preparation and application of a lentiviral vector that improves safety and expresses the codon-optimized Anti-MART-1 TCR gene. The safety of this vector is achieved by replacing the WPRE element of the original lentiviral vector with an OPRE element, the sequence of which is shown in SEQ ID No.: 1, and inserting codon-optimized Anti-MART into the vector's multiple cloning site -1 TCR nucleotide sequence, the sequence is shown in SEQ ID No.:2. The OPRE element has no significant impact on the transfection efficiency and packaging titer of lentivirus compared with WPRE; the homology between the codon-optimized Anti-MART-1 TCR gene and the wild-type gene is 79%, and in T cell genes The expression level of Anti-MART-1 TCR can be significantly increased during modification; after T cells genetically modified to express Anti-MART-1 TCR are co-cultured with melanoma cell lines, their IFN-γ secretion levels are also significantly increased; this T cell The cells can also significantly extend the survival time of tumor-bearing mice implanted with melanoma and inhibit the growth of melanoma. The transformed pRRLSIN.cPPT.MSCV‑CoAnti‑MART‑1 / GFP.OPRE vector can be used for the construction of TCR‑T cells in cell therapy.
Owner:杨晶
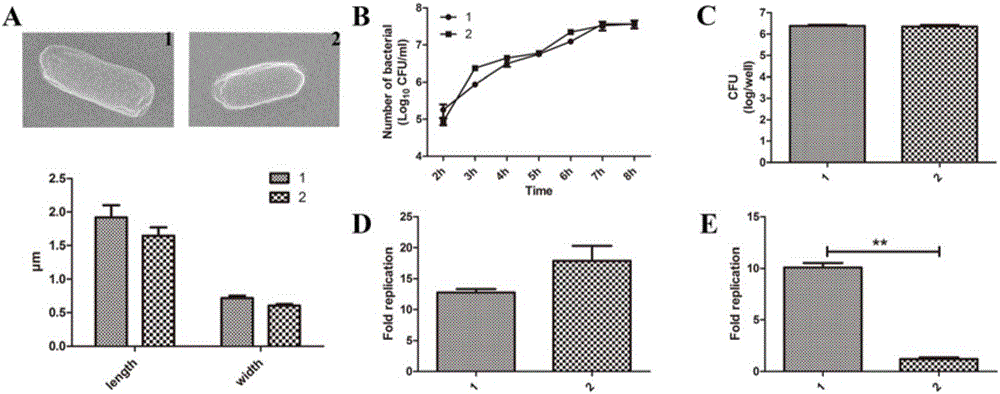
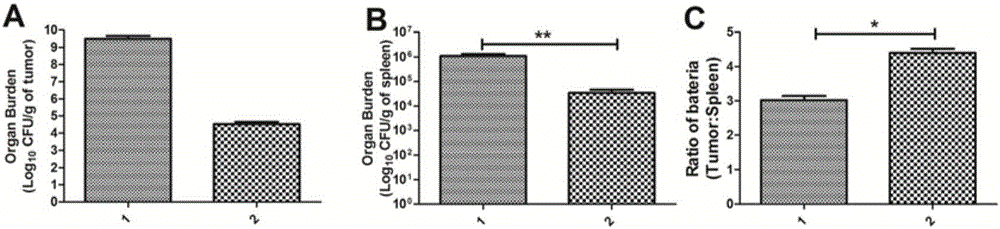
Gene deleted attenuated salmonella typhimurium mutant strain and construction method thereof and application of gene deleted attenuated salmonella typhimurium mutant strain in improvement of melanoma targeting performance
ActiveCN106434511ALower titerImprove tumor targetingBacteriaMicroorganism based processesSalmonellaMutant strain
The invention discloses an attenuated salmonella typhimurium mutant strain with STM3120 genes knocked out and application thereof. The attenuated salmonella typhimurium mutant strain is a polynucleotide fragment with attenuated salmonella typhimurium VNP20009 knocked out, the sequence of the polynucleotide fragment is SEQ ID NO:2, and the polynucleotide fragment is replaced with a polynucleotide fragment with the sequence of SEQ ID NO:3. Application of the attenuated salmonella typhimurium mutant strain with the STM3120 genes knocked out in targeted mouse melanoma is disclosed. The mouse melanoma targeted attenuated salmonella typhimurium mutant strain which is high in targeting performance, brand-new, safe, low in price and convenient to use and application thereof are provided.
Owner:NANJING UNIV
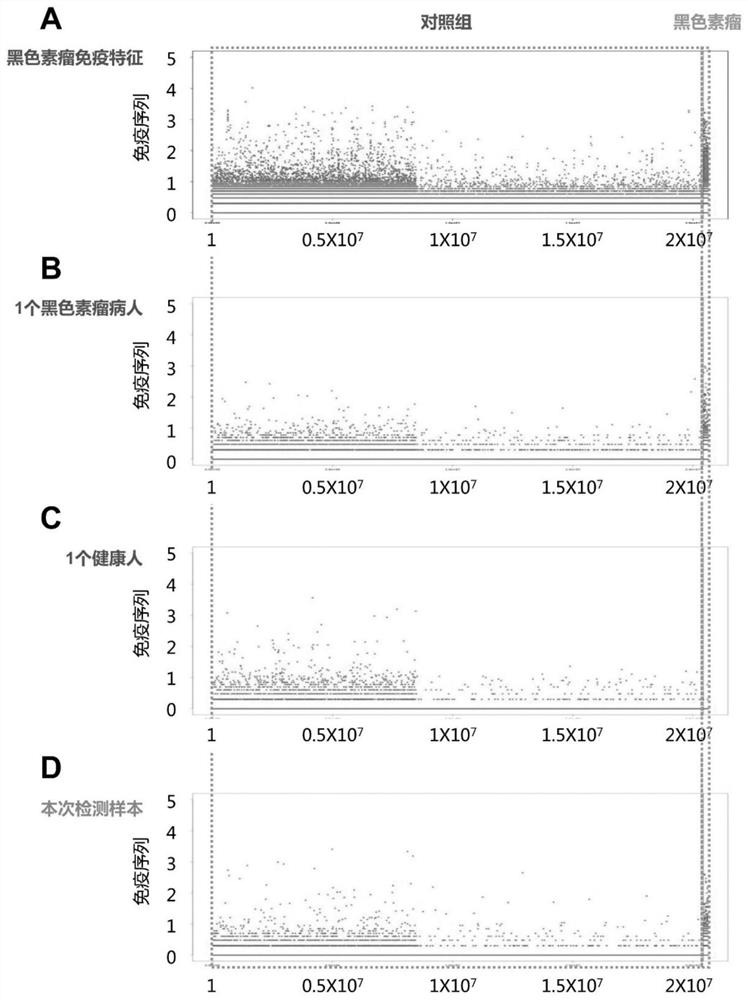
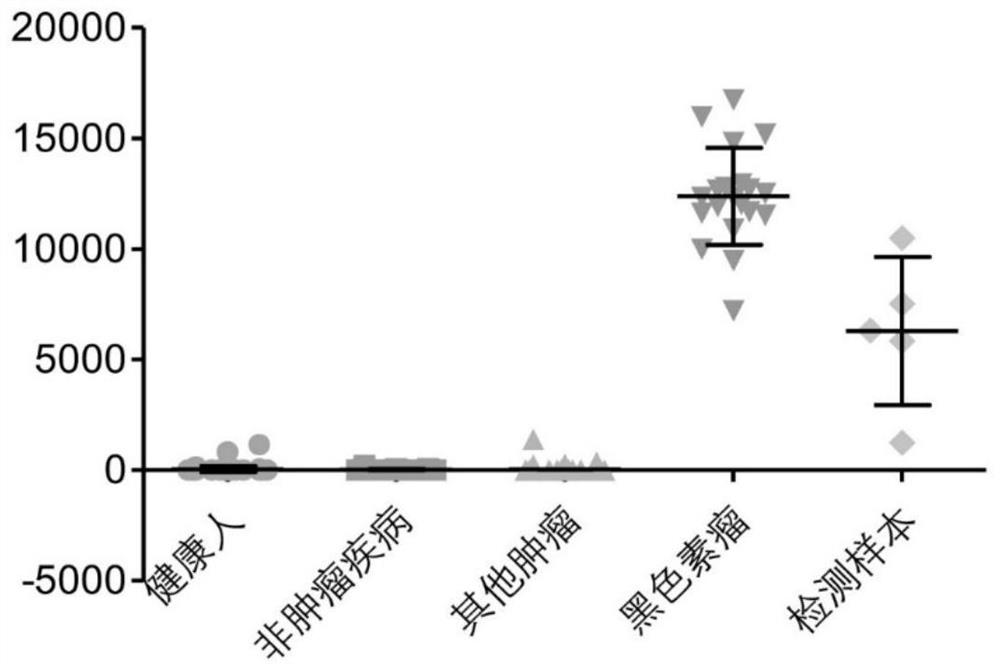
Peripheral blood TCR marker of melanoma as well as detection kit and application of peripheral blood TCR marker
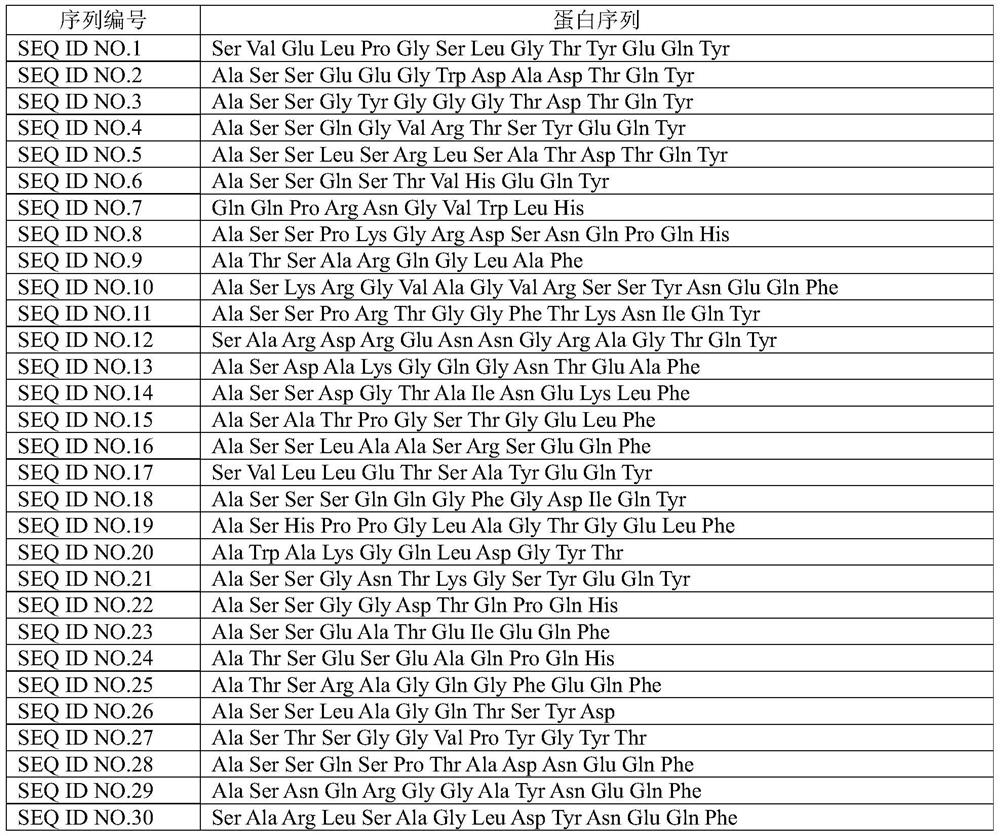
PendingCN111693702AImprove featuresImprove accuracyBiological material analysisAntibody medical ingredientsStage melanomaHigh throughput sequence
The invention discloses a peripheral blood TCR marker of melanoma as well as a detection kit and application of the peripheral blood TCR marker. The marker comprises at least one of proteins of whichthe sequences are shown as SEQ ID NO. 1-100. The sequence is based on a high-throughput sequencing method; only a small amount of peripheral blood needs to be collected; RNA (Ribonucleic Acid) is extracted, a sample is treated to establish an immune map library, high-throughput sequencing and TCR data analysis are carried out, a characteristic TCR sequence in peripheral blood of melanoma is determined, and then a test result of a sample to be tested is compared with the characteristic TCR sequence so as to determine whether the melanoma exists or not. According to the kit, a huge number of melanoma specific TCR sequences can be compared at the same time, and compared with single detection of one or more markers, the kit has higher specificity and accuracy, and the diagnosis efficiency is improved.
Owner:CHENGDU EXAB BIOTECH CO LTD
ACE (angiotensin converting enzyme) inhibition peptide with nutrition repairing and cell function activation and preparation method
InactiveCN110950925APowerfulSmall side effectsPeptide/protein ingredientsAntinoxious agentsDendritic cellStage melanoma
The invention provides an ACE (angiotensin converting enzyme) inhibition peptide with nutrition repairing and cell function activation and a preparation method. The peptide is a small-molecule peptidewhich is separated and extracted from black foods. It finds that the peptide self is an ACR (anti-hypertension) inhibitor, and experiment shows that the peptide has an activation function on TIDCs (tumorindiltrating dendritic cells) induced by melanoma cells, and has a repairing function on A549 cell injury caused by hydrogen peroxide; a medicine made of the small-molecule peptide has the characteristics of being remarkable in clinical effect and small in toxic and side effect; and the preparation method of the food small-molecule peptide extracted by the invention is simple and practical.
Owner:JILIN UNIV +1
Methods for treatment of melanoma
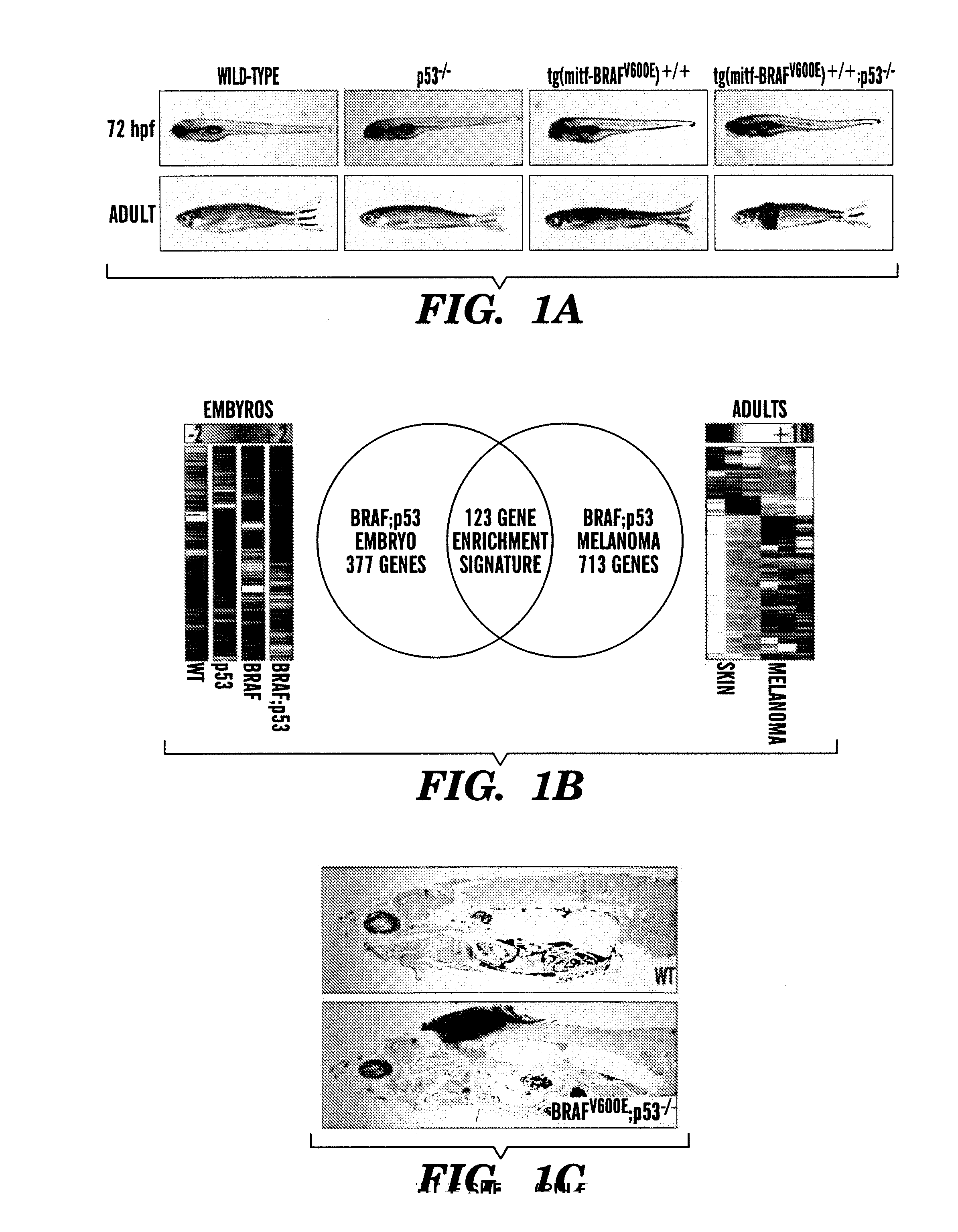
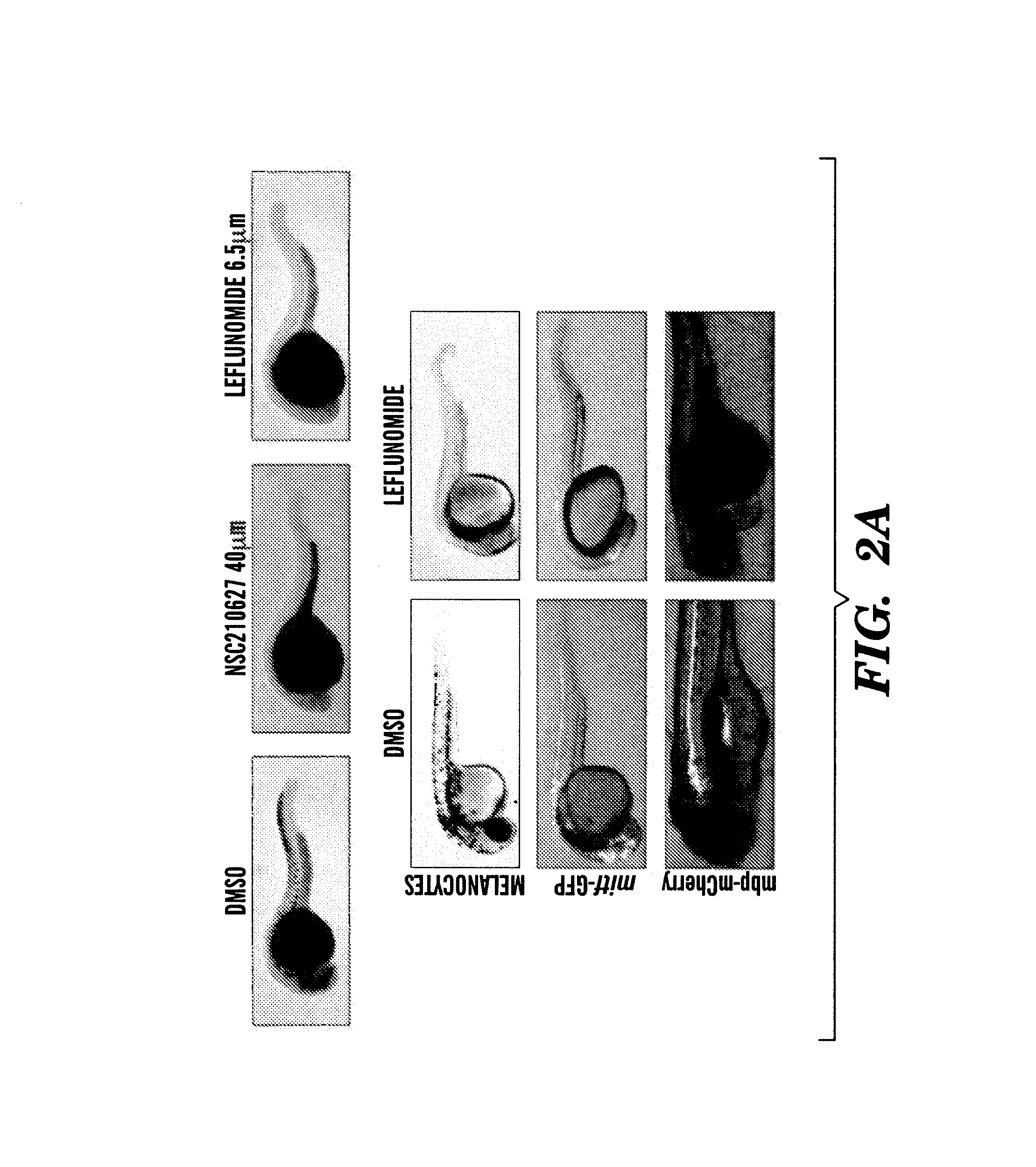
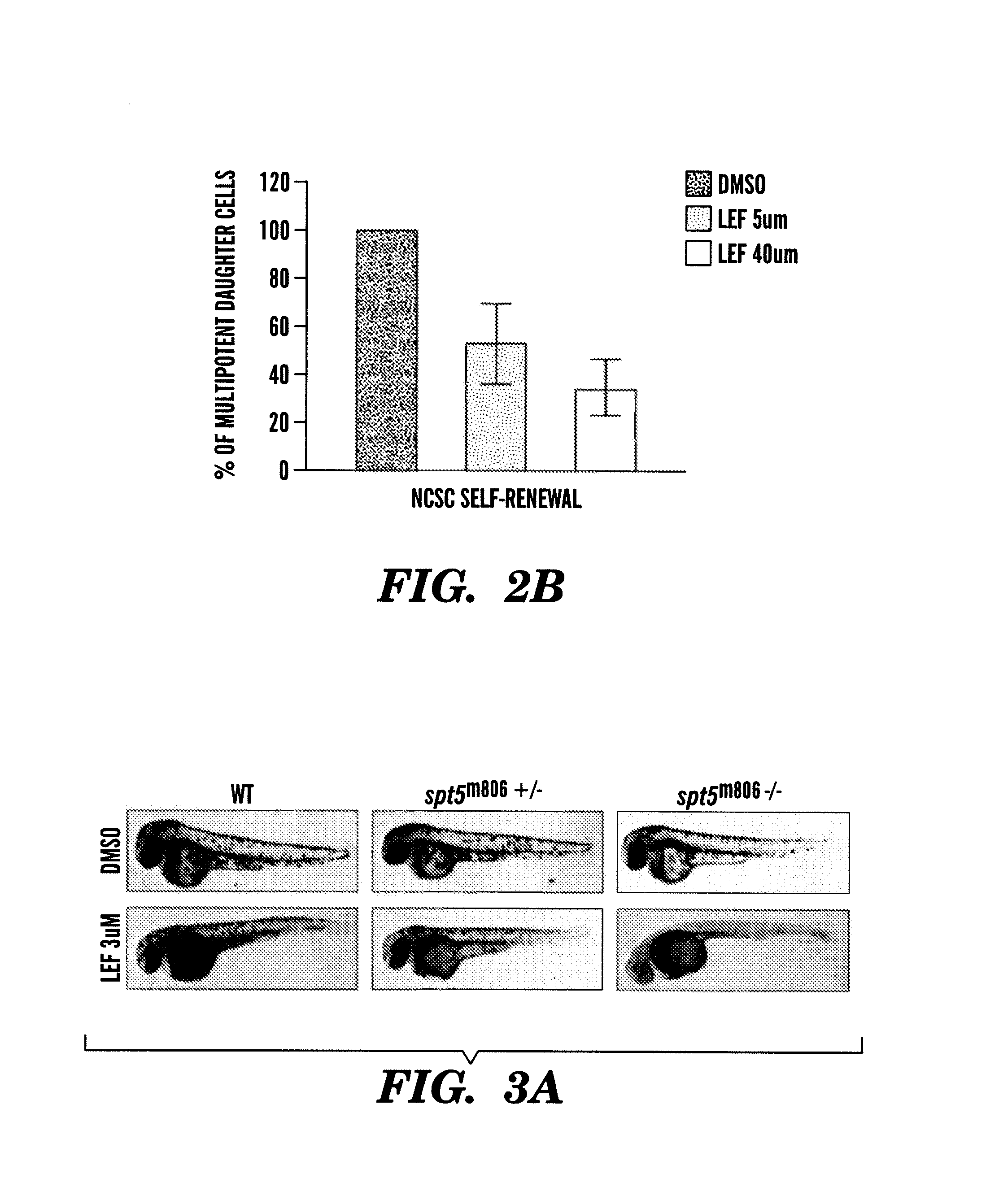
InactiveUS20140031383A1Utility in treatmentUseful in treatmentBiocideMicrobiological testing/measurementProgenitorNeural crest
The present invention is directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods herein are directed to screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
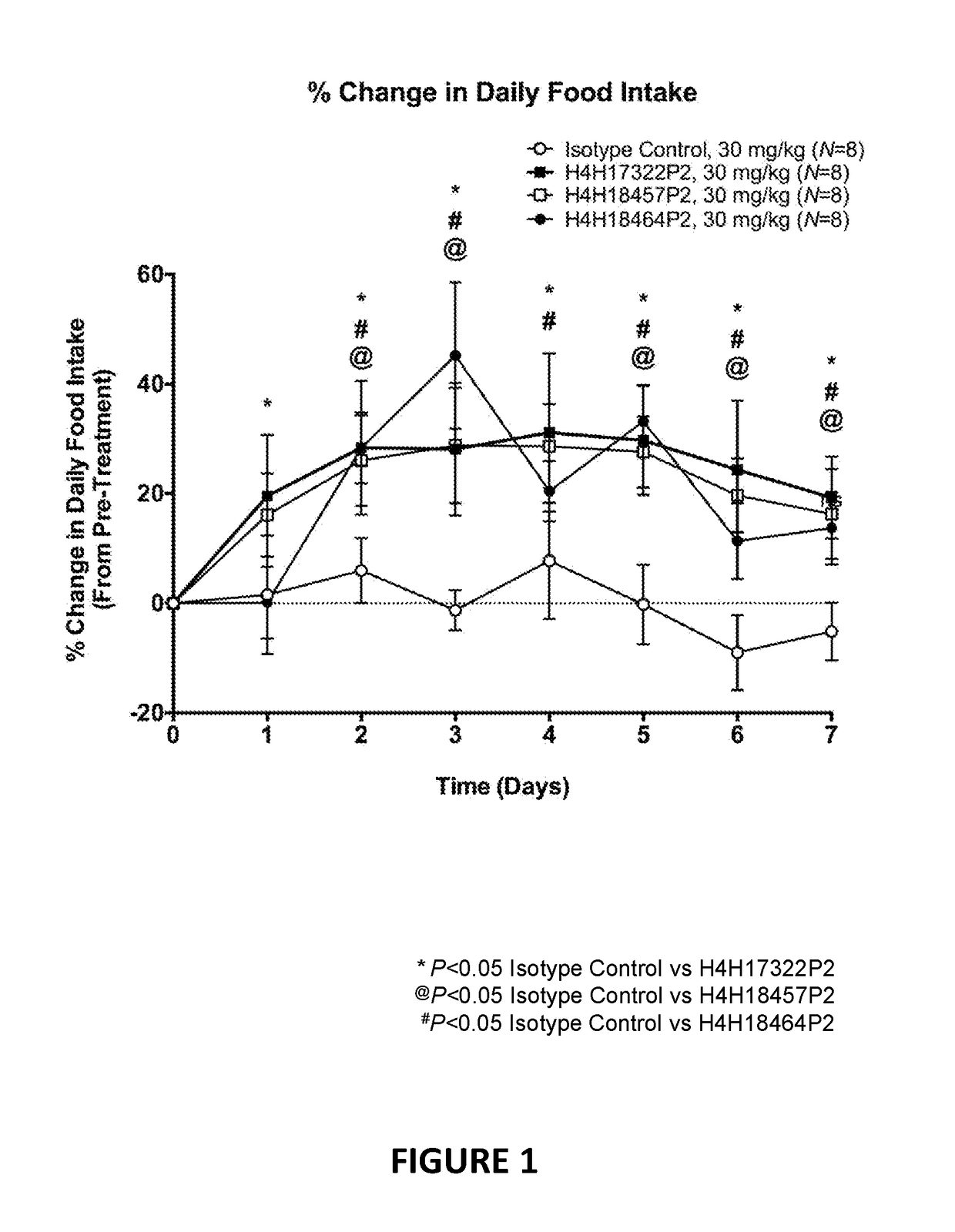
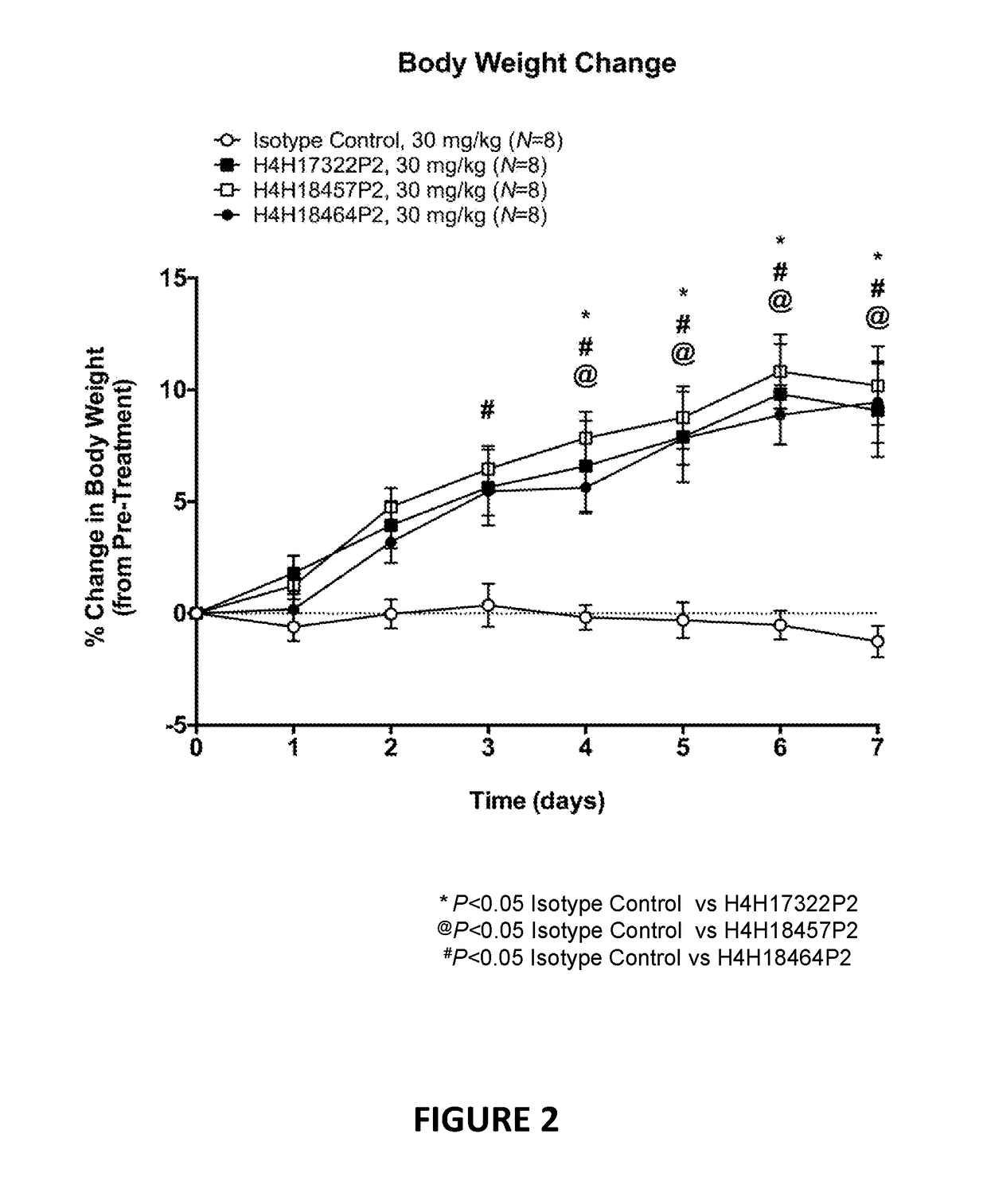
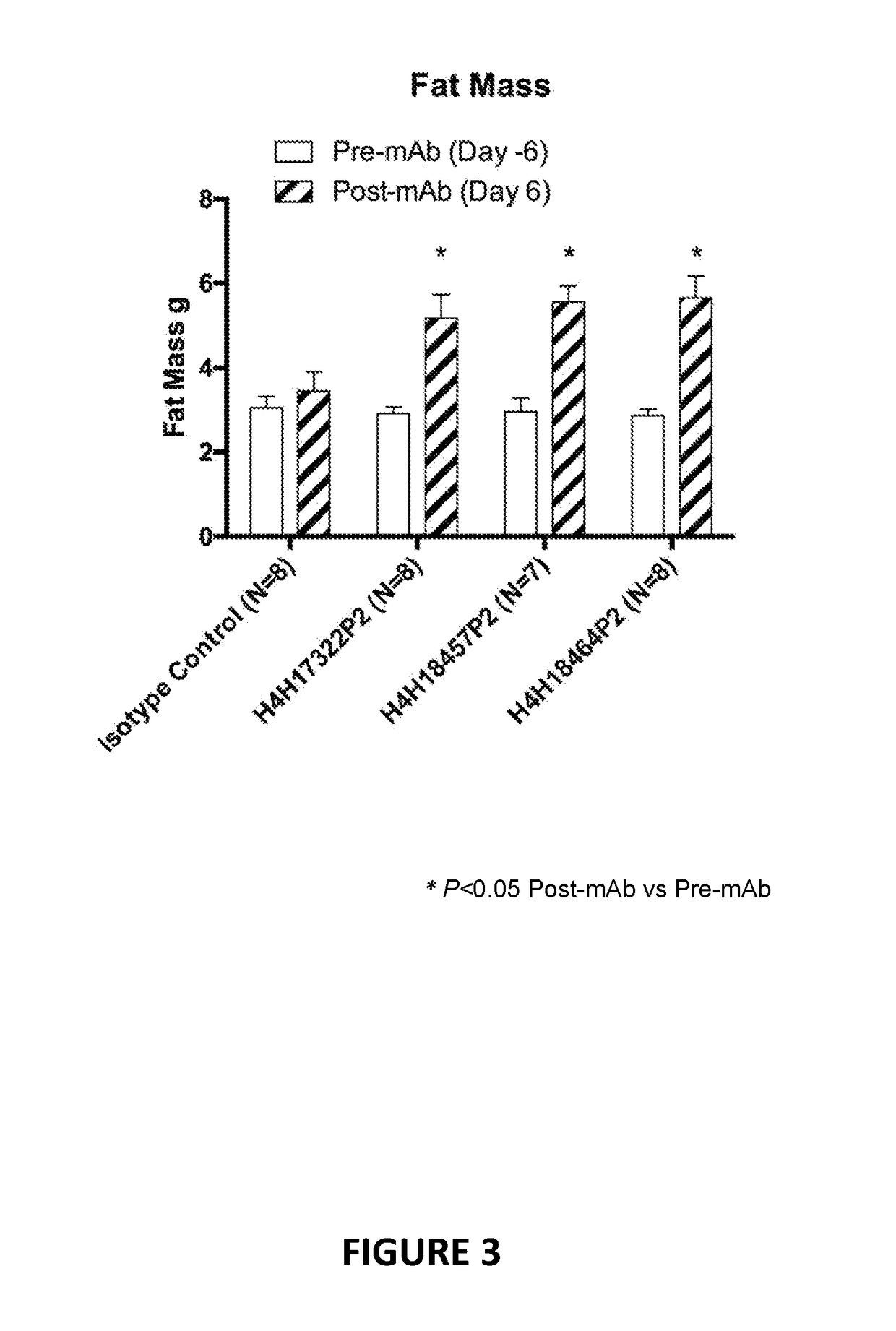
Antigen-binding proteins that antagonize leptin receptor
ActiveUS20180127508A1Weak partial agonist activityUseful in therapyMetabolism disorderImmunoglobulins against cell receptors/antigens/surface-determinantsMelanomaInflammatory Bowel Diseases
The present invention provides antibodies and antigen-binding fragments of antibodies that bind to leptin receptor (LEPR), and methods of using the same. According to certain embodiments, the invention includes antibodies and antigen-binding fragments of antibodies that bind LEPR and antagonize LEPR signaling. In certain embodiments, the invention includes antibodies and antigen-binding fragments of antibodies that bind LEPR in the presence or absence of leptin. In other embodiments, the invention includes antibodies and antigen-binding fragments of antibodies that exhibit partial agonism of LEPR signaling. The antibodies and antigen-binding fragments of the present invention are useful for the treatment of various conditions, including but not limited to congestive heart failure cachexia, pulmonary cachexia and cancer cachexia, autoimmune disorders such as inflammatory bowel disease, lupus erythematosus, multiple sclerosis, psoriasis, cardiovascular diseases, elevated blood pressure, neurodegenerative disorders, depression, cancer such as hepatocellular carcinoma, melanoma, breast cancer, and other diseases and disorders associated with or caused by elevated leptin signaling.
Owner:REGENERON PHARM INC
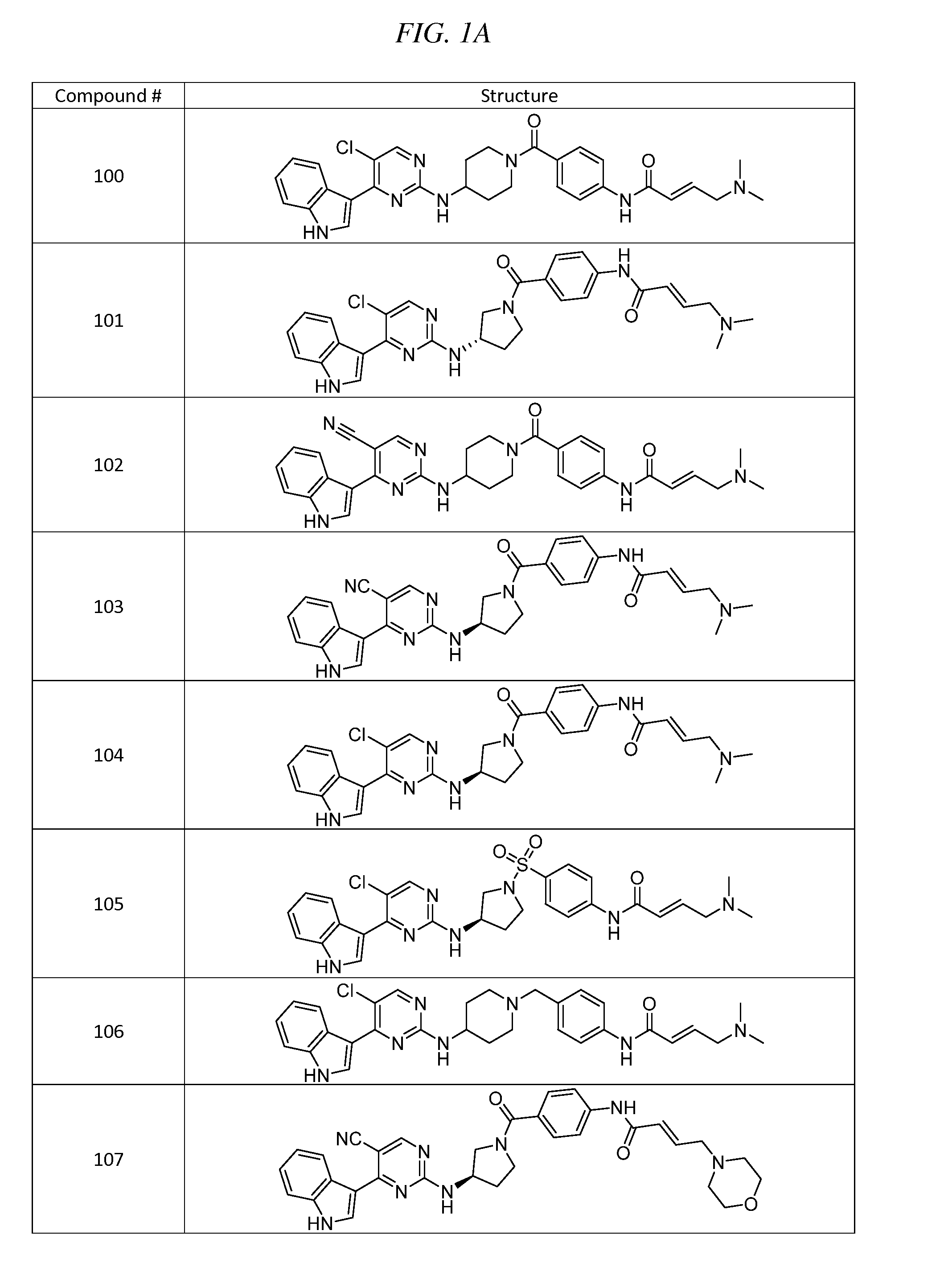
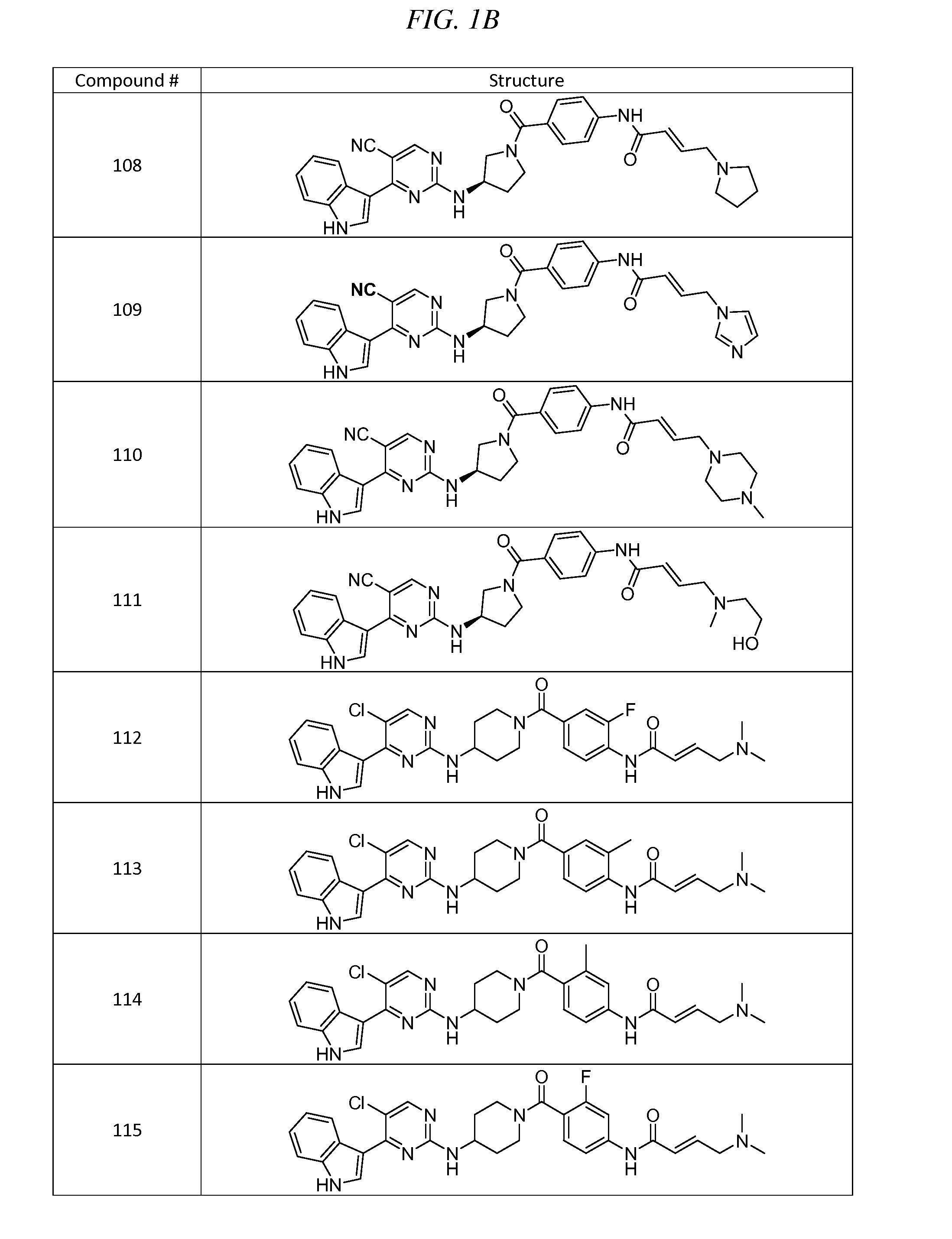
Acyl benzo[d]thiazol-2-amine and their methods of use
InactiveUS20190209532A1Increasing serotonin activityOrganic active ingredientsOrganic chemistryMelanomaNervous system
Disclosed are acyl benzo[d]thiazol-2-amines having a structure according to formula (I):including enantiomers, diastereomers, hydrates, solvates, pharmaceutically acceptable salts, and complexes thereof. The compounds may be useful for the treatment of various diseases including, for example, neurological disorders such as amyotrophic lateral sclerosis, bipolar disorder, treatment resistant and major depression, general anxiety disorder, and cancers such as melanoma, breast cancer, brain cancer, and prostate cancer.
Owner:BIOHAVEN THERAPEUTICS LTD
E2f reporter melanoma cells
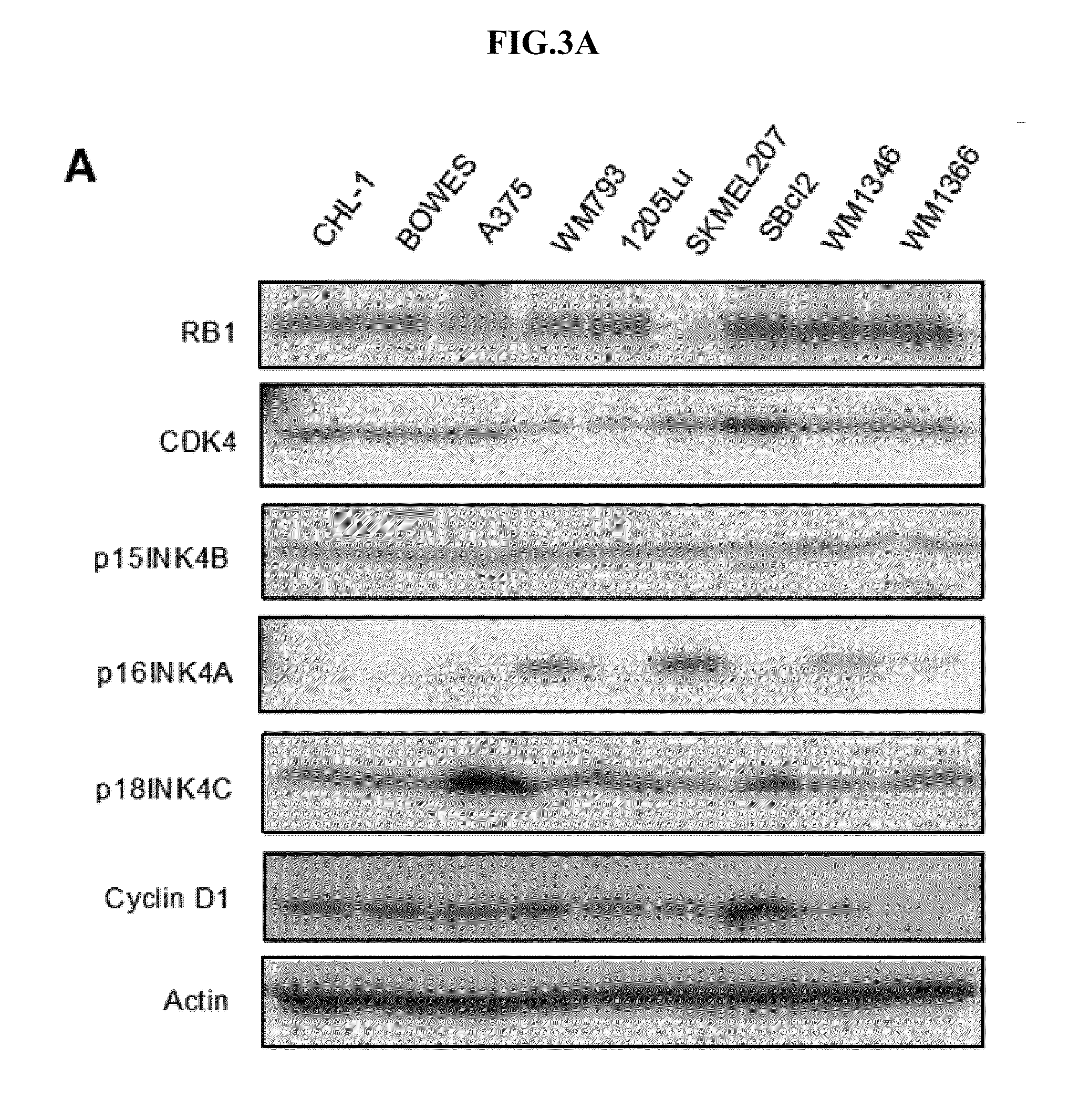
ActiveUS20160216251A1Compounds screening/testingBiological material analysisNon invasiveReporter gene
Owner:THOMAS JEFFERSON UNIV
Total saponins of psammosilene tunicoids (TSPT) with antitumor activity, and preparation thereof
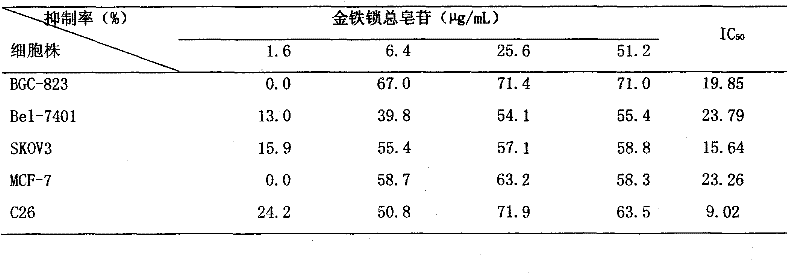
The invention discloses a new application of the antitumor effect of a Miao ethnomedicine psammosilene tunicoids, and simultaneously discloses a preparation method and preparation of the pharmaceutical part of psammosilene tunicoids with the antitumor activity. The preparation method mainly comprises the steps of: extracting by ethanol, concentrating, extracting by n-butyl alcohol, purifying by macroporous absorption resin, and the like to obtain the total saponins of psammosilene tunicoids (TSPT). The total saponins are used as raw materials to prepare clinical commonly used pharmaceutical preparations such as tablets, capsules, injections and the like. A method of pharmacodynamic evaluation both in vivo and in vitro is adopted, wherein shown by in vitro tests, the TSPT has stronger inhibitory actions on 6 tumor cells of stomach cancer, liver cancer, colon cancer, ovarian cancer, breast cancer and melanoma, but has unobvious inhibition on the normal splenic lymphocyte; shown by integral animal in vivo experimental results, the TSPT has an inhibition rate as high as 72% on S180 solid tumors and has high action valence, but has small influence on the body weight of mice; and compared with a positive drug cis-platinum, the TSPT has lower side effect. Shown by the experimental results, the TSPT has strong anticancer valence, low side effect and the advantage of original innovation.
Owner:王学勇
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/56ad8e42-9fd9-4d02-ba70-fb173ab812ce/US07951819-20110531-C00001.png)
![Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/56ad8e42-9fd9-4d02-ba70-fb173ab812ce/US07951819-20110531-C00002.png)
![Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/56ad8e42-9fd9-4d02-ba70-fb173ab812ce/US07951819-20110531-C00003.png)

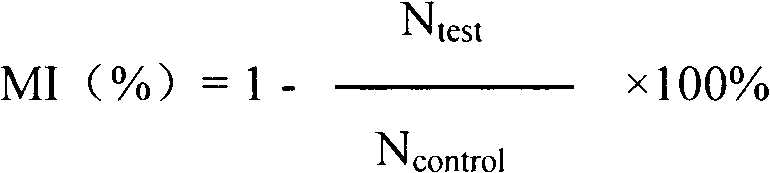
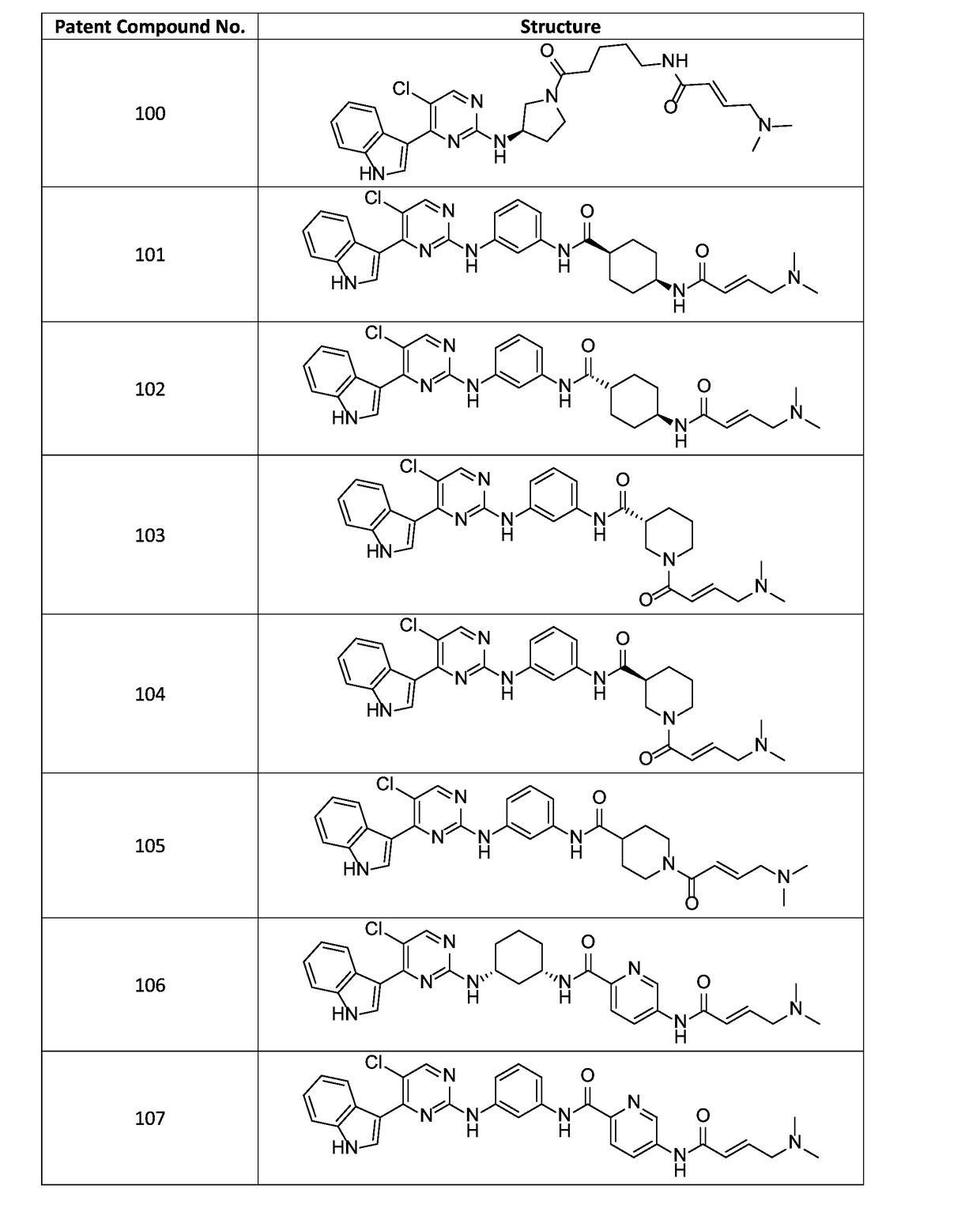
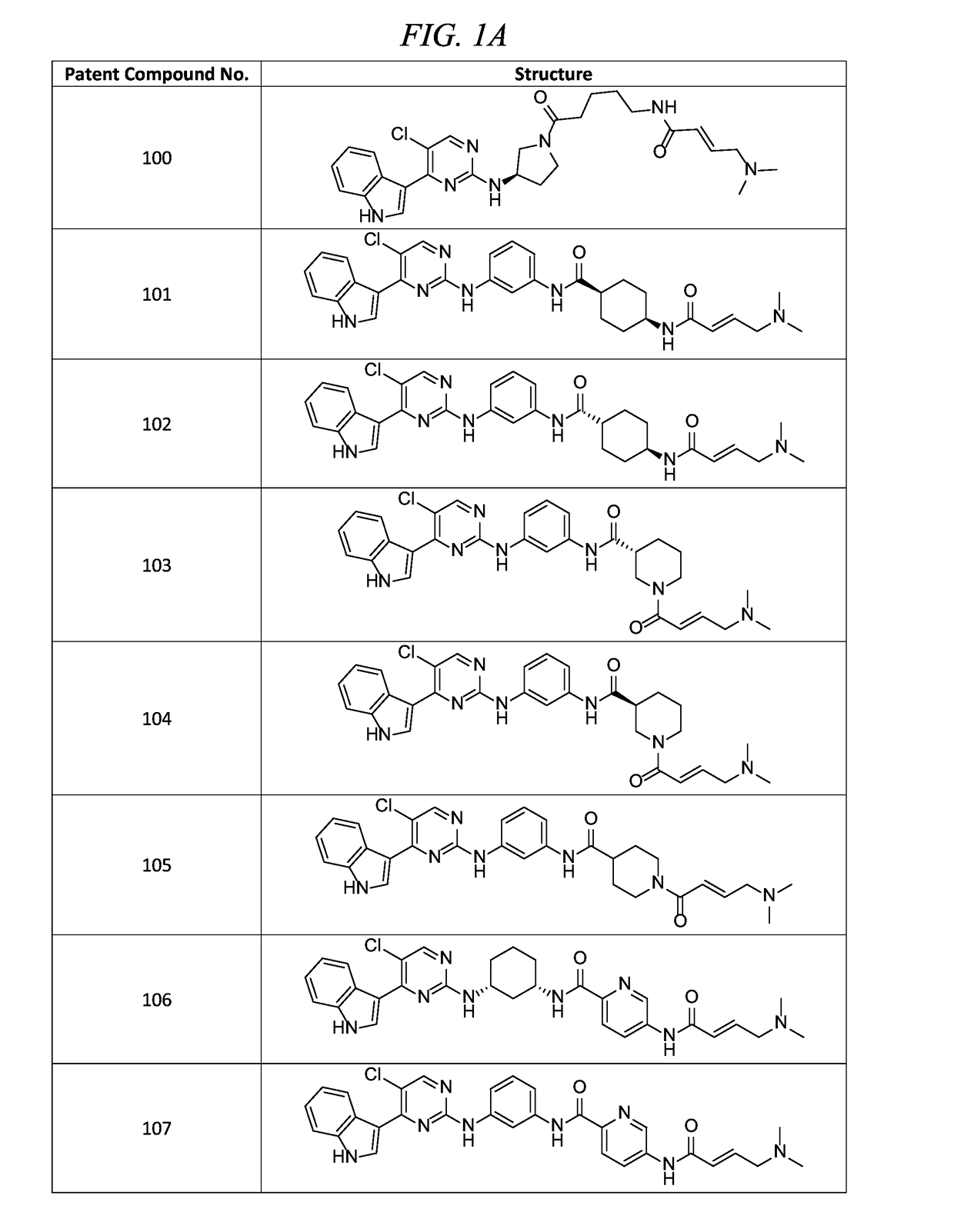
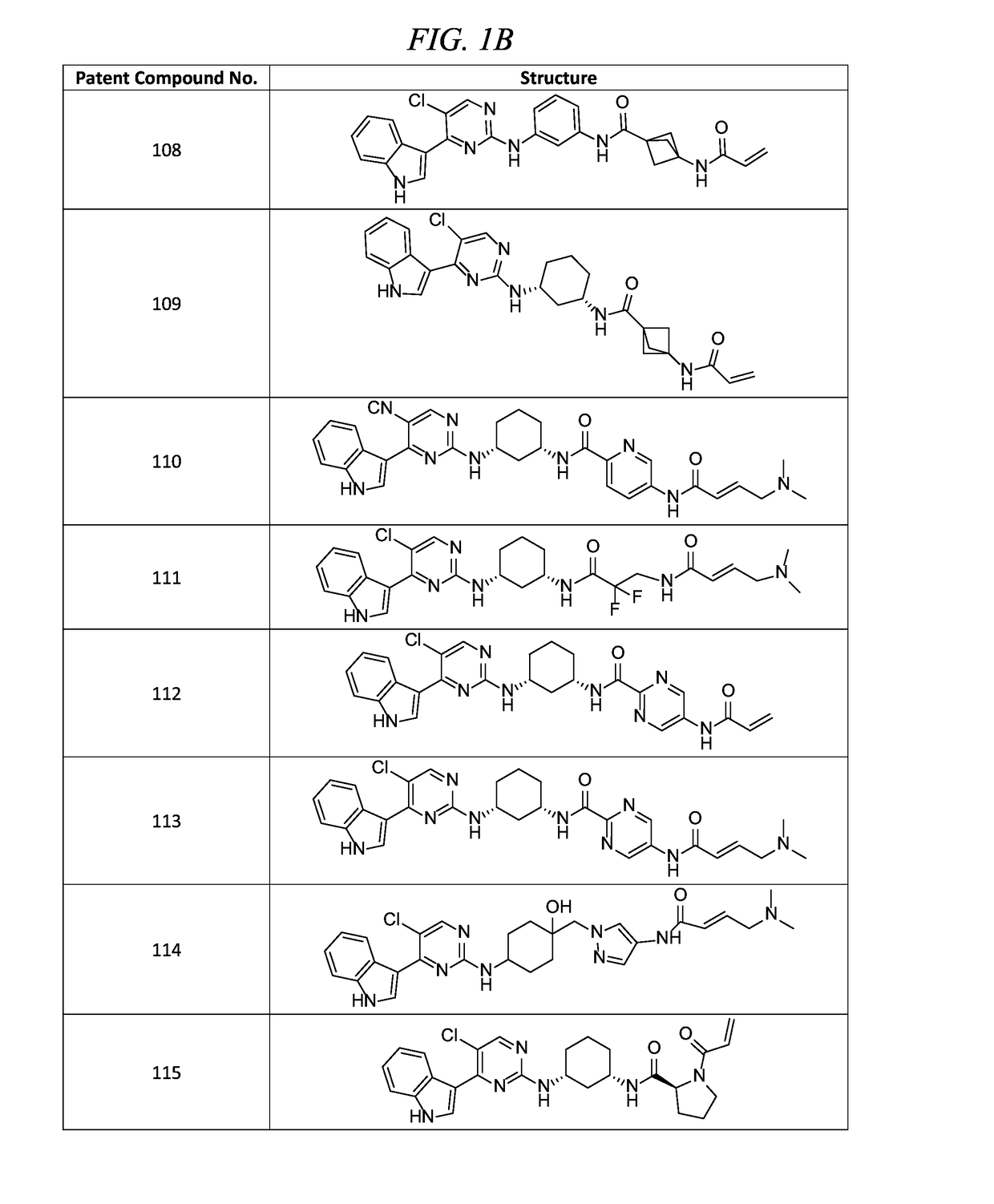
![Acyl benzo[d]thiazol-2-amine and their methods of use Acyl benzo[d]thiazol-2-amine and their methods of use](https://images-eureka.patsnap.com/patent_img/05f8ab5e-bbf3-4c71-9951-bace728b96f1/US20190209532A1-C00001.png)
![Acyl benzo[d]thiazol-2-amine and their methods of use Acyl benzo[d]thiazol-2-amine and their methods of use](https://images-eureka.patsnap.com/patent_img/05f8ab5e-bbf3-4c71-9951-bace728b96f1/US20190209532A1-C00002.png)
![Acyl benzo[d]thiazol-2-amine and their methods of use Acyl benzo[d]thiazol-2-amine and their methods of use](https://images-eureka.patsnap.com/patent_img/05f8ab5e-bbf3-4c71-9951-bace728b96f1/US20190209532A1-C00003.png)