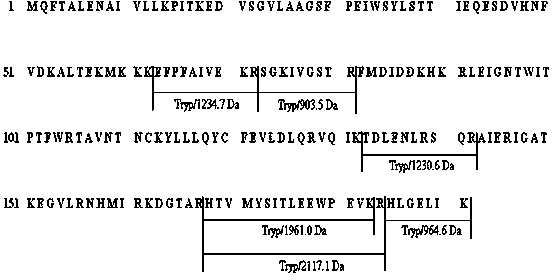
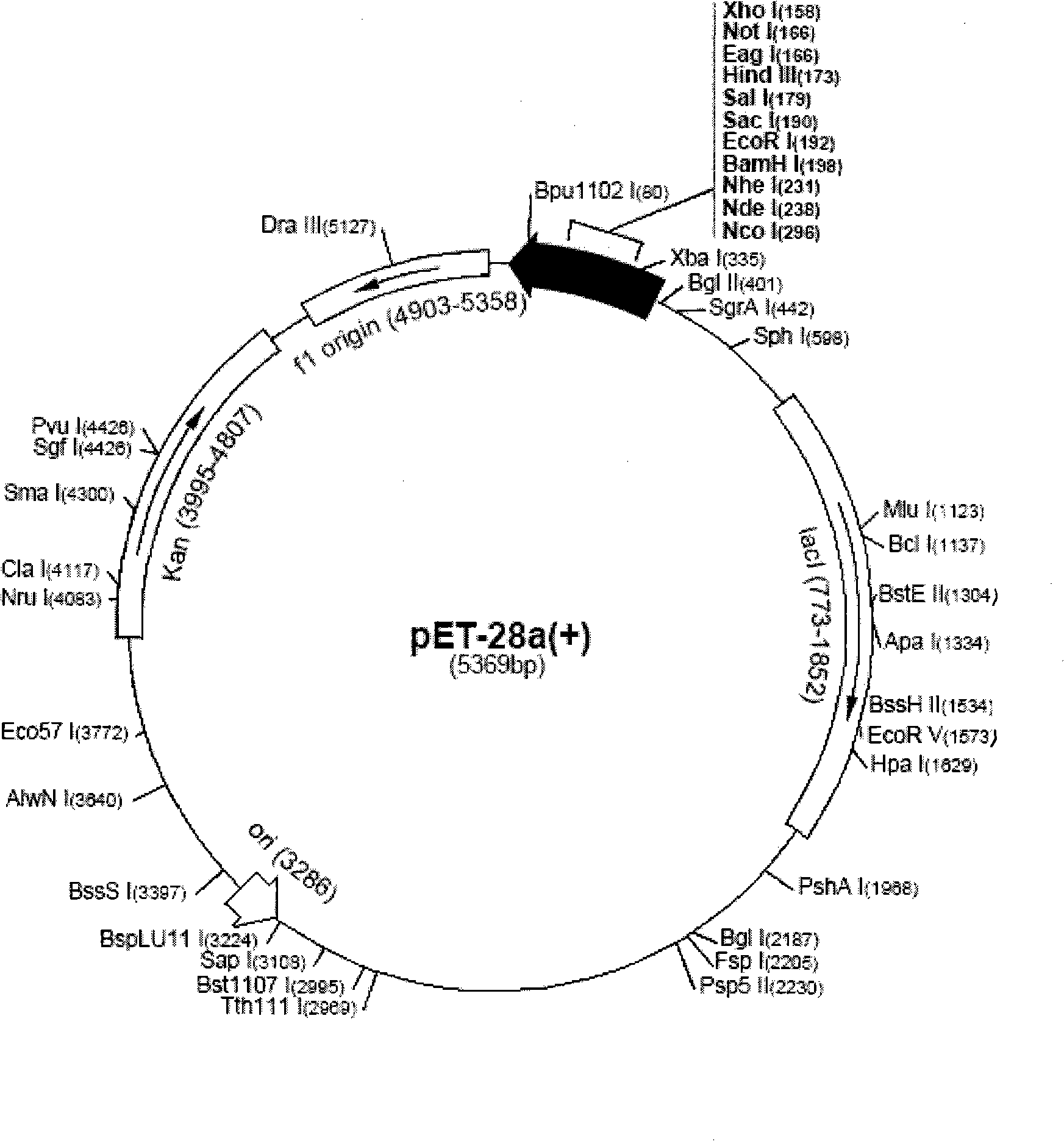
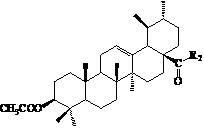
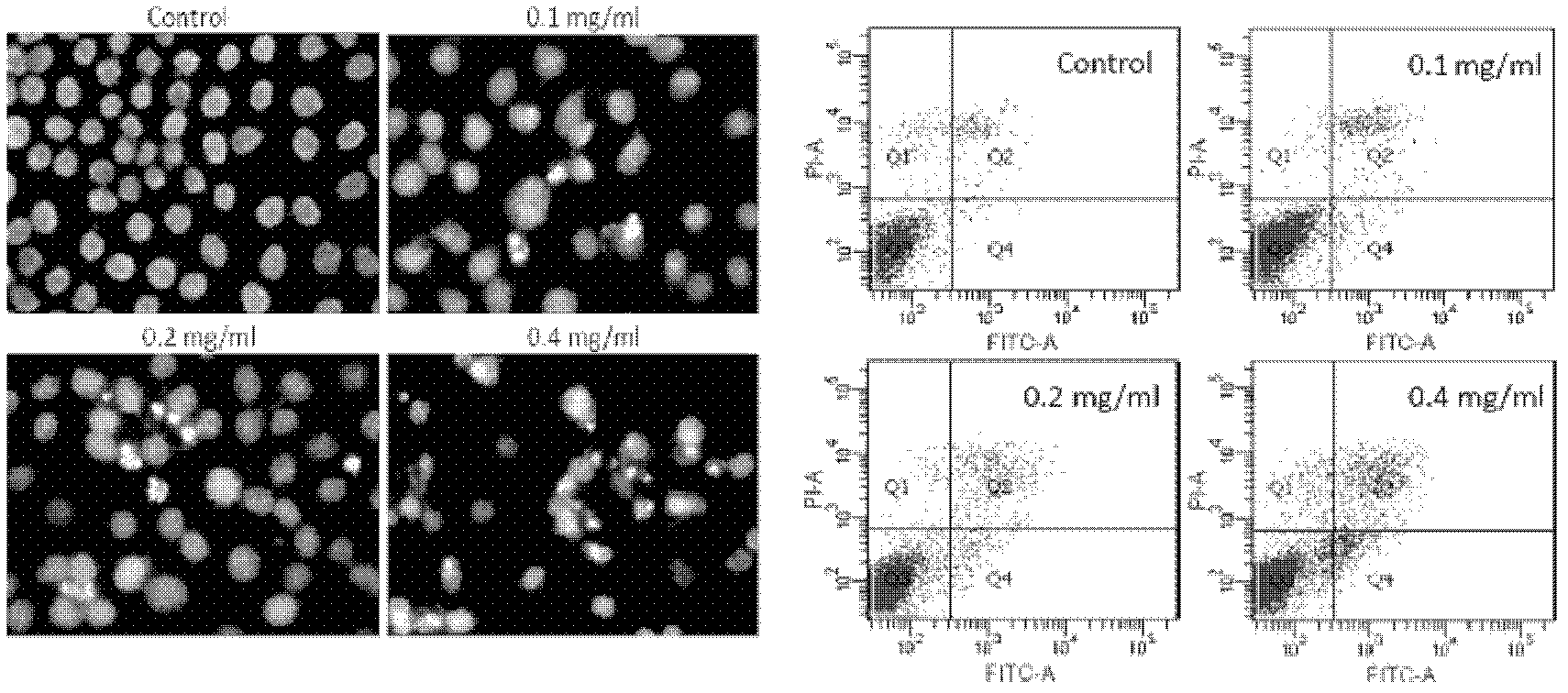
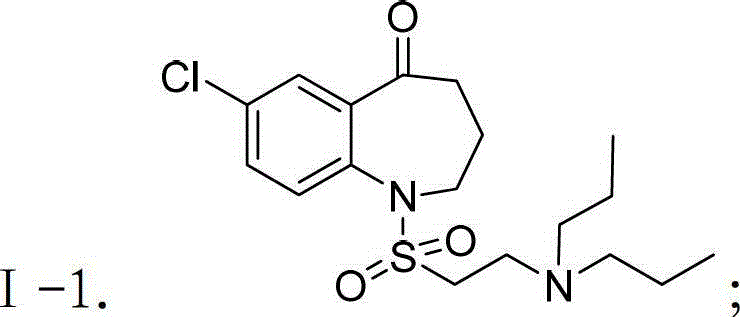
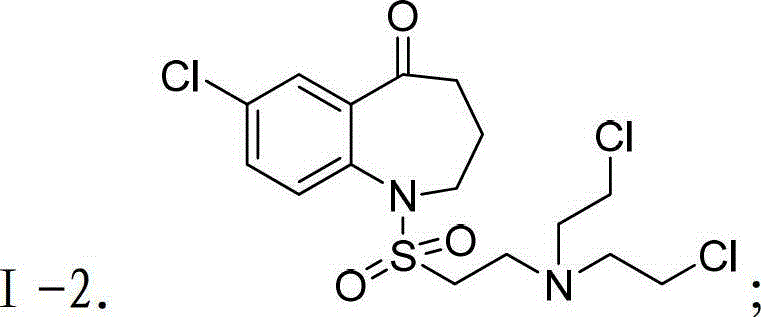
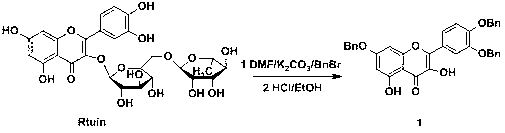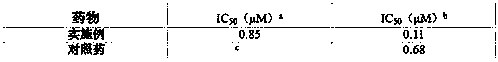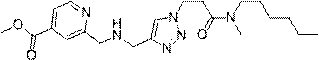Patents
Literature
172 results about "Human gastric carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel anti-tumor application of penicillium enol A1 from penicillium citrinum
InactiveCN103865808AGood antitumor activityOrganic active ingredientsFungiHuman gastric carcinomaPenicillium cainii
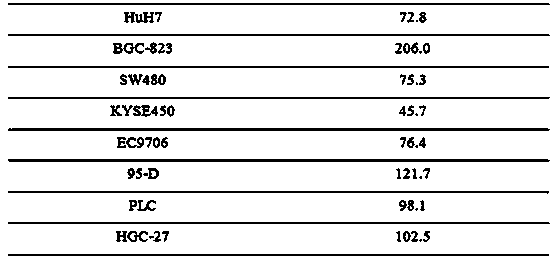
The invention relates to novel anti-tumor application of an alkaloid compound penicillium enol A1 from penicillium citrinum. The penicillium citrinum IBPT-5 is collected in the China Center for Type Culture Collection (CCTCC), which is located in Wuhan University and has the collection number of CCTCC NO:M2013713, on December 25, 2013. Experiments prove that the compound has better anti-tumor activity on various tumor cells, and can be used for preparing cell proliferation inhibition medicines or anti-tumor medicines for anti-tumor study, wherein tumor cells comprise human colon cancer cells SW620, human hepatoma cells Huh7, human gastric carcinoma cells BGC-823, human colon cancer cells SW480, human esophageal squamous carcinoma cells KYSE450, human esophageal cancer cells EC9706, human highly metastatic lung carcinoma cells 95-D, human hepatoma cells PLC and human gastric carcinoma cells HGC-27.
Owner:FUZHOU UNIV
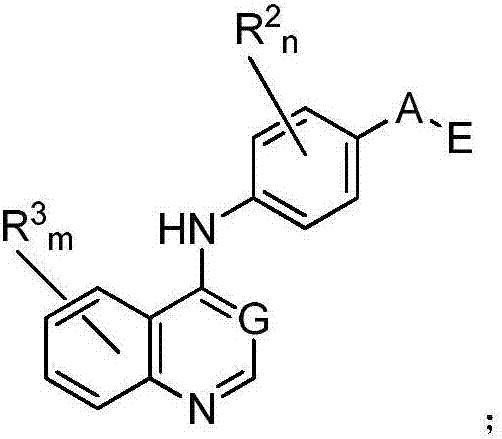
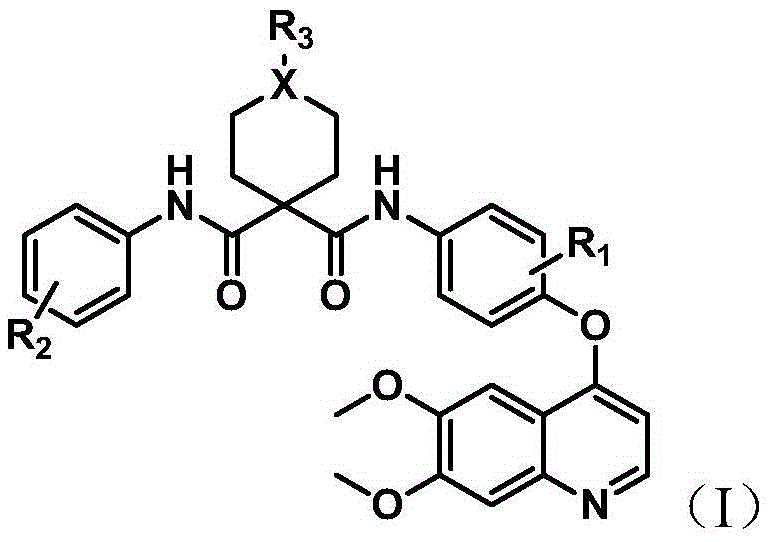
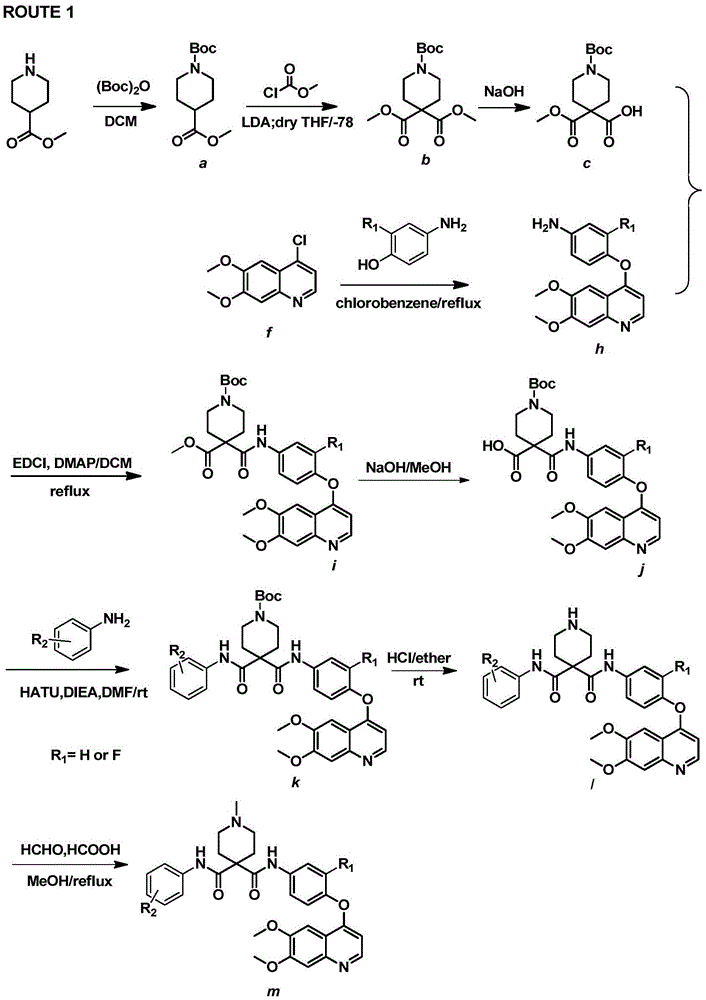
Nitrogenous heterocyclic compound, preparation method, intermediates, composition and application
ActiveCN107141293ASmall toxicityStrong inhibitory activityOrganic active ingredientsOrganic chemistryNitrogenous heterocyclic compoundHuman gastric carcinoma
The invention discloses a nitrogenous heterocyclic compound, a preparation method, intermediates, a composition and application. The nitrogenous heterocyclic compound is as shown in the formula I, has high inhibitory activity to ErbB2 tyrosine kinase, has relatively good inhibitory activity to ErbB2 high-expression human breast cancer cells BT-474, human gastric carcinoma cells NCI-N87 and the like, meanwhile, has relatively weak inhibitory activity to EGFR kinase, is a small-molecule inhibitor with good selectivity to ErbB2 target spots, therefore, is high in safety and can effectively enlarge the safety window in the medicine administration process.
Owner:SHANGAI PHARMA GRP CO LTD
Gastric cancer cell line for expressing green fluorescent protein and luciferase and application thereof in cancer model
InactiveCN102399748AMeet actual needsFermentationIn-vivo testing preparationsYellow fluorescent proteinTail vein
The invention relates to the field of biomedical research, in particular to a gastric cancer cell line for expressing green fluorescent protein and luciferase, which is established by introducing the green fluorescent protein and the luciferase into a cell, and an application thereof in a cancer model. The gastric cancer cell line is characterized in that the gastric cancer cell line for stably expressing green fluorescent protein and luciferase is obtained by using a human gastric cancer cell line as mother cell and bringing the green fluorescent protein (GFP) and firefly luciferase (Fireflyluciferase, LUC) genes into the cell by using a slow virus infection method; and different gastric cancer models are established for the cell line by using hypodermic injection, tail vein injection and in situ injection methods. The invention provides establishment and application of the human gastric cancer cell line; and the gastric cancer cell line for expressing green fluorescent protein and luciferase is established by using the slow virus infection method, applied to the cancer model and used for gastric cancer preclinical studies.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Novel anti-tumor application of penicillium enol B1 from penicillium citrinum
InactiveCN103865809AGood antitumor activityOrganic active ingredientsFungiHuman gastric carcinomaPenicillium cainii
The invention relates to novel anti-tumor application of an alkaloid compound penicillium enol B1 from penicillium citrinum. The penicillium citrinum IBPT-5 is collected in the China Center for Type Culture Collection (CCTCC), which is located in Wuhan University and has the collection number of CCTCC NO:M2013713, on December 25, 2013. The compound has good anti-tumor activity to various tumor cells, and can be used for preparing cell proliferation inhibition medicines or anti-tumor medicines for anti-tumor study, wherein tumor cells comprise human colon cancer cells SW620, human hepatoma cells Huh7, human gastric carcinoma cells SGC-7901, human gastric carcinoma cells BGC-823, human colon cancer cells SW480, human esophageal cancer cells EC9706, human highly metastatic lung carcinoma cells 95-D, human hepatoma cells PLC, human gastric carcinoma cells HGC-27 and human lymphoma cells RAJI.
Owner:FUZHOU UNIV
Phellinus baumii fruit body anticancer activity polysaccharide PBPP and preparation method thereof
ActiveCN105777924AHigh purityGood inhibitory effectAntineoplastic agentsIon exchangeHuman gastric carcinoma
The invention discloses phellinus baumii fruit body anticancer activity polysaccharide PBPP and a preparation method thereof.The method comprises the steps that crude polysaccharide is extracted from phellinus baumii fruit body powder through ultrasonic wave assisted hot water, precipitation is conducted with ethyl alcohol, acidic polysaccharose components are separated out through DEAE ion exchange chromatography, chromatographic purification is conducted with sephair conditioning unitryl S-100, S-200 and S-400 sequentially, eluent corresponding to eluting peaks is collected, vacuum concentration is conducted, freeze vacuum drying is conducted and a product is obtained.According to the polysaccharide PBPP and the method thereof, the purity is uniform, adverse effects on growth of normal cells do not exist, obvious inhibiting effects on growth of human cervical carcinoma cells HELA and human gastric carcinoma cells SGC-7901 are achieved, and the polysaccharide PBPP can be used for developing anticancer products.
Owner:ZHEJIANG UNIV
Quinoline multi-target kinase inhibitor with antitumor activity and preparation method thereof
InactiveCN105541798AStrong in vitro inhibitory activityStrong inhibitory activityOrganic chemistryAntineoplastic agentsPositive controlHuman gastric carcinoma
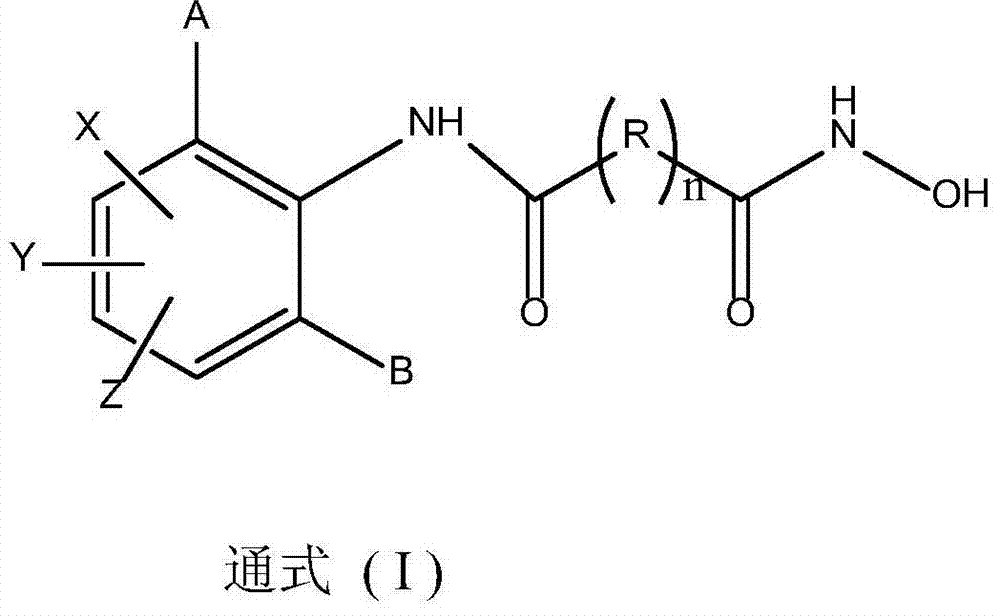
The invention relates to a quinoline multi-target kinase inhibitor with antitumor activity and a preparation method thereof. A general structural formula of the compound is shown in a formula (I) described in the specification. In vitro cell experiments verify that the compound provided by the invention has strong in vitro inhibitory activity on five common tumor cell lines, namely human thyroid carcinoma SW579, human hepatic carcinoma HepG2, human lung adenocarcinoma A549, human colorectal adenocarcinoma HCT116 and human gastric carcinoma MKN45, antitumor activities of most of target compounds are better than or equivalent to that of a positive control drug Cabozantinib, and the in vitro cell experiments verify that the compound provided by the invention has strong inhibitory activity on two kinases KDR and MET, so that the compound provided by the invention has a broad application prospect in preparation of a new antitumor drug.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
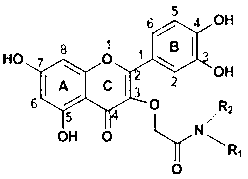
Preparation method and use of quercetin amide derivative
InactiveCN103214445AStrong proliferation inhibitory effectOrganic active ingredientsOrganic chemistryHuman gastric carcinomaQuercitrin
The invention discloses a preparation method of a quercetin amide derivative and application of the quercetin amide derivative in preparing an antitumor drug. The preparation method of the quercetin amide derivative comprises the following steps of: carrying out benzyl selective protection, a Williamson ether forming reaction, a DCC (Dicyclohexylcarbodiimide) condensation reaction, catalytic hydrogenation and the line by using cheap rutin as the material. The preparation method of the quercetin amide derivative can be used for realizing the orientation and efficient modification of the quercetin, and is gentle in reaction conditions, simple to operate, simple in post-treatment and convenient for industrial production. The obtained quercetin amide derivative obviously has a stronger inbibitional effect to proliferation of a human esophageal squamous carcinoma cell EC (Ethyl Cellulose)109, a human esophageal squamous carcinoma cell EC9706, a human gastric carcinoma cell SGC (Soluble Guanylyl Cyclase) 7901 and a mouse melanoma cell B16-f10 relative to the quercetin, and therefore, the quercetin amide derivative is an anti-tumor candidate compound with great potential.
Owner:ZHENGZHOU UNIV
Inula wissmannian extract and preparation thereof and application in preparation of antitumor drug
The invention provides an inula wissmannian extract and preparation thereof and application in preparation of an antitumor drug. The extract is gemma alkane type sesquiterpene lactone inula wissmannian lactone methyl, ethyl, propyl, butyl and amyl or sheepear inula herb lactone. The extract has the following chemical structural formula and absolute configuration that: due to the toxicity killing function experiment of inula wissmannian lactone methyl, ethyl, propyl, butyl and amyl or sheepear inula herb lactone on human hepatoma cells HepG2, human prostatic cancer cells PC-3, human gastric carcinoma cells MGC-803, human leukemia cells K562, human nasopharynx cancer cells KB and normal human hepatocytes LO2, the result shows that the gemma alkane type sesquiterpene lactone has strong tumor cell killing function, and proliferation of tumor cells can be inhibited, so that the tumor cells are killed. The animal in-vivo experiment proves that the inula wissmannian plant extract and monomeric compounds have the anti-tumor effect and can serve as active ingredients for preparing the antitumor drug. A novel cancer treatment medicine is provided clinically, and high clinical application values and social benefits are generated.
Owner:SHANGHAI JIAO TONG UNIV
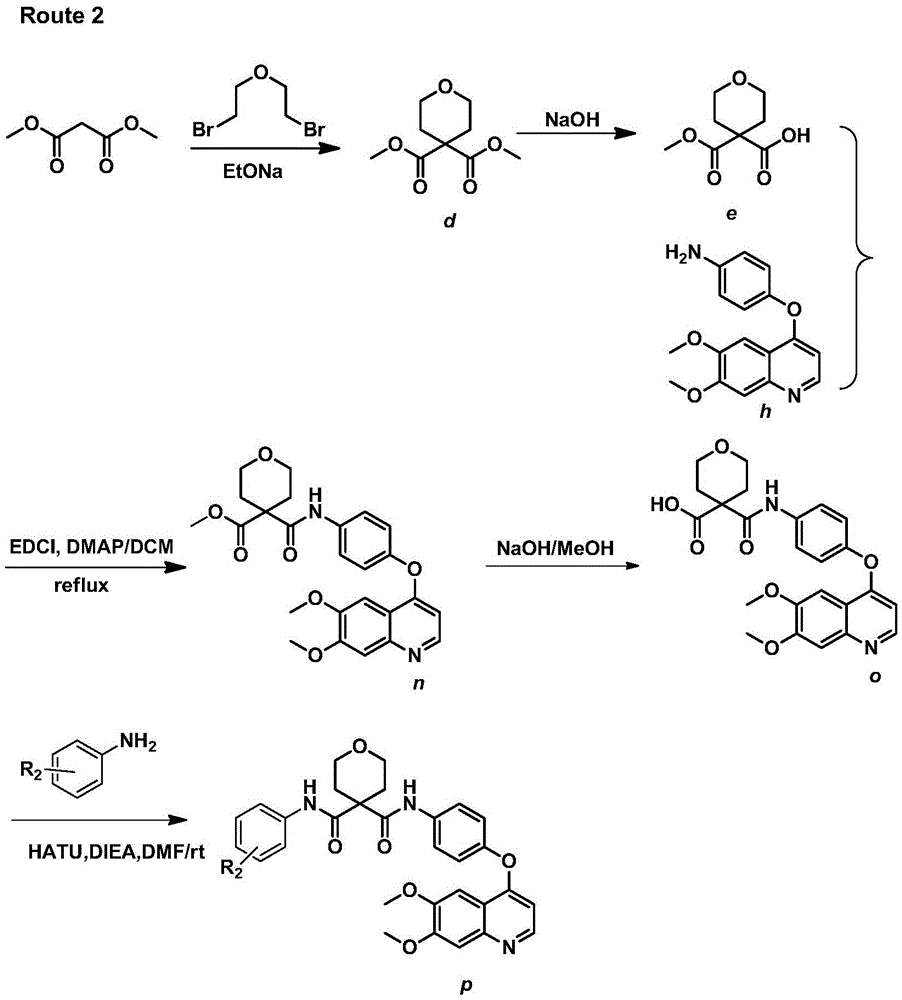
Nitrogen heterocyclic ring compound, preparation method, midbody, composition and application
ActiveCN109422755ASmall toxicityStrong inhibitory activityOrganic active ingredientsGroup 5/15 element organic compoundsNitrogenous heterocyclic compoundHuman gastric carcinoma
The invention discloses a nitrogen heterocyclic ring compound, a preparation method, a midbody, a composition and an application. The invention provides a nitrogen heterocyclic compound as shown in formula I, a pharmaceutically-acceptable salt, an enantiomer thereof, diastereoisomer, a tautomer, a solvent compound, a metabolism product or a drug precursor. The compound has high inhibition activityfor ErbB2 tyrosine kinase, and good inhibition actitivity for ErbB2 highly-expressed human breast cancer cell BT-474 and human gastric carcinoma cells NCI-N87, and has relatively weak inhibition activity for EGFR kinase. The nitrogen heterocyclic ring compound is an EGFR / ErbB2 double-target inhibitor capable of weakening the inhibition activity of EGFR kinase or a small-molecular inhibitor with selectivity for ErbB2 target. (Shown in the description).
Owner:SHANGAI PHARMA GRP CO LTD
Crocetin amides derivative and preparation method and application thereof
InactiveCN103724219AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsOrganic compound preparationHuman gastric carcinomaCrocetin
The invention relates to a crocetin amides derivative. The crocetin amides derivative is characterized by having the following general formula: (img file='DSA00000789203800011.tif' wi='1009' he='177' / ), wherein R in the formula is (img file='DSA00000789203800012.tif' wi='1529' he='751' / ). The crocetin amides derivative has obvious inhibiting effect on human lung cancer cells A549 and human gastric carcinoma cells SGC7901. Therefore, the crocetin amides derivative can be applied to preparing anti-cancer drug. The invention also discloses a preparation method of the crocetin amides derivative.
Owner:NANJING UNIV
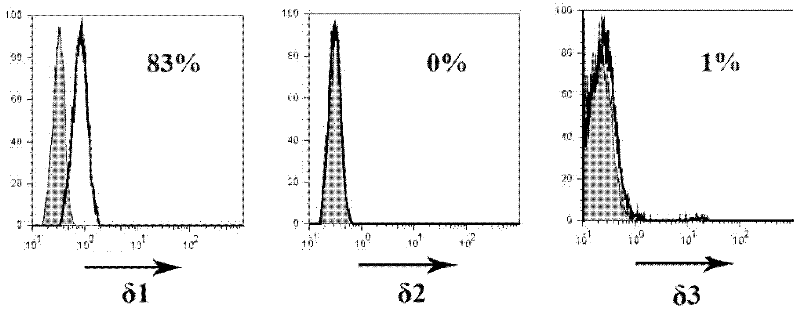
Dominant sequence of delta 1 chain complementary determining region (CDR) 3 in gamma delta T lymphocytes, and T cell receptor (TCR) transfected cells and application thereof
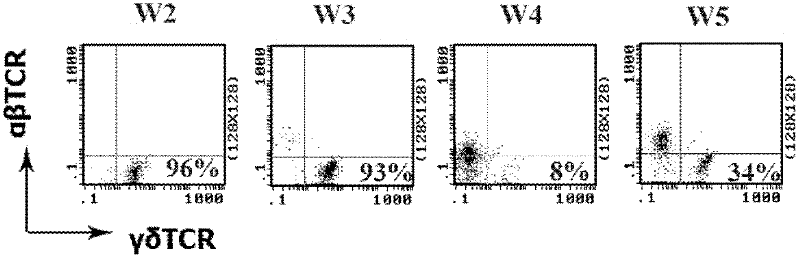
The invention discloses a dominant sequence GTM of a delta 1 chain complementary determining region (CDR) 3 in human gastric cancer tissue-derived tumor-infiltrating gamma delta T lymphocytes, a T cell receptor (TCR) containing the sequence, and TCR transfected cells. The amino acid residue sequence of the GTM is shown as SEQ ID NO.1 in a sequence table. Experiments prove that GTM peptide can be specifically combined with tumor cells, tumor tissue and V delta 1 T cell ligand MICA protein; and a J.RT3-T3.5 cell platform for expressing the TCR containing the dominant sequence GTM of the delta 1 chain CDR3 in the human gastric cancer tissue-derived tumor-infiltrating gamma delta T lymphocytes on a J.RT3-T3.5 surface is established by a lentivirus expression system, gamma delta 1 tumor-infiltrating lymphocytes (TIL) and a tumor identifying and killing mechanism thereof are simulated in the cell level, the killing activity of the transfected cells TCR gamma 4 delta 1 on the tumor cells is obviously increased, and the killing effect is TCR-dependent. The invention plays an important role in the development of medicines for treating malignant tumors and the adoptive treatment of malignant tumors, and has wide application prospects.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Grifola frondosa mycelium anti-tumor glycoprotein and preparation method
ActiveCN103509091AHas antitumor activityHigh yieldPeptide preparation methodsDepsipeptidesHuman gastric carcinomaHuman breast
The invention discloses a grifola frondosa mycelium anti-tumor glycoprotein and a preparation method, and belongs to the technical field of bioengineering. The glycoprotein is prepared by the following steps: using submerged fermented grifola frondosa mycelium as a raw material, homogenating and crushing, extracting with cold water, centrifuging, taking supernatant, precipitating with ammonium sulfate, dialyzing, and carrying out DEAE (diethyl-aminoethanol) -sepharose Fast Flow and superdexTM 75 prepgrad column chromatography and other steps to systematically separate and purify. The glycoprotein is a compound of polysaccharide and protein, wherein polysaccharide is 2-10% in content and composed of four monosaccharides including arabinose, fructose, mannose and glucose; the protein is 30-90% in content and composed of 17 amino acids including aspartic acid, methionine, glutamic acid and so on; the molecular weight of the glycoprotein is 30-90 KDa. The glycoprotein can inhibit growth of human gastric carcinoma cell SGC-7901 and human breast cancer cell MCF-7, and can be used for preparing a possible anti-cancer drug. Besides, the grifola frondosa mycelium anti-tumor glycoprotein can be generally applied to separation and purification of glycoprotein obtained from mycelium of various officinal and edible fungi through submerged fermentation.
Owner:JIANGSU UNIV
Coumarin-modified BODIPY (boron dipyrromethene) derivative as well as preparation and application thereof
ActiveCN108440586AGood anticancer effectSingle structureEnergy modified materialsGroup 3/13 element organic compoundsResearch ObjectSynthesis methods
The invention discloses a coumarin-modified BODIPY (boron dipyrromethene) derivative as well as preparation and an application thereof. Chemotherapy drug coumarin covalent bonds with clear curative effects are linked to a BODIPY photosensitizer for PDT (photon dynamic treatment), so that a PDT and chemotherapy combined anti-tumor drug, namely, the coumarin-modified BODIPY derivative, is obtained.Meanwhile, the coumarin-modified BODIPY derivative is taken as a research object, a human gastric carcinoma cell BGC-823 is taken as a tested cell line, the in-vitro anti-cancer activity research is carried out, an efficient anti-cancer prodrug is screened out, and a foundation is laid for PDT and chemotherapy combination of the coumarin-modified BODIPY derivative. The synthesis method of the compound is simpler, raw materials are easy to obtain, the cost is low, few side reactions exist, the yield is higher, purification is easy, and industrial production is facilitated.
Owner:FUZHOU UNIV
CIAPIN1 monoclone antibody for multidrug resistant differential diagnosis of stomach cancer and method for preparing the same
InactiveCN101492506AImprove the detection rateBacteriaImmunoglobulins against animals/humansBALB/cStomach cancer
The invention relates to a mouse anti-human CIAPIN1 monoclonal antibody for confirming multi-drug resistant gastric cancer, and a preparation method thereof. The invention is characterized in that the monoclonal antibody is obtained by adopting the hybrid tumor technology; the heavy chain is 1gG1; the light chain is k; the molecular weight is 39KD; and the affinity is 3.6x10. The preparation method comprises the following steps of: (1) synthesizing primers; (2) synthesizing single-chain DNA by carrying out reverse transcription on SGC-7901 / VCR general RNA of a human gastric-cancer multi-drug resistant cell; (3) expanding human CIAPIN1 gene cDNA by using the primers F and R and by adopting a PCR method; (4) constructing CIAPIN1 gene induction expression plasmids pET28-CIAPIN1 and pGEX-CIAPIN1; (5) constructing engineering bacteria B21-CIAPIN1 and DE3-CIAPIN1; (6) using the engineering bacteria B21-CIAPIN1 and DE3-CIAPIN1 to carry out prokaryotic expression and purification on CIAPIN1 protein; (7) using CIAPIN1 protein to immunize a 4-week-old Balb / C mouse so that mouse anti-human CIAPIN1 antibody is generated; and (8) using the serum of the immunized mouse to prepare the mouse anti-human CIAPIN1 monoclonal antibody. The invention can better identify multi-drug resistant gastric cancer and non-multi-drug resistant gastric cancer of clinical gastric cancer patients, and predict clinical prognosis of the gastric cancer patients.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Ursolic acid nitrogen heterocyclic ring structure modifiers with antitumor activity and preparation method for ursolic acid nitrogen heterocyclic ring structure modifiers
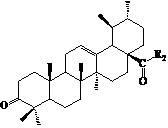
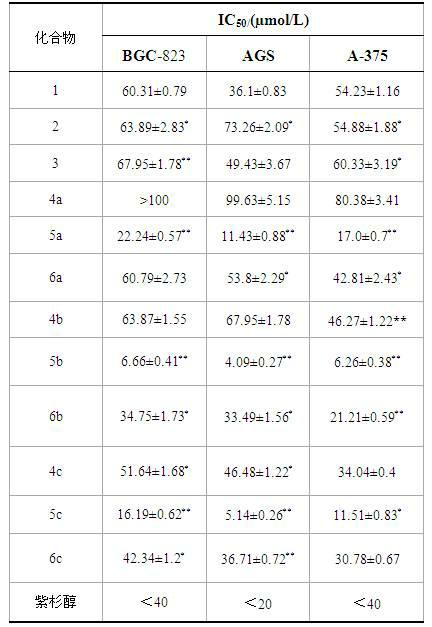
InactiveCN102675406AGood antitumor effect in vitroProliferative Inhibitory Effect in VitroOrganic active ingredientsSteroidsCancer cellStage melanoma
The invention relates to the transformation of a chemical structure of a natural medicinal plant, namely ursolic acid, in particular to ursolic acid nitrogen heterocyclic ring structure modifiers with antitumor activity and a preparation method for the ursolic acid nitrogen heterocyclic ring structure modifiers. The ursolic acid nitrogen heterocyclic ring structure modifiers are ursolic acid nitrogen heterocyclic ring derivatives shown as a general formula (I). The preparation method comprises the following steps of: oxidizing or acetylating a C-3 hydroxyl group of the ursolic acid, performing condensation reaction of a C-17 carboxyl group and piperazine or a derivative of the piperazine so as to achieve the structural transformation of the ursolic acid, and thus obtaining a series of ursolic acid nitrogen heterocyclic ring structure modifiers. The in-vitro inhibitory activities of the ursolic acid nitrogen heterocyclic ring structure modifiers on human malignant melanoma A-375 cells, human gastric carcinoma AGS and BGC-823 cells are determined; and methyl thiazolyl tetrazolium (MTT) data show that the modifiers have a certain effect of inhibiting the proliferation of three kinds of cancer cells, and the inhibitory activity of partial compounds is even superior to that of paclitaxel.
Owner:FUZHOU UNIV
Sansevieria trifasciata saponin, preparation method and application thereof
InactiveCN101804132AEasy to operateLow costAntineoplastic agentsPlant ingredientsProcess equipmentHuman gastric carcinoma
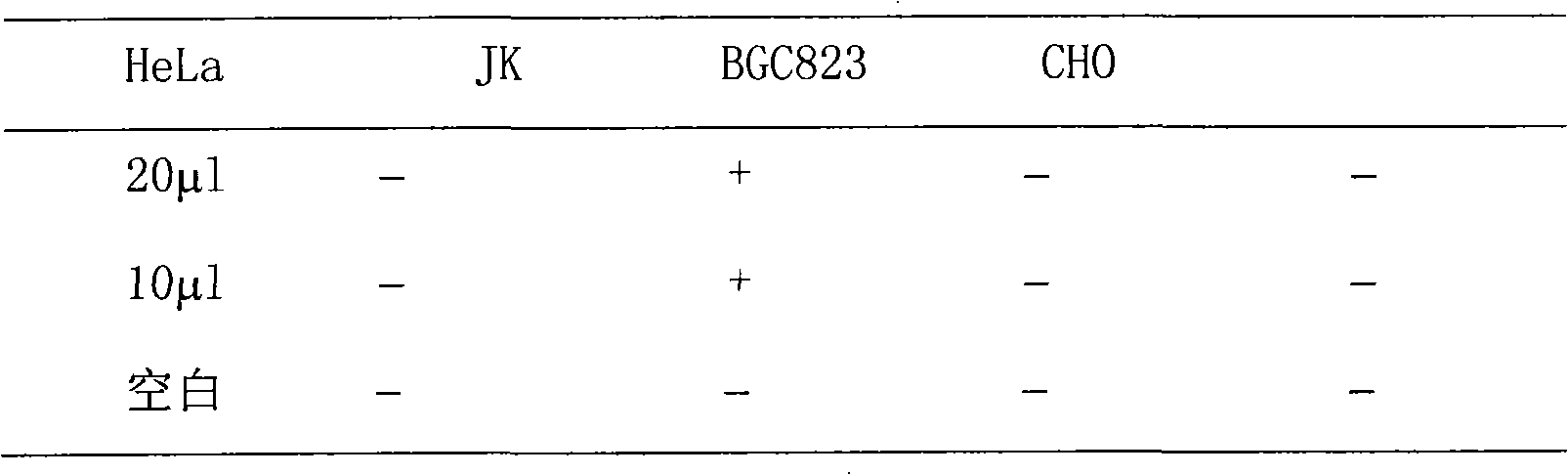
The invention discloses sansevieria trifasciata saponin, a preparation method and application thereof. The method comprises the following steps of: making the sansevieria trifasciata into dry powder, extracting by using water, alcohol or methanol, extracting by using petroleum ether or ether, and extracting by using butyl alcohol or pentanol to obtain the sansevieria trifasciata total saponin extract. The method for preparing the sansevieria trifasciata saponi optimizes the process conditions, has simple operation and low cost, has low requirement on process equipment, can reduce the consumption of solvents and enhance the leaching rate. The sterilized sansevieria trifasciata has obvious inhibition function on the growth of JurKet, has no activity killing effect on human cervical carcinoma cells (HeLa) and human gastric carcinoma cells (BGC823), and has no injury to normal Chinese Hamster Ovary (CHO), and the cell grows normally without any obvious change.
Owner:JILIN AGRICULTURAL UNIV
Screening of aptamers specially targeting gastric cancer cells and aptamers obtained after screening
InactiveCN103627701AImprove featuresHigh selectivityMicrobiological testing/measurementDNA preparationAptamerCancers diagnosis
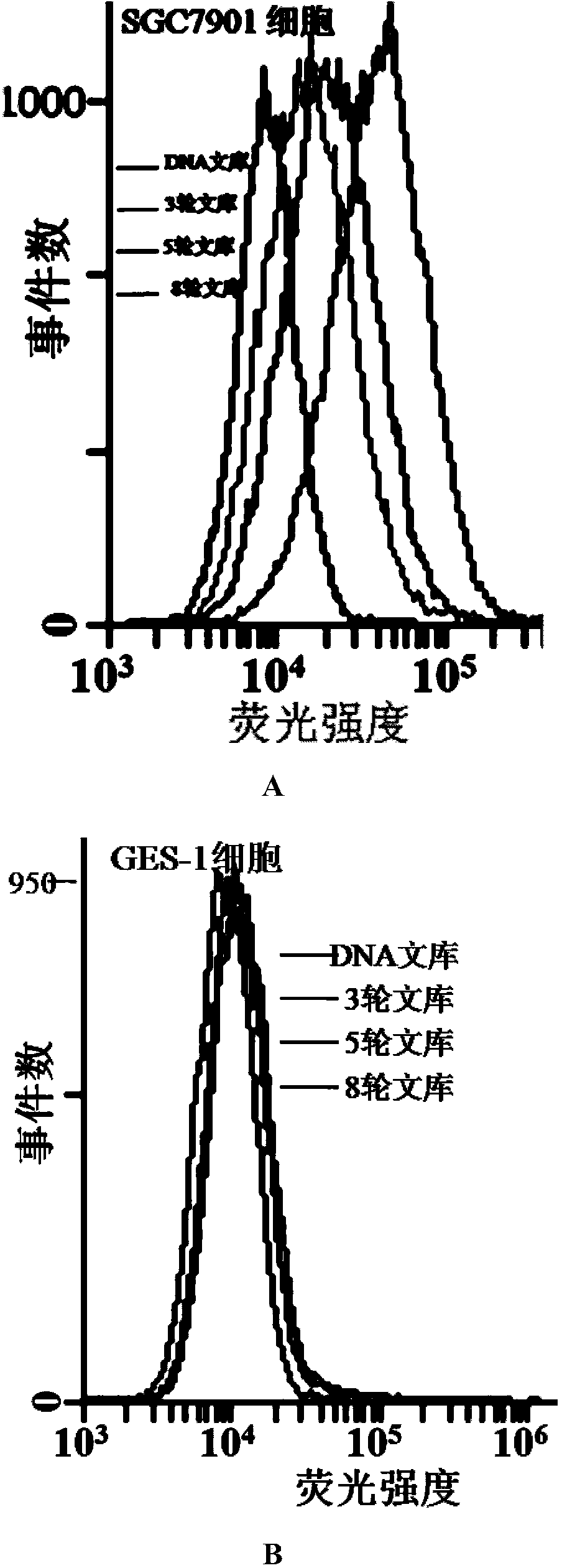
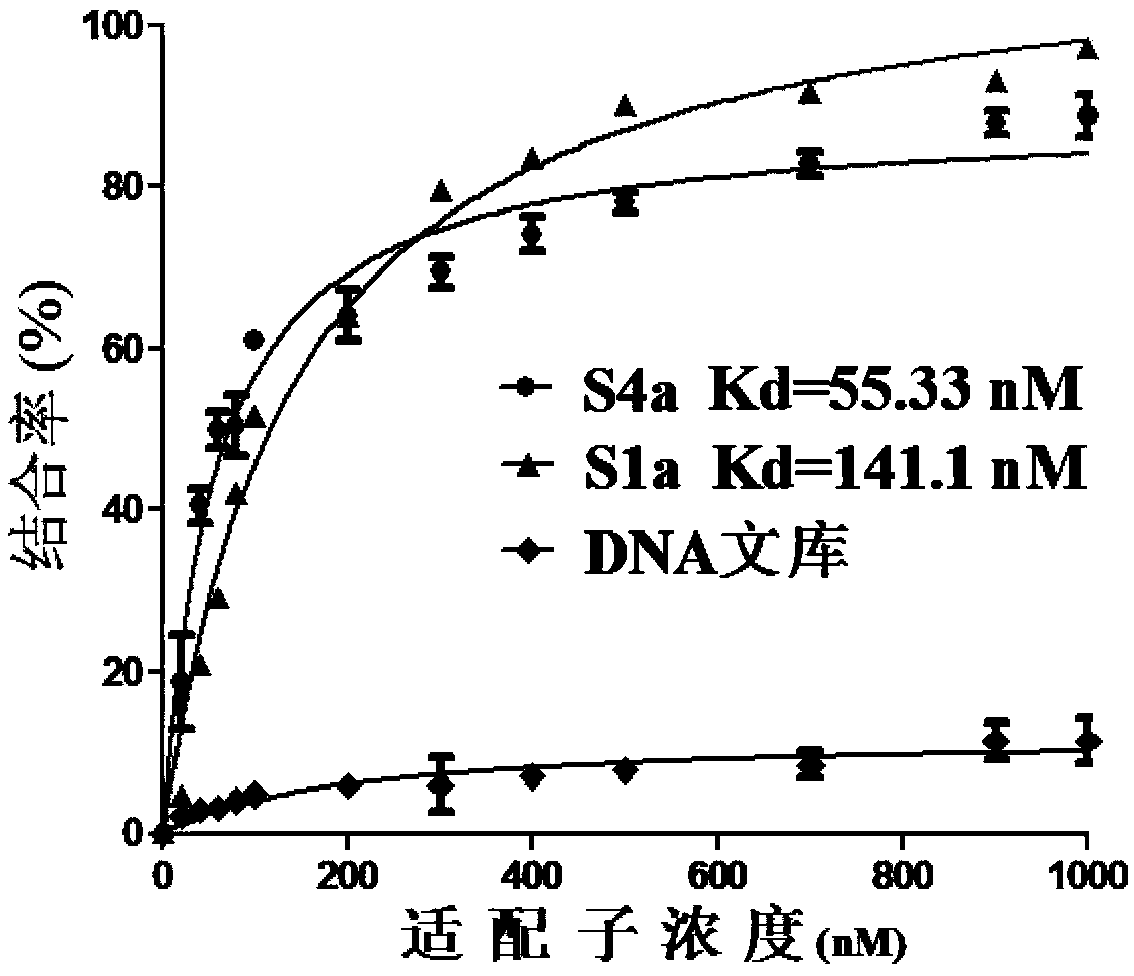
The invention discloses screening of aptamers specially targeting gastric cancer cells and aptamers obtained after screening, and belongs to the fields of bioanalysis and genetic engineering. The screening method provided by the invention is based on a cell-SELEX technology, SGC 7901 cells human gastric carcinoma cells are adopted as target cells, GES-1 cells of human gastric mucosa cells are adopted as negative-screening cells; during the negative-screening, the amount, elution time and volume of eluant are continuously increased; meanwhile, the incubating time of target cells is gradually shortened, and the incubating time of negative-screening cells is properly prolonged; through screening for several times, a sequence specially bound with the gastric cancer cells and high in binding force can be obtained, namely, the aptamers for special targeted gastric cancer cells can be obtained. The sequences for the aptamers are shown in SEQ ID NO.1-NO.6, and the aptaamers have good binding force and specificity, can be bound with and target the gastric cancer cells specially, and can be used for diagnosis of gastric cancer and preparation of gastric cancer diagnosis drugs.
Owner:WUHAN UNIV
Hydroxamic acid compounds and application thereof in preparation of medicines for inhibiting cancer cell proliferation and/or treating cancers
The invention relates to the field of medicinal chemistry and particularly relates to hydroxamic acid compounds and application thereof in preparation of medicines for inhibiting cancer cell proliferation and / or treating cancers. The hydroxamic acid compounds provided by the invention is capable of inhibiting cancer cell proliferation so as to treat cancers and especially has excellent activity of inhibiting proliferation of melanoma cell (A375), human lung cancer cell (A549), human gastric carcinoma cell (MGC80-3), human esophageal carcinoma cell (TE-1) and cell hepatoma carcinoma cell (HepG2).
Owner:BEIJING UNIV OF CHEM TECH
Semen lepidii anti-tumor effective component extract, as well as preparation method and application thereof
ActiveCN103006741AGrowth inhibitionHas anti-tumor effectAntineoplastic agentsPlant ingredientsHuman gastric carcinomaOncology
The invention discloses a semen lepidii anti-tumor effective component, a preparation method of the semen lepidii anti-tumor effective component and an application of the semen lepidii anti-tumor effective component in the field of anti-tumor. The preparation method comprises the following steps of: extracting semen lepidii (roasted) with water; and extracting with ethyl acetate and water saturated n-butyl alcohol sequentially; collecting water saturated n-butyl alcohol extract; separating with a silica (200 to 300 meshes) column; and performing gradient elution with dichloromethane-methanol in different proportions, wherein the dichloromethane-methanol (5:1) elution part is a semen lepidii anti-tumor effective part. The part has a remarkable effect of inhibiting U251 (glioma cell line), HepG2 (human hepatoma cell line), SGC (human gastric carcinoma cell), mcf-7 (human breast cancer cell) and SCLC (lung carcinoma cell), and can be used for preparing an anti-tumor medicament.
Owner:JIANGSU UNIV
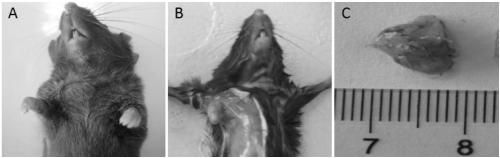
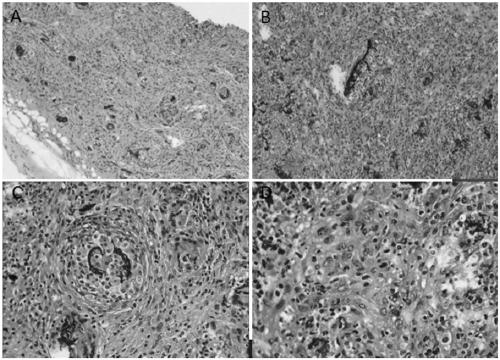
Method for constructing tumor xenograft model in mice immunized with gastric cancer based on organ-like method, and application thereof
InactiveCN109182271AConvenient researchNormal food intakeTumor/cancer cellsGeneral culture methodsWilms' tumorStomach cancer
The invention discloses a method for constructing a tumor xenograft model in mice immunized with gastric cancer based on an organ-like method, and an application thereof. The invention relates to a medical tumor animal model, based on an organ-like method. For the first time, the present invention employs a quasi-organ technique, that is, using a microcarrier as substrate. The composite human gastric cancer cell line SGC-7901 / MKN-45 is transplanted subcutaneously into C57BL / 6 mice to establish a tumor model of human gastric cancer with normal immune function. The tumor model constructed by this method has good tumorigenicity, and the tumorigenicity rate is up to 75%. The HE staining and the immunohistochemistry of the transplanted tumor accord with the characteristics of human gastric cancer. Compared with the existing immunodeficient mice transplanted tumor model, the model can better study and further clarify the mechanism of tumor occurrence and development in the normal immune system, and also provides a more valuable new animal model for the research and development of anticancer drugs. The invention is applied to the tumor model field.
Owner:上海美峰生物技术有限公司
Method of establishing animal model of HER2 positive tumor by using normal mouse
PendingCN107137424AEasy to operateOvercome limitationsCompounds screening/testingMammal material medical ingredientsCell culture mediaHuman gastric carcinoma
The invention discloses a method of establishing animal model of an HER2 positive tumor by using a normal mouse. The method comprises the following steps: (1) cell cultivation: cultivating a human gastric carcinoma cell NCI-N87 in a cell culture medium and collecting cells in a logarithmic phase; (2) inoculation: inoculating the human gastric carcinoma cell NCI-N87 to the normal mouse; (3) evaluation: after inoculation for two weeks, the diameter of the tumor is greater than or equal to 0.6cm and the tumor formation rate is greater than or equal to 80%, showing that the animal model of the HER2 positive tumor is successively established, wherein an immunosuppressive step of the normal mouse is not included. The method of directly inoculating the normal mouse to prepare the animal model by means of the NCI-N87 is simple to operate and stable and reliable, the test cost can be greatly lowered, and many limitations of current other tumor vaccine animal models are overcome. The method of detecting the treatment potency or preventing potency of the HER2 positive tumor vaccine can be used for screening tumor vaccine candidates in a lab and has a wide application prospect.
Owner:LINK HEALTH GRP
Semen momordicae extractive as well as preparation method and use thereof
InactiveCN103169752AGrowth inhibitory effectHigh purityAntineoplastic agentsPlant ingredientsCancer cellApoptosis
The invention belongs to the field of medical examination, and relates to a semen momordicae extractive as well as a preparation method and anti-tumour use thereof. The semen momordicae extractive having anti-tumour activity is prepared from corresponding components separated and purified by taking high performance liquid chromatography as the method after a sample to be measured and extracted is quenched. Found from in-vitro pharmacological experiments, the semen momordicae extractive is capable of obviously inhibiting proliferation of cancer cells by inducing cell apoptosis and retarding cell growth cycle, has an obvious inhibiting effect on human gastric carcinoma cells and other tumour cells, and can be used as the active component for preparing medicines for treating solid tumours, particularly medicines for treating human gastric carcinoma, human prostatic cancer or colon cancer. The preparation method disclosed by the invention is simple, convenient, low in cost and high in yield; and the extractive is high in component purity and good in reproducibility.
Owner:FUDAN UNIV
Anti-cancer activity indole derivative, synthesis method and uses thereof
The present invention relates to an indole derivative and uses of the indole derivative in preparation of anti-cancer drugs for tumor inhibition, wherein a cyano indole compound and a boric acid compound can be subjected to one-step synthesis in the presence of a catalyst, a ligand and an additive so as to obtain the derivative. According to the present invention, the derivative has the excellent anti-cancer activity, and results of the activity test show that the derivative provides good tumor cell growth inhibition effects on human gastric cancer cell line (SGC-7901), human lung cancer cell line (H446) and human gastric cancer cell line (HGC-27), such that the derivative can be adopted as the anti-tumor drug in the medicine field and has good medical research prospects and industrial application values.
Owner:WENZHOU UNIVERSITY
Diosgenin amino acid derivative and application thereof to antitumor drug
The invention discloses a series of amino acid derivatives prepared by using diosgenin as a lead compound through corresponding chemical reaction. Solubility test finds that the solubility of derivatives is increased compared with the raw material medicine of diosgenin; and the pharmacological experiments prove that the diosgenin amino acid derivatives have in vitro antitumor activity, have different degrees of growth inhibition on human colon cancer cell Caco-2, human gastric carcinoma cell MGC-709, human lung adenocarcinoma cell SPC-A-1 and human neuroblastoma cell SH-SY5Y, and have anti tumor activity similar to that of a nuclear parent compound diosgenin.
Owner:JILIN AGRICULTURAL UNIV
Benzo-azepine type derivative and preparation method and purpose thereof
InactiveCN102911118AEnhanced inhibitory effectNovel chemical structureOrganic chemistryAntineoplastic agentsHuman gastric carcinomaOxygen
The invention discloses a benzo-azepine type derivative and a preparation method and purposes thereof. The benzo-azepine type derivative has the structure of a general formula I, wherein in the formula I, R1 is any one among hydrogen, halogen, or halogen substituted C1-C4 alkyl, R2 is oxygen or hydroxyl, and R3 is any one among hydrogen, C1-C4 alkyl, C1-C4 alkoxy, halogen or hydroxyl. According to the invention, compounds or pharmaceutically acceptable salts having the structure of the formula I are novel in chemical constitution, and have an obvious restraining effect, the same as fluorouracil generally used in clinical trials, for tumors, in particular to human lung adenocarcinoma cells, human breast cancer cells and human gastric carcinoma cells.
Owner:TIANJIN UNIV OF COMMERCE
Application of fingerprint atlas composed of microRNAs in gastric cancer
ActiveCN105985955AEasy to distinguishHigh sensitivityNucleotide librariesMicrobiological testing/measurementNormal tissueStomach cancer
The invention relates to an application of a fingerprint atlas composed of microRNAs in diagnosis and treatment of human gastric cancer. The fingerprint atlas, which is composed of a plurality of microRNAs, can quite effectively distinguish gastric cancer tissues from tissues (normal tissues) adjacent to the gastric cancer tissues; and the fingerprint atlas, which is high in sensitivity and strong in specificity, is applicable to the effective diagnosis of the gastric cancer.
Owner:SHANGHAI XIANGQIONG TECHNOLOGY LTD
Hydroxamic acid group-containing diarylethene LSD1/HDACs double-target inhibitors as well as preparation method and application thereof
ActiveCN111592487AStrong dual inhibitory activityHigh activityOrganic compound preparationCarboxylic acid esters preparationHydroxamic acidHuman gastric carcinoma
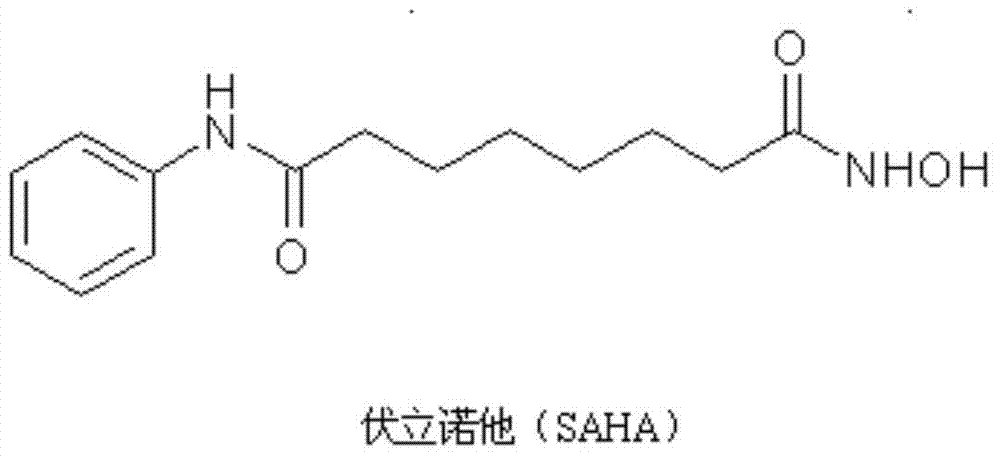
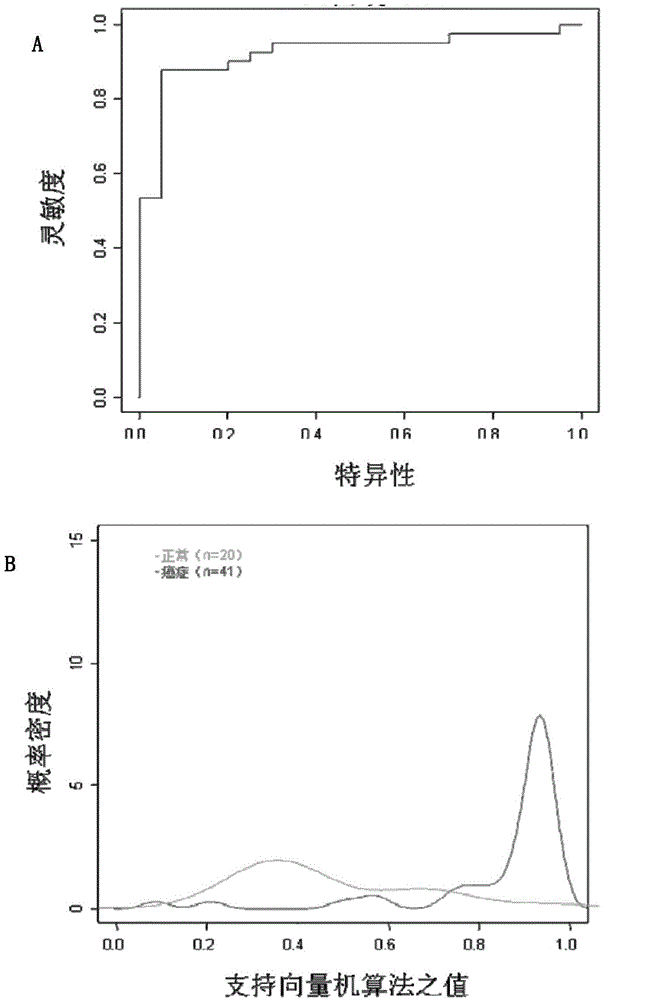
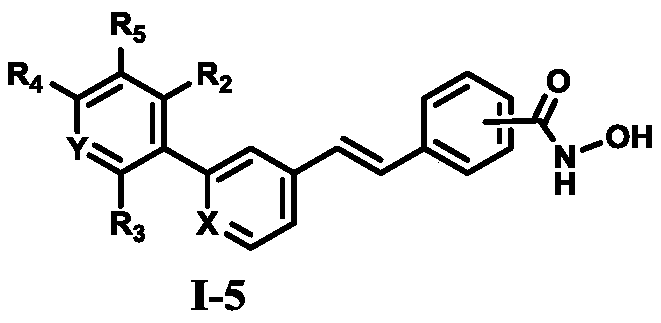
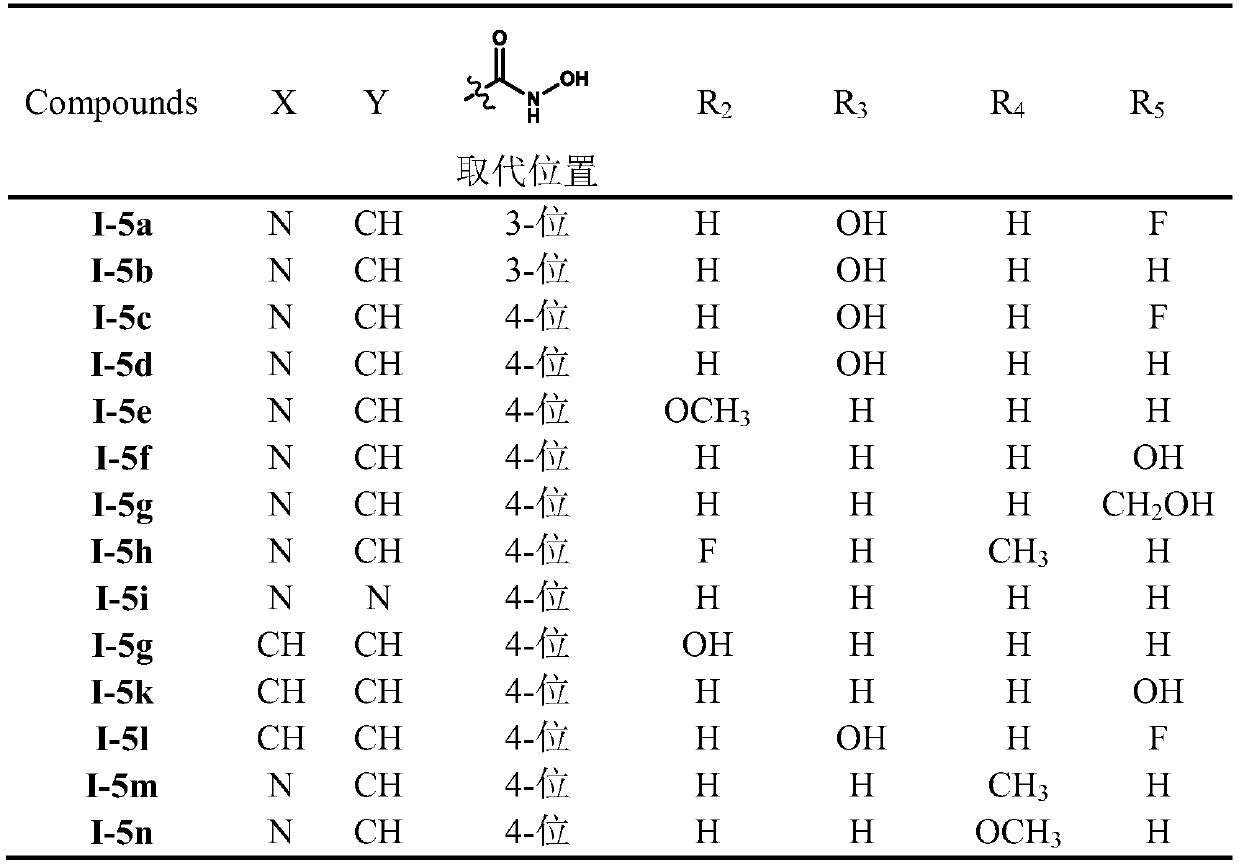
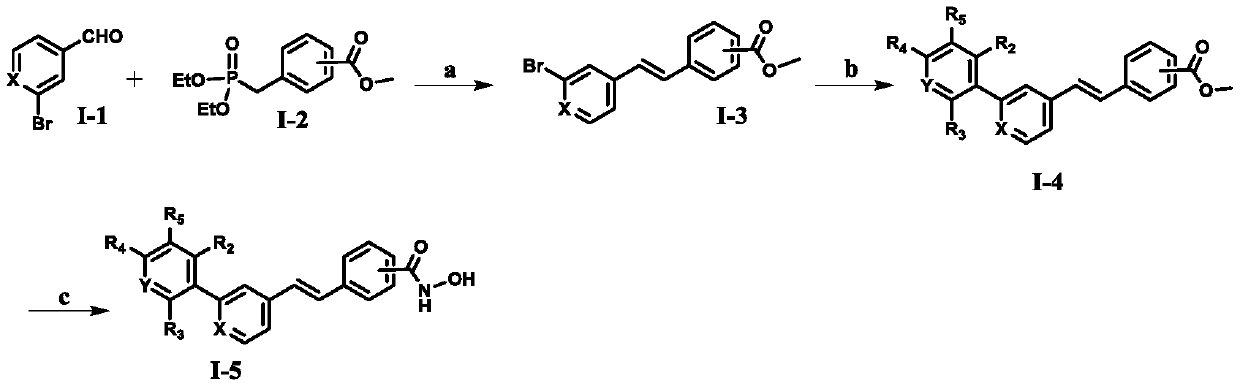
The invention relates to hydroxamic acid group-containing diarylethene LSD1 / HDACs double-target inhibitors, a preparation method thereof and application thereof in preparation of antitumor drugs, andbelongs to the technical field of medicinal chemistry. The compounds have a general formula shown in the specification, wherein 3-position or 4-position monosubstitution is preferred; R2 is preferablyOH, OCH3, F or H; R3 is preferably selected from H and CH2OH; R4 is preferably selected from H, CH3, F, or OCH3; R5 is preferably selected from H, OH, CH2OH, CH3, F and OCH3; X is N or CH; Y is N orCH. The compounds provided by the invention have relatively strong inhibitory activity on LSD1 and HDAC1 / 6, and have very good selectivity on LSD1, and the in vitro antitumor activity of the multiplecompounds on human colon cancer HCT-116 cell strains and human gastric cancer MGC-803 cell strains is superior to that of commercially available drug SAHA. A basis is provided for research and development of LSD1 / HDACs double-target inhibitor drugs, and the compounds can be used as candidates for further development or lead compounds for development of antitumor treatment drugs.
Owner:XINXIANG MEDICAL UNIV
Pyridine compound, preparation method thereof and application thereof in resisting gastric cancer
ActiveCN109369620ANovel structureReduced ability to migrateOrganic active ingredientsOrganic chemistryCancer drugsReactive site
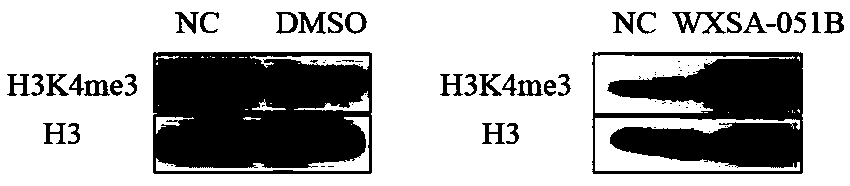
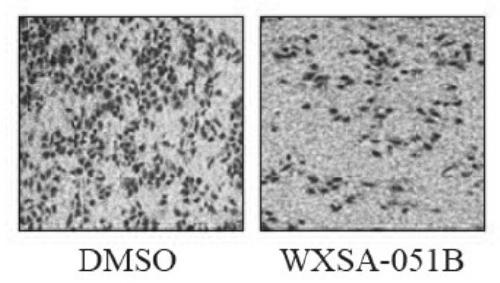
The invention provides a pyridine compound WXSA-051B which is designed and synthesized for aiming at an enzyme active site to inhibit RBP2 enzyme activity, and also provides a preparation method of the pyridine compound. Tests prove that the compound can obviously increase the level of H3K4me3 protein in a human gastric cancer cell BGC-823, has an obvious effect of inhibiting RBP2 enzyme activity,and can reduce the migration ability of the human gastric cancer cell BGC-823. The compound provided by the invention can be used as a gastric cancer inhibitor for preparing an anti-gastric cancer drug. The structure of the compound is as shown in the description.
Owner:SHANDONG UNIV
Water-soluble monodemethoxycurcumin derivative, and preparation method and use of water-soluble derivative
ActiveCN104447322AEnhanced inhibitory effectOrganic active ingredientsOxygen-containing compound preparationSolubilityHepatocellular carcinoma
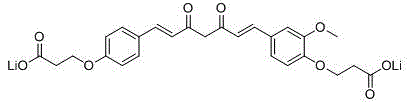
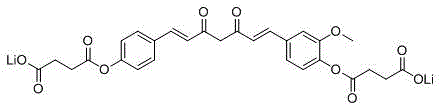
The invention provides a water-soluble monodemethoxycurcumin derivative as shown in general formula I and a pharmaceutically acceptable salt thereof. The compound has higher water solubility as well as high antitumor activity, has a function of inhibiting human gastric carcinoma cells MGC-803, human hepatocellular carcinoma cells Bel-7402, human chronic leukemia granulocytes K562 and human glioma U87 to different extents, and can be used for preparing an antitumor medicament, and the IC50 (Inhibitory Concentration 50) reaches the level of 1 [mu]M.
Owner:石磊 +2
Trichothecene-type compound and effect thereof
The invention provides a trichothecene-type compound, namely myrothecinol roridum. The structural formula of the compound is as follows (please see the specifications for the formula), the median inhibitory concentration of the trichothecene-type compound, namely the myrothecinol roridum to human stomach cancer cell lines MGC803 is 5.4+ / -0.5 [mu]m, and the median inhibitory concentration of the trichothecene-type compound, namely the myrothecinol roridum to human stomach cancer cell lines NCI-H1975 is 28.7+ / -1.3 [mu]m. The trichothecene-type compound can be applied to prodrugs for treating stomach cancer and lung cancer, and the material basis is laid for the next step of drug efficacy study of drugs for stomach cancer and lung cancer.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com