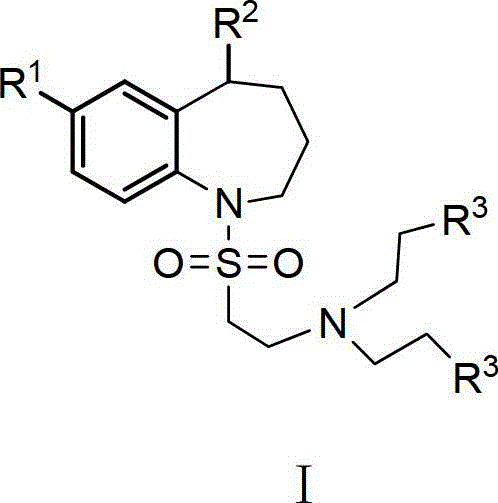
Benzo-azepine type derivative and preparation method and purpose thereof
A technology of benzonitrogen and its derivatives, which is applied in the field of medicine and can solve problems such as low cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation of Intermediate III-1:
[0071]
[0072] Add 3.91g (20mmol) of 7-chloro-3,4-dihydro-1H-benzo[b]azepine-5(2H)-one and 50mL of pyridine into the reaction flask equipped with stirring, thermometer and condenser , stir to dissolve, add 2.91g (24mmol) of 2-chloroethylsulfonyl chloride dropwise at 0°C, keep stirring at -10°C-5°C for 4h, TLC detection shows that the reaction is complete (developing agent: ethyl acetate: petroleum ether = 1: 3).
[0073] The reaction solution was poured into 200ml of 25°C cold water, stirred, and solids were precipitated. After filtering, the filter cake was washed with water and dried to obtain a brownish yellow solid crude product. The crude product was recrystallized with isopropanol to obtain an off-white solid which is intermediate III-1, with a purity of 98.1% (HPLC normalization method), and a yield of 81.0%. ES I-MS: 321.0.
Embodiment 2
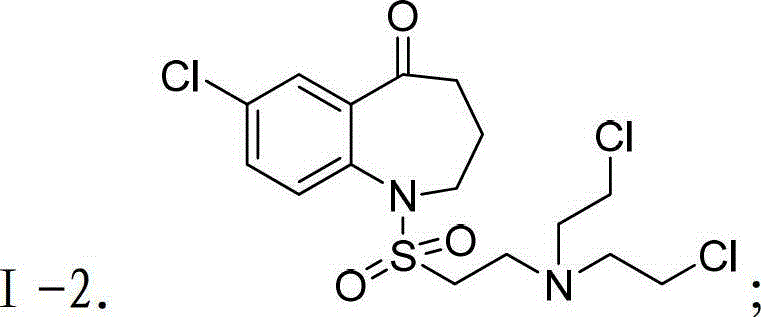
[0075] Preparation of Intermediate III-2:
[0076]
[0077] Add 4.58g (20mmol l ), add acetonitrile 50mL, potassium bicarbonate 5.0g (50mmol) and stir, stir at 0°C, add 2.91g (24mmol) of 2-chloroethylsulfonyl chloride dropwise, keep stirring at 5°C-20°C for 3h, TLC detection shows The reaction ends (developing solvent: ethyl acetate: petroleum ether = 1:3).
[0078] The reaction solution was poured into 200 mL of distilled water at 10°C, extracted with ethyl acetate three times, 50 mL each time, dried over anhydrous sodium sulfate, evaporated to dryness, and silica gel column chromatography to obtain a white solid as Intermediate III-2 with a purity of 99.7% (HPLC Normalization method), the yield was 85.8%. ES I-MS: 355.1.
Embodiment 3
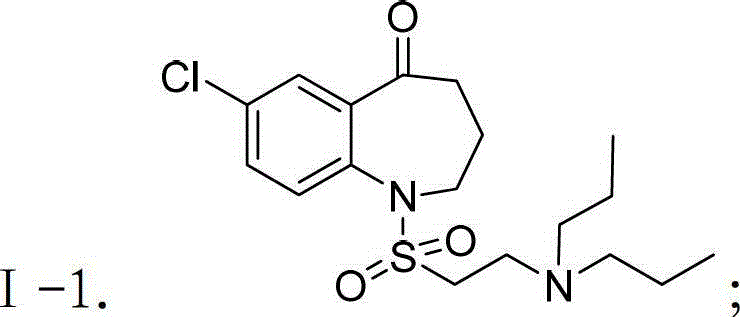
[0080] 1-(2-(Dipropylamino)ethylsulfonyl)-7-chloro-3,4-dihydro-1H-benzo[b]azepine-5(2H)-one and compound Ⅰ-1 Preparation of:
[0081]
[0082] Add 3.22g (10mmol) intermediate III-1, 2.0g (20mmol) potassium bicarbonate, 2.02g (20mmol) dipropylamine and 50mL acetonitrile into the reaction flask equipped with stirring, thermometer and condenser, and stir , react at 80°C-90°C for 12 hours, cool to room temperature naturally, filter out insoluble matter, pour the filtrate into 100mL distilled water, extract 3 times with ethyl acetate, 50mL each time, combine the organic phases, and wash the organic phases with saturated brine 3 times , 50 mL each time, dried over anhydrous sodium sulfate, evaporated the solvent under reduced pressure, obtained white solid compound Ⅰ-1 by silica gel column chromatography, purity 99.2% (HPLC normalization method), yield 81.6%, HRMS (m / z)[M+H] + : 387.1504.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com