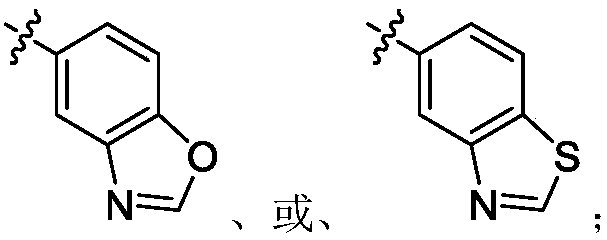
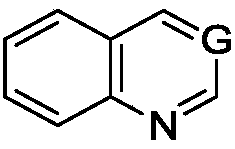
Nitrogen heterocyclic ring compound, preparation method, midbody, composition and application
A nitrogen heterocyclic compound and compound technology, applied in the field of nitrogen-containing heterocyclic compounds, can solve the problems of poor compound effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0408]
[0409] N-(3-(4-((4-([1,2,4]triazolo[4,3-c]pyrimidin-7-yloxy)-3-methylphenyl)amino)quinazole Synthesis of olin-6-yl)cyclohexyl-3-en-1-yl)acrylamide
[0410] Step A: Preparation of 2-chloro-6-hydrazinopyrimidine: 2,6-dichloropyrimidine (25g, 167.81mmol) was dissolved in 350mL isopropanol, and hydrazine hydrate (29.5g, 503.44 mmol, 85%), exothermic during the dropwise addition and a white solid precipitated, and stirred at room temperature for 1 hour after the addition. The solvent was removed under reduced pressure, the residue was stirred with water (50 mL) for 30 minutes, filtered, the filter cake was washed with water, and dried to obtain 22.4 g of a white solid with a yield of 92.3%.
[0411] Step B: Preparation of 7-chloro-[1,2,4]triazolo[4,3-c]pyrimidine: 2-chloro-6-hydrazinopyrimidine (21 g, 145.27 mmol) was dispersed in 210 mL of trimethylorthoformate The ester was stirred overnight at 60°C, and the reaction liquid became clear. Add p-toluenesulfonic acid ...
Embodiment 2
[0420]
[0421] N-(4-([1,2,4]triazolo[4,3-c]pyrimidin-7-yloxy)-3-methylphenyl)-6-(3-amino-1H-pyridine Synthesis of oxazol-5-yl)quinazolin-4-amine
[0422] Step A: Preparation of 5-bromo-3-((tert-butoxycarbonyl)amino)-1H-pyrazole-1-carboxylic acid tert-butyl ester: 5-bromo-1H-pyrazol-3-amine (250 mg, 1.54mmol), triethylamine (627mg, 6.19mmol) and di-tert-butyl dicarbonate (846mg, 3.87mmol) were added to dichloromethane (10mL), and then 4-dimethylaminopyridine (38mg, 0.31mmol ), the resulting reaction solution was stirred at room temperature for 16 hours. After the reaction was completed, the reaction solution was concentrated under reduced pressure to obtain a residue which was separated by column chromatography to obtain 135 mg of a white solid with a yield of 24%.
[0423]Step B: (E)-(5-(3-cyano-4-(((dimethylamino)methylene)amino)phenyl)-1H-pyrazol-3-yl)carbamate tert-butyl ester Preparation: tert-butyl 5-bromo-3-((tert-butoxycarbonyl)amino)-1H-pyrazole-1-carboxylate (1...
Embodiment 3
[0427]
[0428] (R)-N-(1-(4-((4-([1,2,4]triazolo[4,3-c]pyrimidin-7-yloxy)-3-methylphenyl) Synthesis of amino)quinazolin-6-yl)piperidin-3-yl)acrylamide
[0429] Step A: Preparation of 2-amino-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile: 2-amino -5-Bromo-benzonitrile (1000mg, 5.08mmol), bis-pinacol borate (1930mg, 7.60mmol), potassium acetate (1490mg, 15.18mmol) and [1,1'-bis(diphenyl Phosphine)ferrocene]dichloropalladium dichloromethane complex (21mg, 0.05eq.) was mixed in 1,4-dioxane (15mL), and the mixture was stirred at 80°C for 16 hours under the protection of argon . Celite was filtered, and the filtrate was concentrated under reduced pressure. The obtained residue was dissolved in ethyl acetate (10 mL), and washed with saturated brine (10 mL). The organic phase was separated and dried with anhydrous sodium sulfate, filtered, concentrated and the residue obtained was separated and purified by silica gel column to obtain 1400 mg of white solid with a y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com