Method of establishing animal model of HER2 positive tumor by using normal mouse
An animal model and mouse technology, applied in the biological field, can solve the problems of animal death, high cost, cumbersome operation, etc., and achieve the effects of wide application prospects, saving test costs, and overcoming limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Screening of human-derived HER2-positive tumor cells
[0044] 10 HER2-positive human tumor cell lines were selected, including 7 breast cancer cell lines: SK-BR-3, MDA-MB-453, BT-474, HCC1008, HCC1569, HCC2218, HCC1419, and 1 ovarian cancer cell line: SKOV- 3. Two gastric cancer cell lines: SGC-7901, NCI-N87, all cells were purchased from ATCC.
[0045] Cell culture: The above cell lines were recovered from cryopreservation tubes, cultured in RPMI 1640 medium, and subcultured until the cells were in a good growth state for later use.
[0046] Inoculation: 60 8-week-old Balb / c mice were randomly divided into 10 groups with 6 mice in each group. After one week of adaptation, the above 10 kinds of tumor cells were inoculated respectively. The inoculated cell concentration was 5×10 6 1 / ml, 0.1ml was inoculated into each mouse, and the inoculation site was the armpit of the left forelimb.
[0047] After tumor inoculation, the tumor growth was observed and record...
Embodiment 2
[0049] Example 2 Optimization of the inoculum amount of human gastric cancer cell NCI-N87 transplanted into mice
[0050] In order to establish the best NCI-N87 animal model, the inoculation amount of human gastric cancer cell NCI-N87 was optimized.
[0051] Twenty-four 8-week-old Balb / c mice were randomly divided into 4 groups, 6 mice in each group. After one week of adaptation, the inoculation concentration was 1.25×10 6 pcs / ml, 2.5×10 6 pcs / ml, 5×10 6 pcs / ml, 1×10 7 0.1 ml of human gastric cancer cell NCI-N87 cells / ml was inoculated at the axilla of the left forelimb. After tumor inoculation, the tumor growth was observed and recorded daily, and 2 weeks after inoculation, all animals were sacrificed to end the experiment, and the test results were evaluated.
[0052] Result: 1.25×10 6 Three animals in the unit / ml group developed tumors, and the tumor formation rate was 50%. 2.5×10 6 pcs / ml, 5×10 6 pcs / ml, 1×10 7 All the animals in the group / ml group have tumor grow...
Embodiment 3
[0053] Example 3 Solid Phase Synthesis of Tumor Vaccine Peptide E75
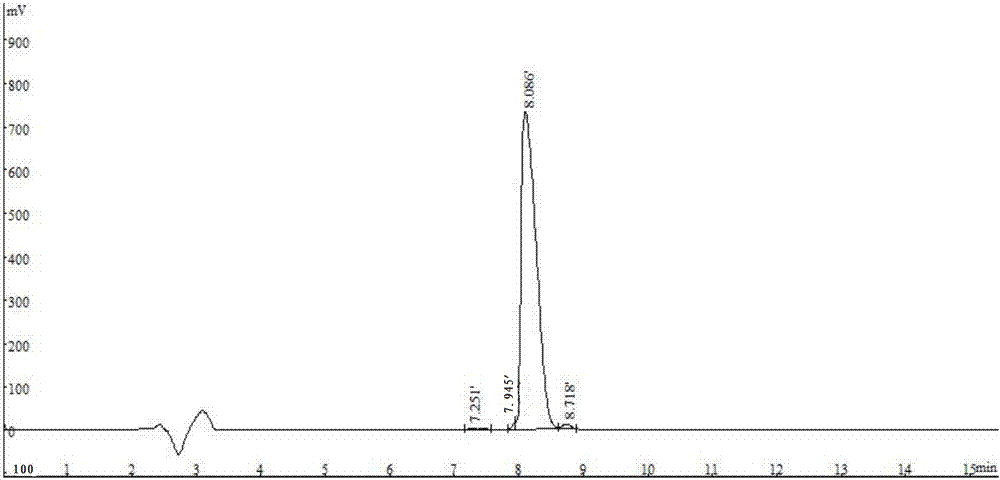
[0054] E75 entrusts Shanghai Aobo Biotechnology Co., Ltd. to synthesize it by conventional solid-phase technology, and the purity of the synthesized peptide is >98%, see figure 1 .
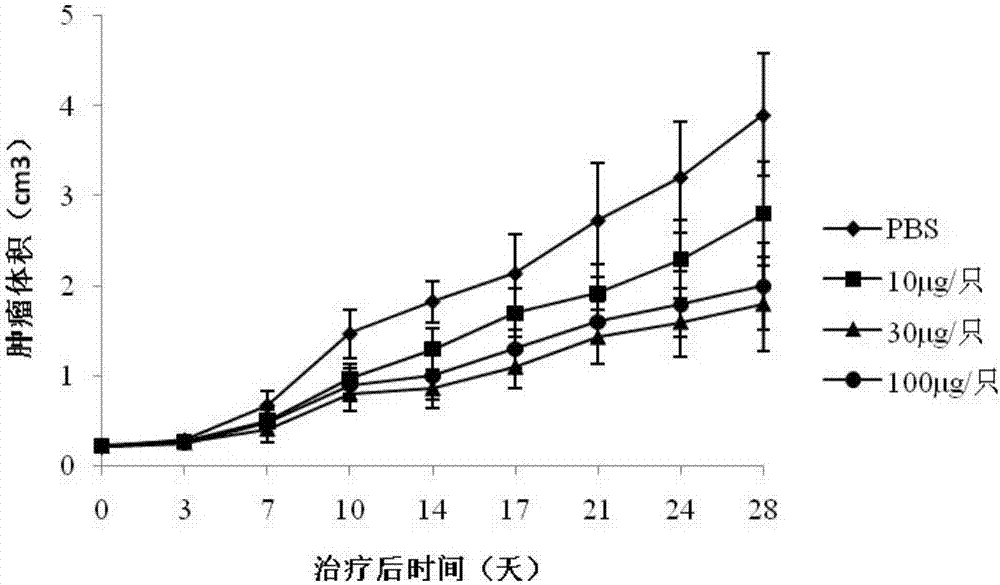
[0055] Evaluation of NCI-N87 animal model for therapeutic effect of tumor vaccine
[0056] Inoculation: 40 8-week-old female Balb / c mice were used in this study. Human gastric cancer cells NCI-N87 in the logarithmic growth phase were collected and prepared into 5×10 6 0.1ml of the cell suspension was planted in the left armpit of the mice; the tumor growth was observed every day, and the immunotherapy was started when the tumor diameter grew to about 8mm.
[0057] Immunization: 40 mice were randomly divided into 4 groups, 10 in each group, respectively negative control group (PBS) and E75 low, medium and high dose groups, E75 respectively with 100, 300, 1000 μg / ml and Freund's incomplete Adjuvant (FIA) was mixed and emulsifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com