Trichothecene-type compound and effect thereof
A technology for trichothecenes and compounds, which are applied to trichothecenes-type compounds and their fields of action, and can solve problems such as activity test reports and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Fermentation and extraction of embodiment 1 bacterial classification
[0028] Myrothecium roridum is deposited in the American Type Culture Collection (ATCC) with the accession number ATCC60379. The strain was revived by streaking, inoculated into PDB solid medium, and activated in a 28°C incubator for 5 days. Use a scalpel to cut out small pieces of colonies from the PDB solid, inoculate them into PDB liquid medium, and cultivate them on a shaker at 28° C. and 160 rpm for 5 days to obtain seed liquid. Then the seed solution was inoculated into the solid medium, and cultured statically for 30 days in a constant temperature and humidity chamber at 28°C. Take the fermented product of rice, add 200 mL of ethyl acetate per 100 g of the fermented product, cold soak and extract twice, combine the extracts and concentrate under reduced pressure until there is no ethyl acetate smell to obtain ethyl acetate extract.
Embodiment 2
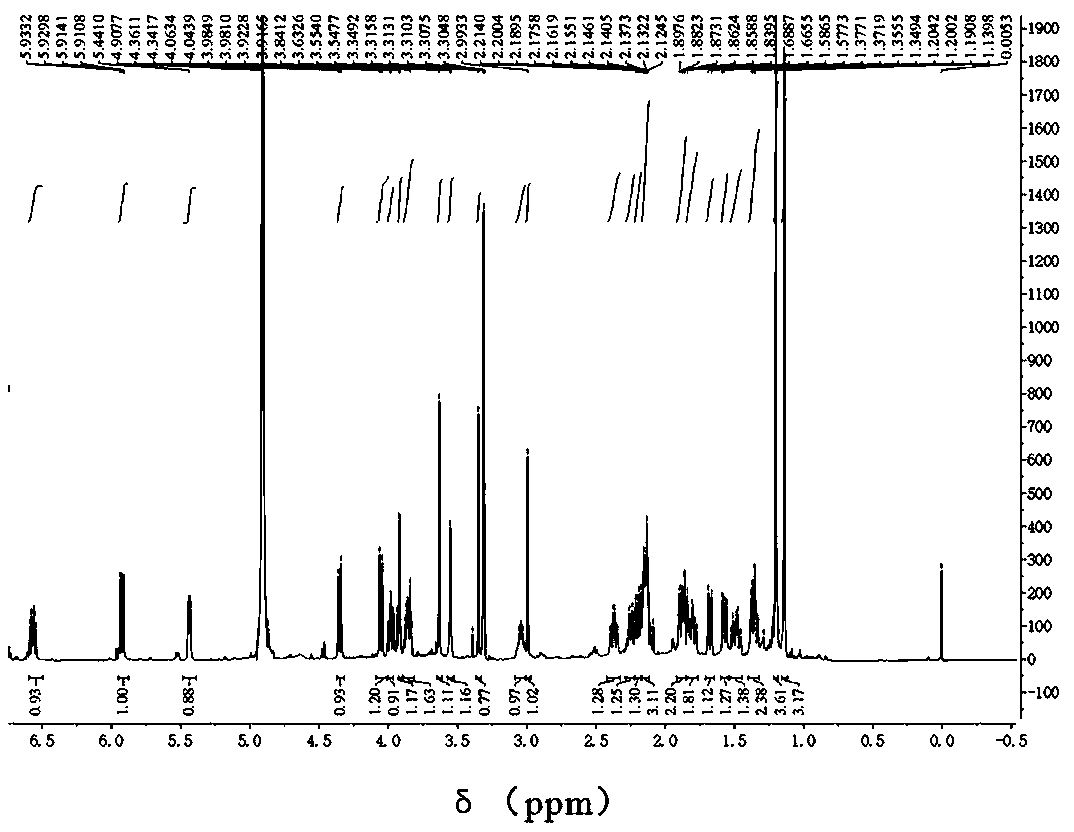
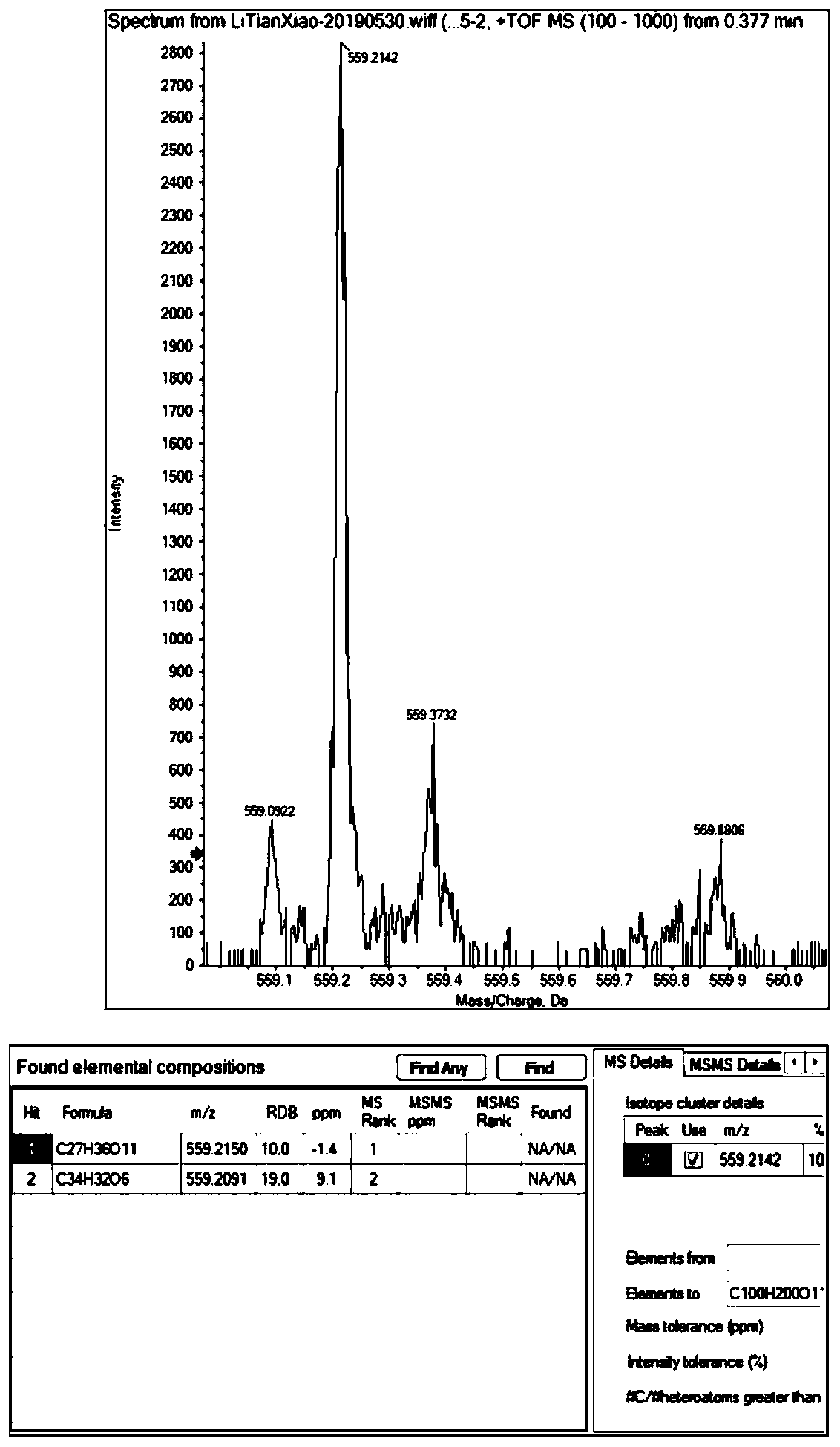
[0030] The extractant was chromatographed on a macroporous resin column, adsorbed overnight, and eluted with 10%, 30%, 50%, 70%, 90%, 100% methanol-water gradient, and each gradient was combined and spin-dried until there was no liquid to obtain 6 a subcomponent. The second subcomponent (30% part) was separated and purified by semi-preparative high performance liquid chromatography, mobile phase: 35% MeOH, flow rate 4mL / min, detection wavelength 210nm, peak time 14.8-15.3min. The peak was picked up and evaporated to complete dryness to obtain 35.0 mg of a white solid. The white solid was subjected to high-resolution mass spectrometry and one-dimensional two-dimensional NMR analysis results as follows Figure 1-8 , the structure of the compound is determined to be
[0031] High resolution mass spectrometry [M+Na] + m / z559.2142, the calculated value is 559.2150 (C 27 h 36 o 11 Na), the molecular formula of the compound is C 27 h 36 o 11 . That 1 H and 13 C NMR data...
Embodiment 3
[0035] Embodiment 3 pharmacological experiment
[0036] Using the MTT method, human gastric cancer cell line MGC803 and human lung adenocarcinoma cell line NCI-H1975 in logarithmic growth phase were taken, rinsed with PBS, digested with 0.25% trypsin, added fresh medium and blown into a single cell suspension. Count with a hemocytometer and adjust the cell concentration to 5-7×10 3 cells / mL, take 190 μL / well and add to 96-well plate, set CO 2 Incubator (37°C, 5% CO 2 , saturated humidity) culture. After 24 hours, 10 μL / well of the drug was added, and the working solution of the sample was diluted with the culture medium to a final concentration of 50, 25, 12.5, 6.25, and 3.125 μM, respectively. Three replicate wells were set for the sample addition group and the blank group. After continuing to culture for 48 h, 20 μL of MTT working solution was added. After continuing to culture for 4 h, discard the medium, add DMSO 150 μL / well, place on a plate shaker and shake at 500 rp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com