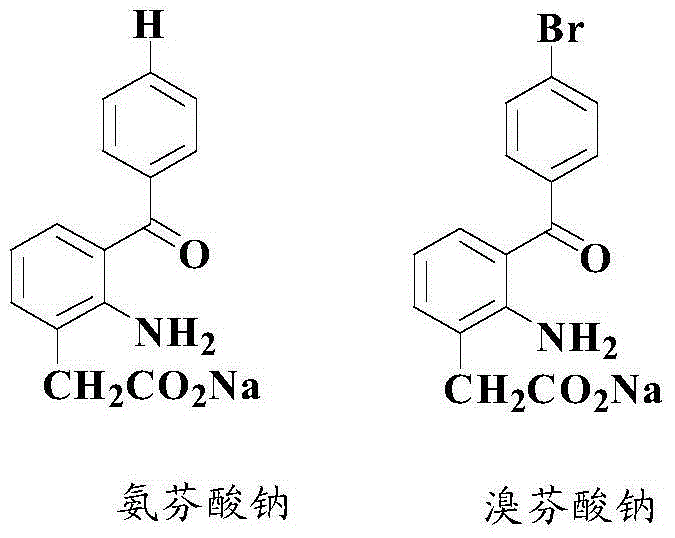
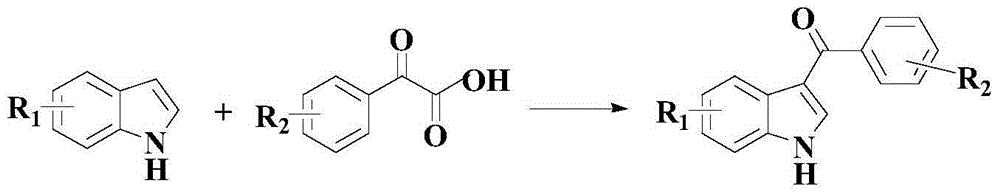
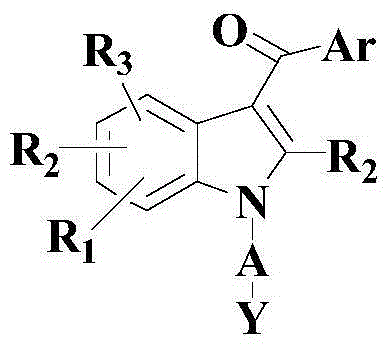
Anti-cancer activity indole derivative, synthesis method and uses thereof
A technology of indole derivatives and anticancer drugs, applied in the direction of organic active ingredients, drug combinations, medical preparations containing active ingredients, etc., can solve the problem of undiscovered anticancer activity, active targets and substituents of indole derivatives There is no clear understanding and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: 7-(4-fluorobenzoyl)indole
[0066]
[0067] At room temperature, add 100ml of solvent (a mixture of 2-MeTHF and water, the volume ratio of which is 2:1), 20mmol of 7-cyanindole, 40mmol of p-fluorophenylboronic acid, 1mmol of palladium trifluoroacetate, 1.5 mmol of the above-mentioned ligand L2 and 200 mmol of methanesulfonic acid; the temperature was raised to 80° C. under stirring, and the reaction was kept for 35 hours.
[0068] After the reaction is finished, add 100ml of water to the reaction mixture, extract 2-4 times with ether, combine the ether layers, and dry with anhydrous sodium sulfate, filter, evaporate and remove the ether for concentration, pass the concentrate through a 300-400 mesh silica gel column , using a mixture of ethyl acetate / petroleum ether as the eluent, wherein the volume ratio of ethyl acetate to petroleum ether was 1:10, and the eluate was concentrated to obtain the title compound as a yellow solid, named A. It has a yield of...
Embodiment 2
[0072] Example 2: 7-(4-Chlorobenzoyl)indole
[0073]
[0074] At room temperature, add 100ml of solvent (a mixture of 2-MeTHF and water, the volume ratio of which is 2:1), 20mmol of 7-cyanindole, 20mmol of p-chlorophenylboronic acid, 0.4mmol of palladium trifluoroacetate to the reaction vessel , 1 mmol of the above-mentioned ligand L2 and 100 mmol of methanesulfonic acid; the temperature was raised to 60° C. under stirring, and the reaction was kept for 40 hours.
[0075] After the reaction is finished, add 100ml of water to the reaction mixture, extract 2-4 times with ether, combine the ether layers, and dry with anhydrous sodium sulfate, filter, evaporate and remove the ether for concentration, pass the concentrate through a 300-400 mesh silica gel column , using a mixture of ethyl acetate / petroleum ether as the eluent, wherein the volume ratio of ethyl acetate to petroleum ether was 1:5, and the eluate was concentrated to obtain the title compound as a yellow solid, name...
Embodiment 3
[0079] Example 3: 7-(3,4,5-trimethoxybenzoyl)indole
[0080]
[0081] At room temperature, add 100ml of solvent (a mixture of 2-MeTHF and water, the volume ratio of which is 2:1), 20mmol of 7-cyanindole, 60mmol of 3,4,5-trimethoxyphenylboronic acid, 1.5 mmol of palladium trifluoroacetate, 2 mmol of the above-mentioned ligand L2 and 300 mmol of methanesulfonic acid; the temperature was raised to 100° C. under stirring, and the reaction was kept for 30 hours.
[0082] After the reaction is finished, add 100ml of water to the reaction mixture, extract 2-4 times with ether, combine the ether layers, and dry with anhydrous sodium sulfate, filter, evaporate and remove the ether for concentration, pass the concentrate through a 300-400 mesh silica gel column , using a mixture of ethyl acetate / petroleum ether as the eluent, wherein the volume ratio of ethyl acetate to petroleum ether was 1:15, and the eluate was concentrated to obtain the title compound as a yellow solid, named as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com