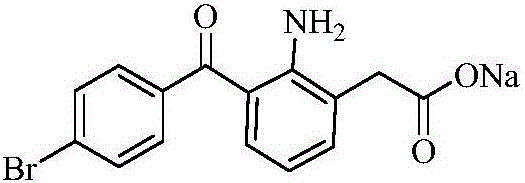
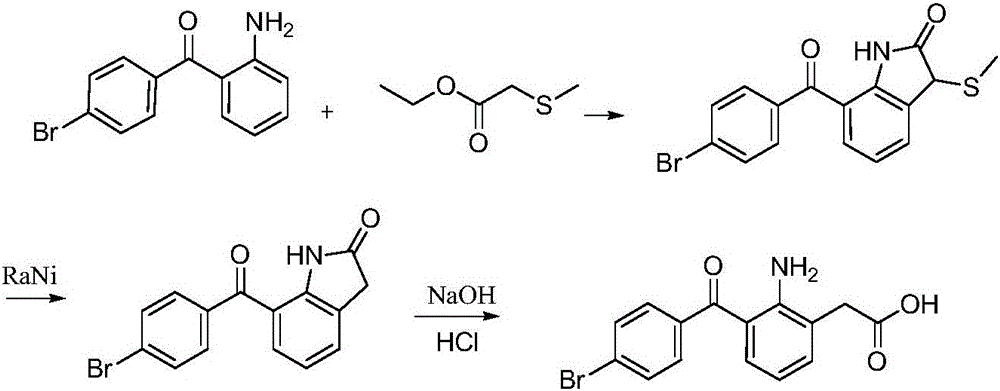
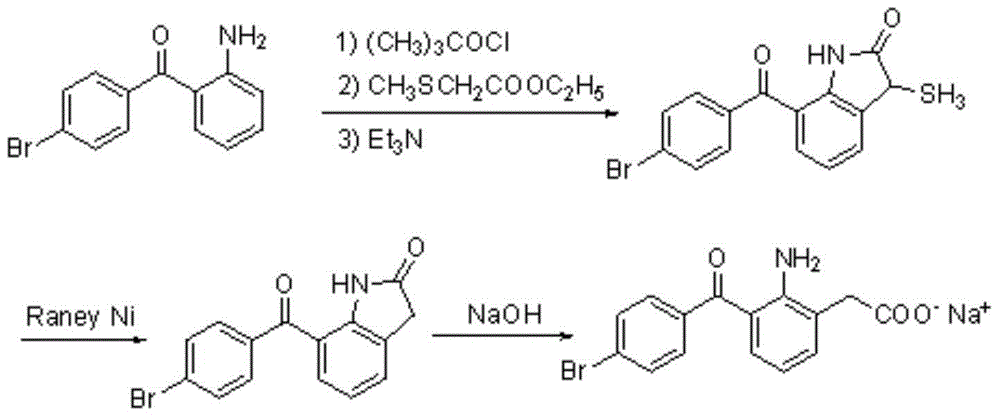
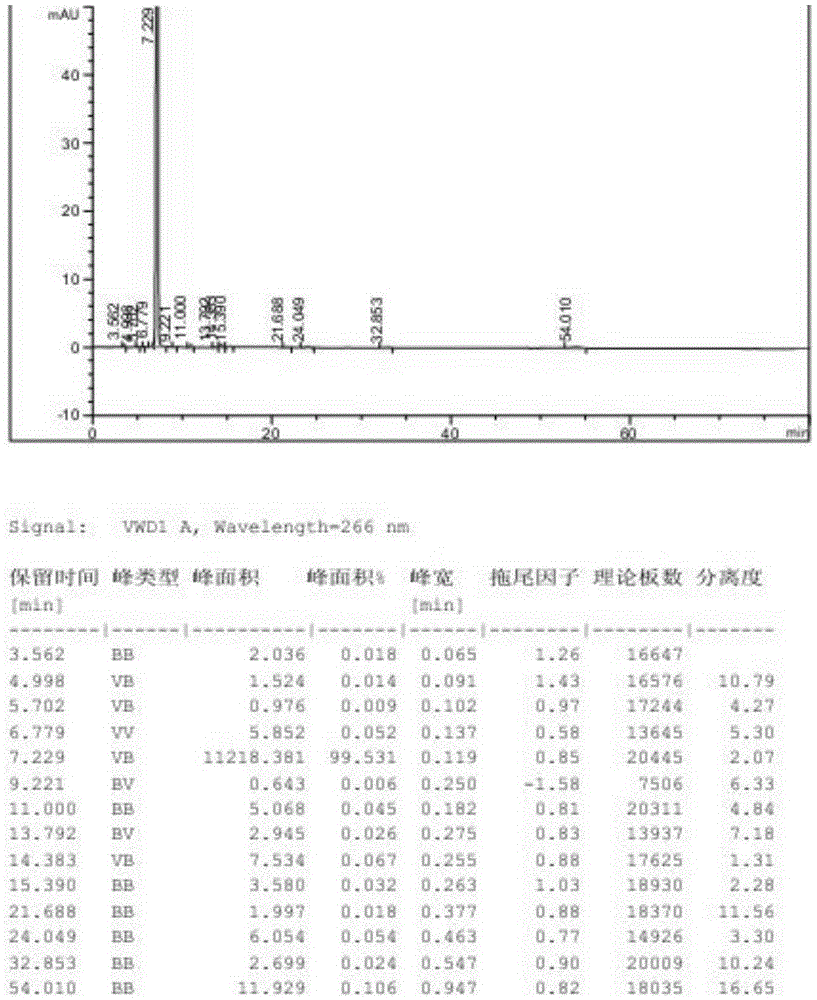
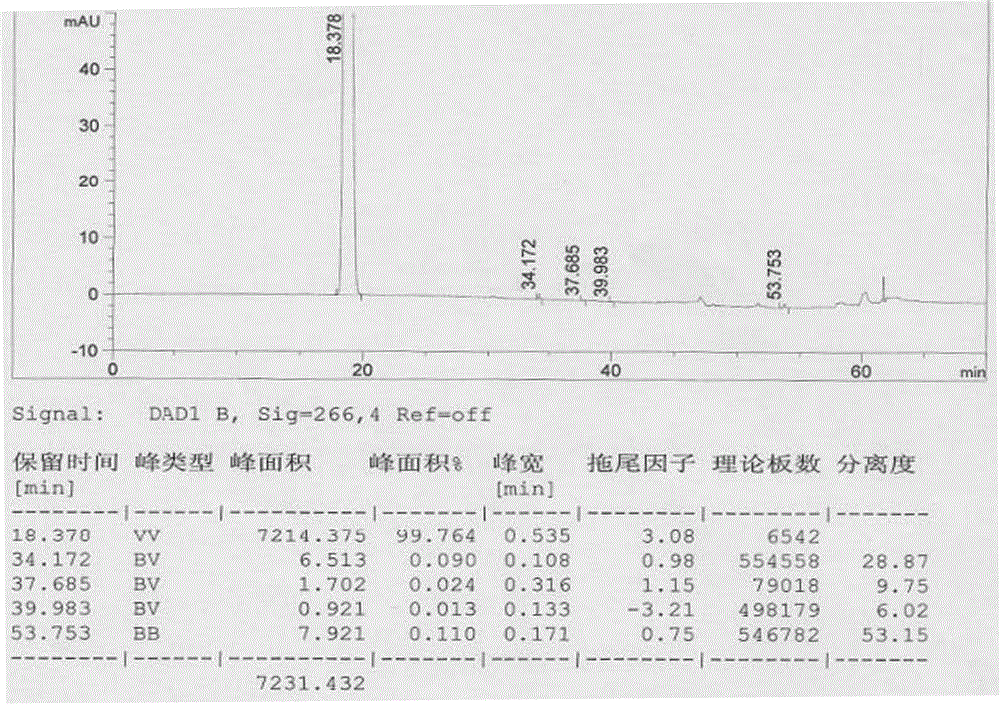
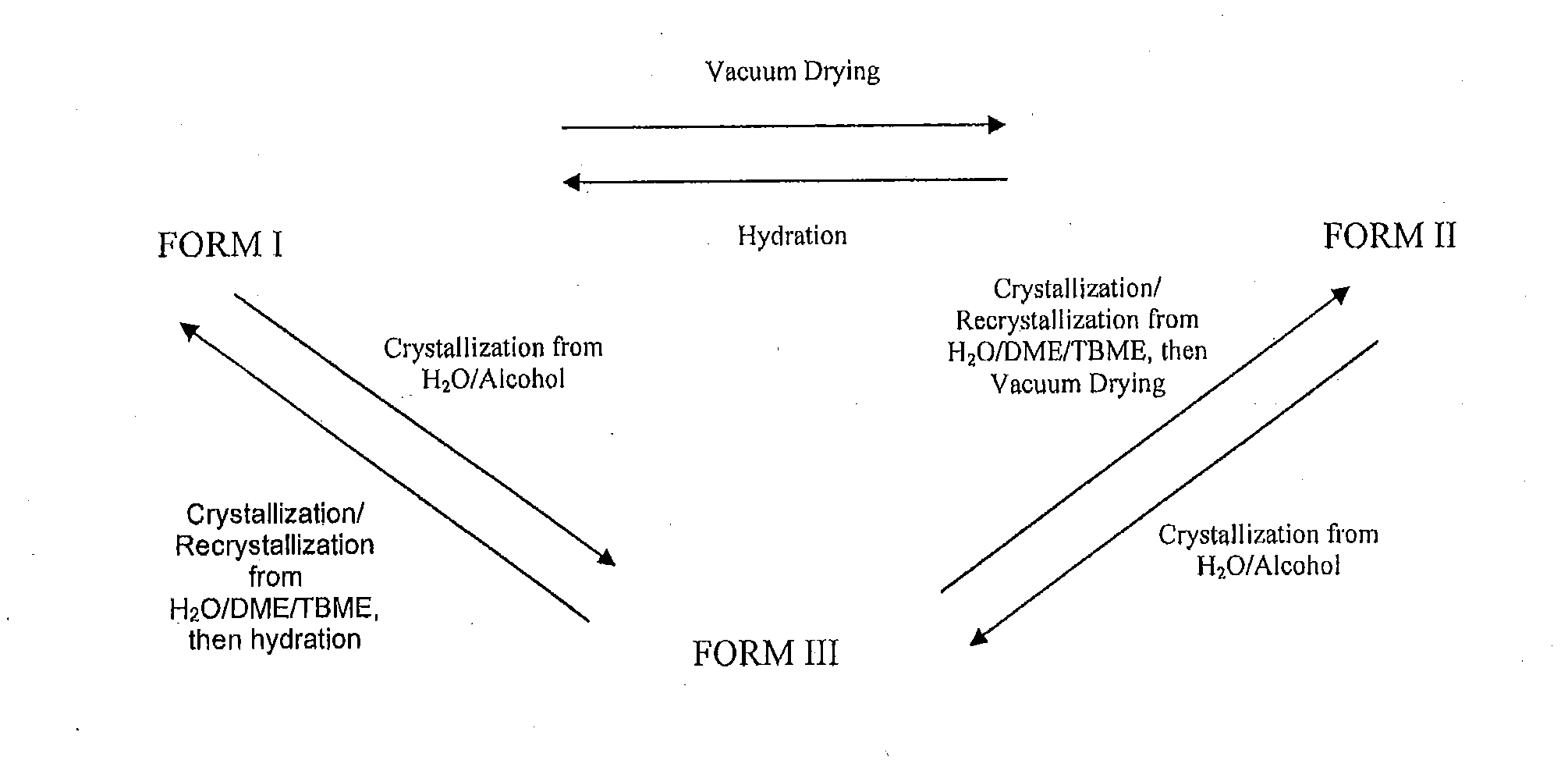
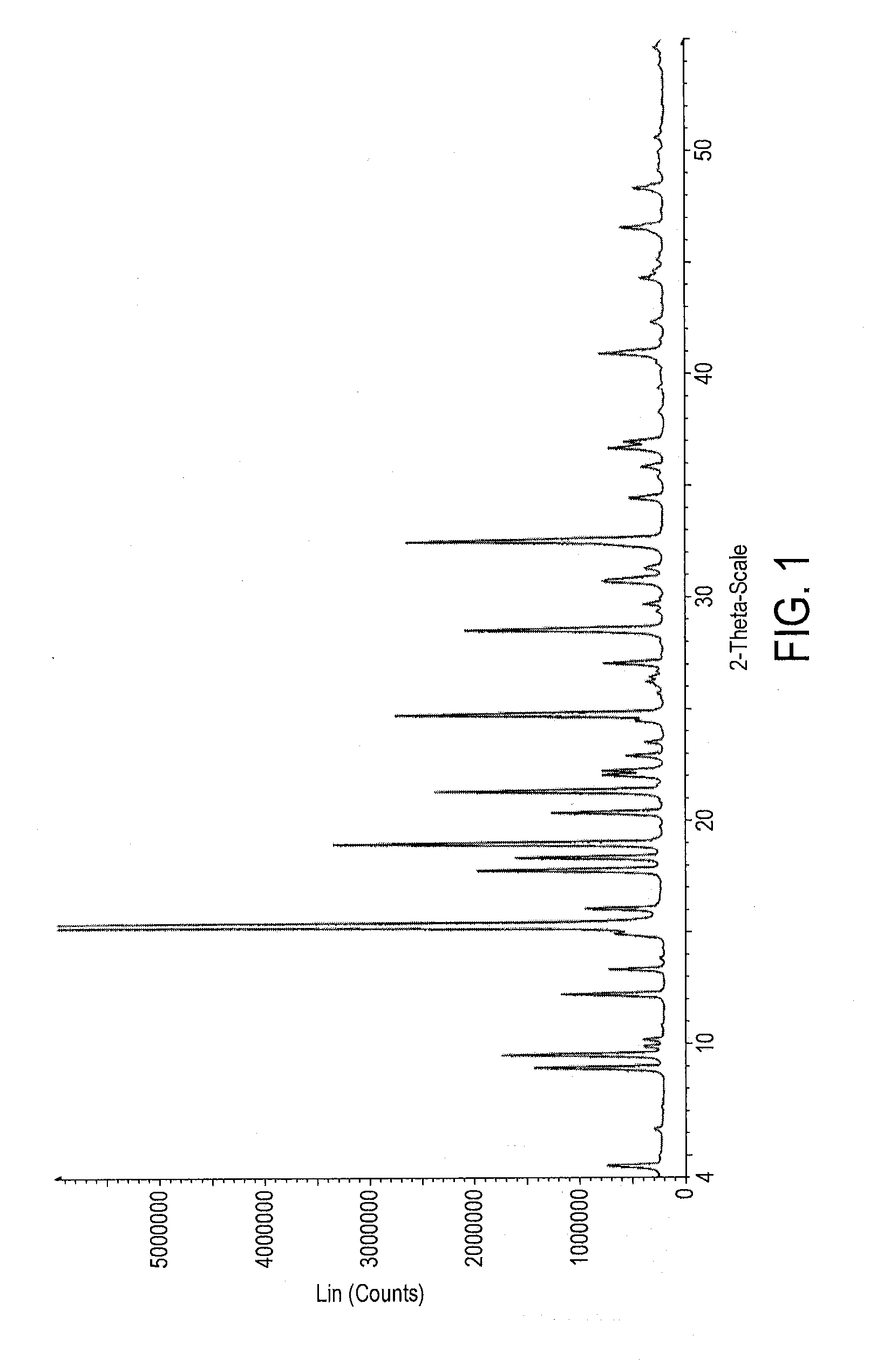
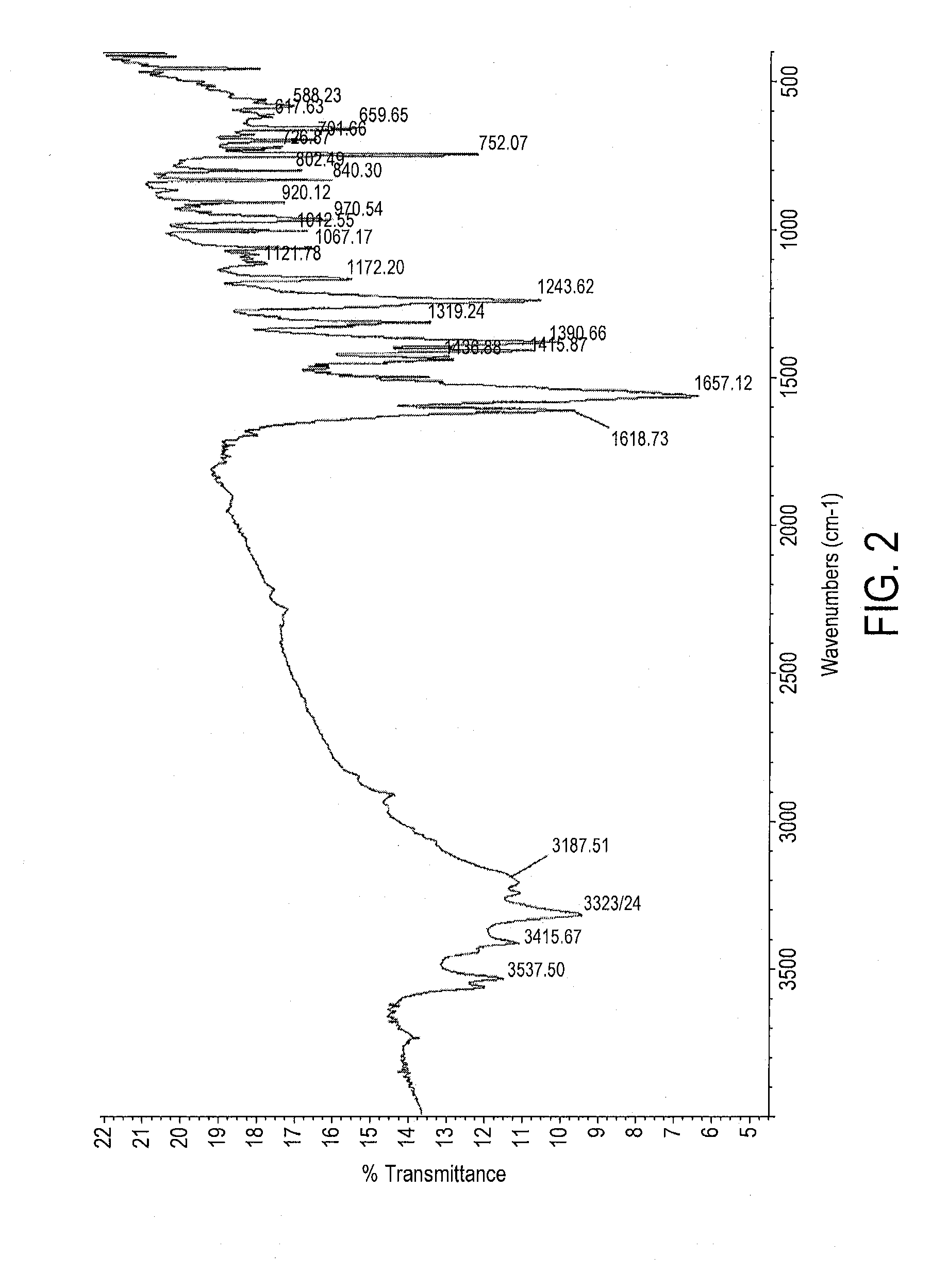
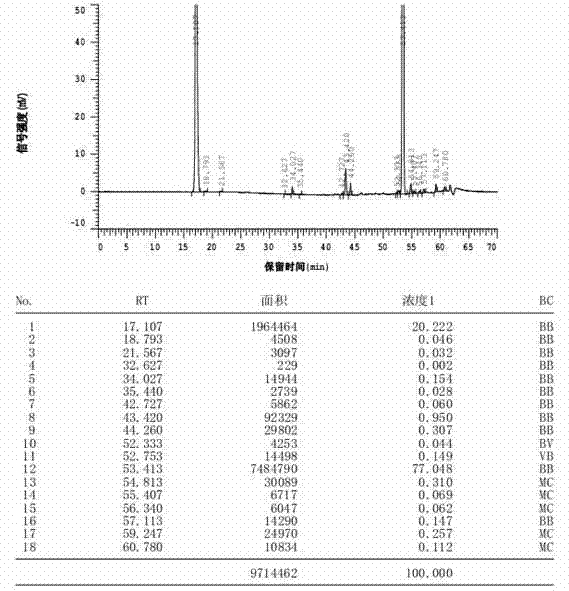
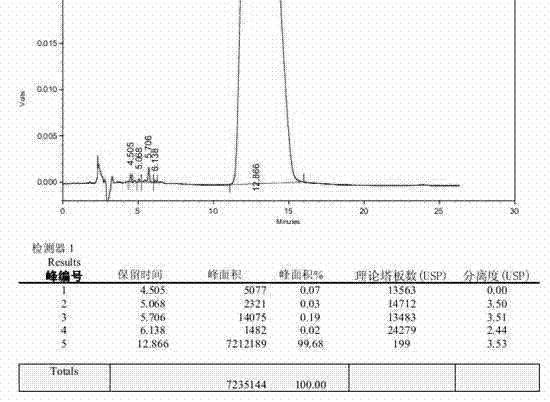
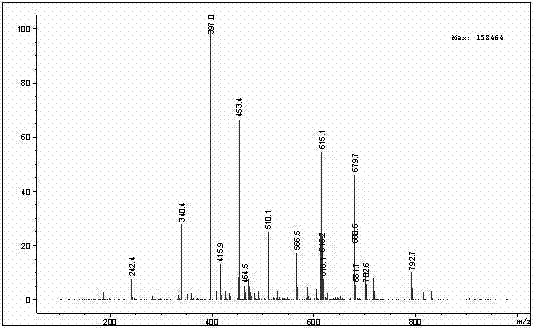
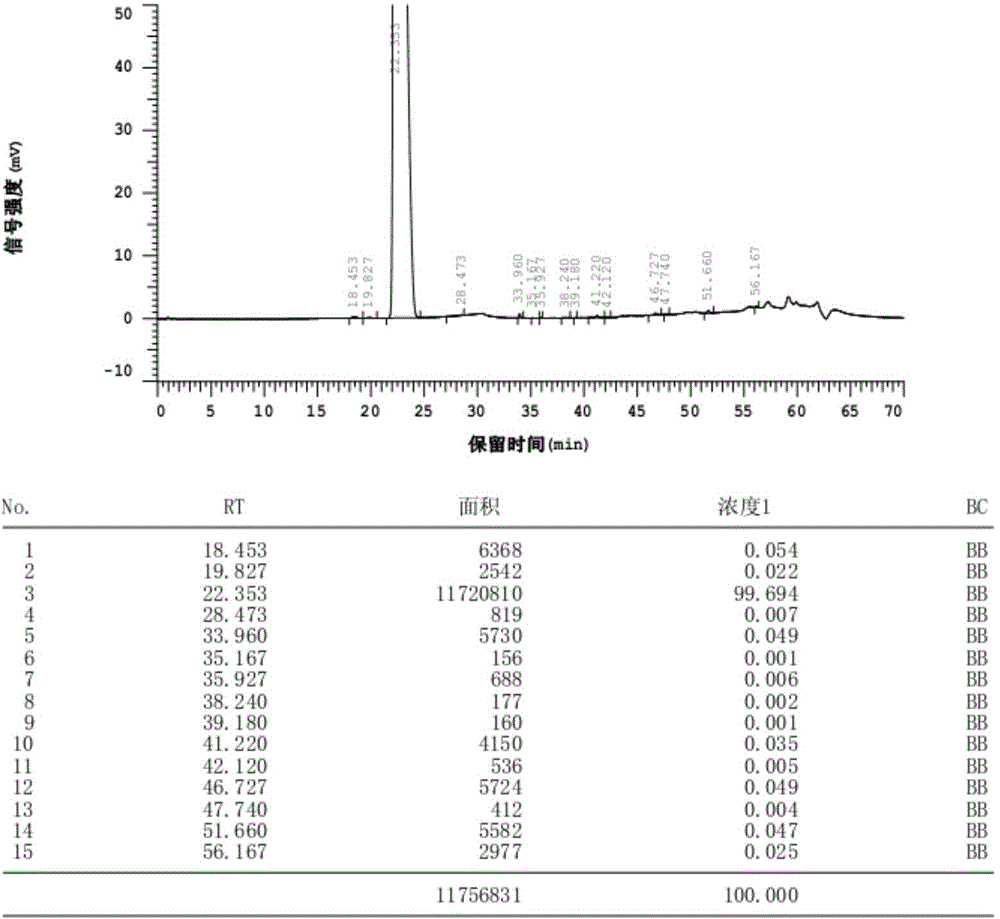
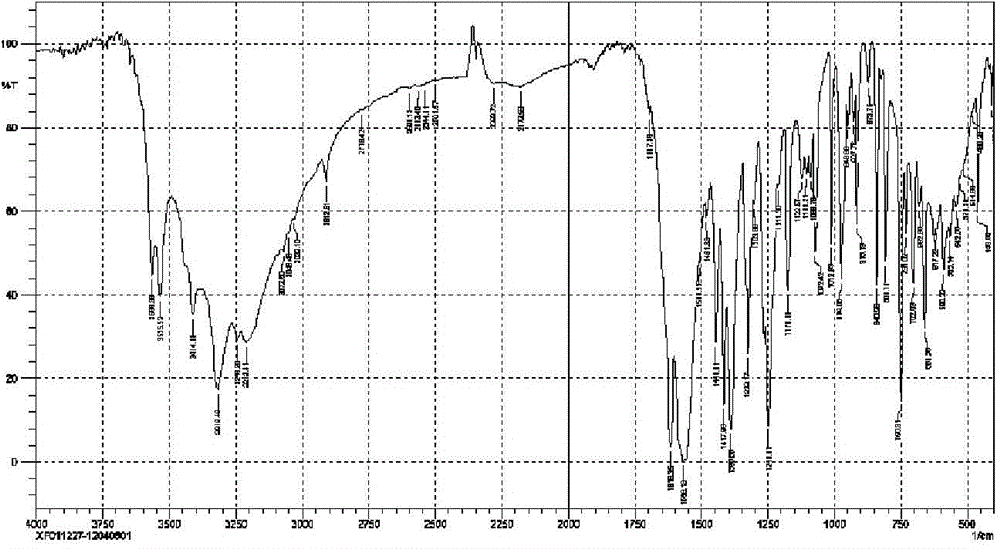
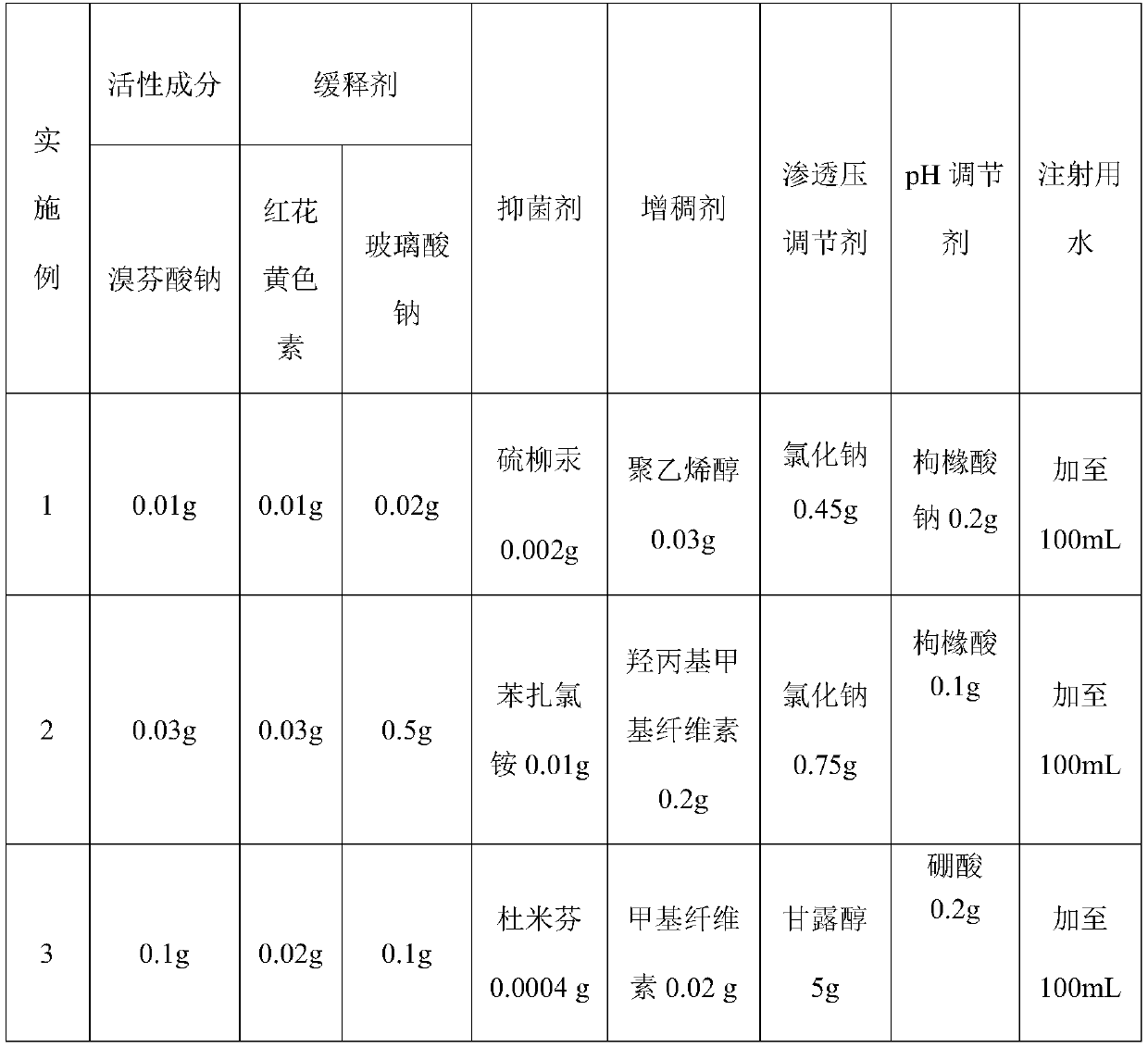
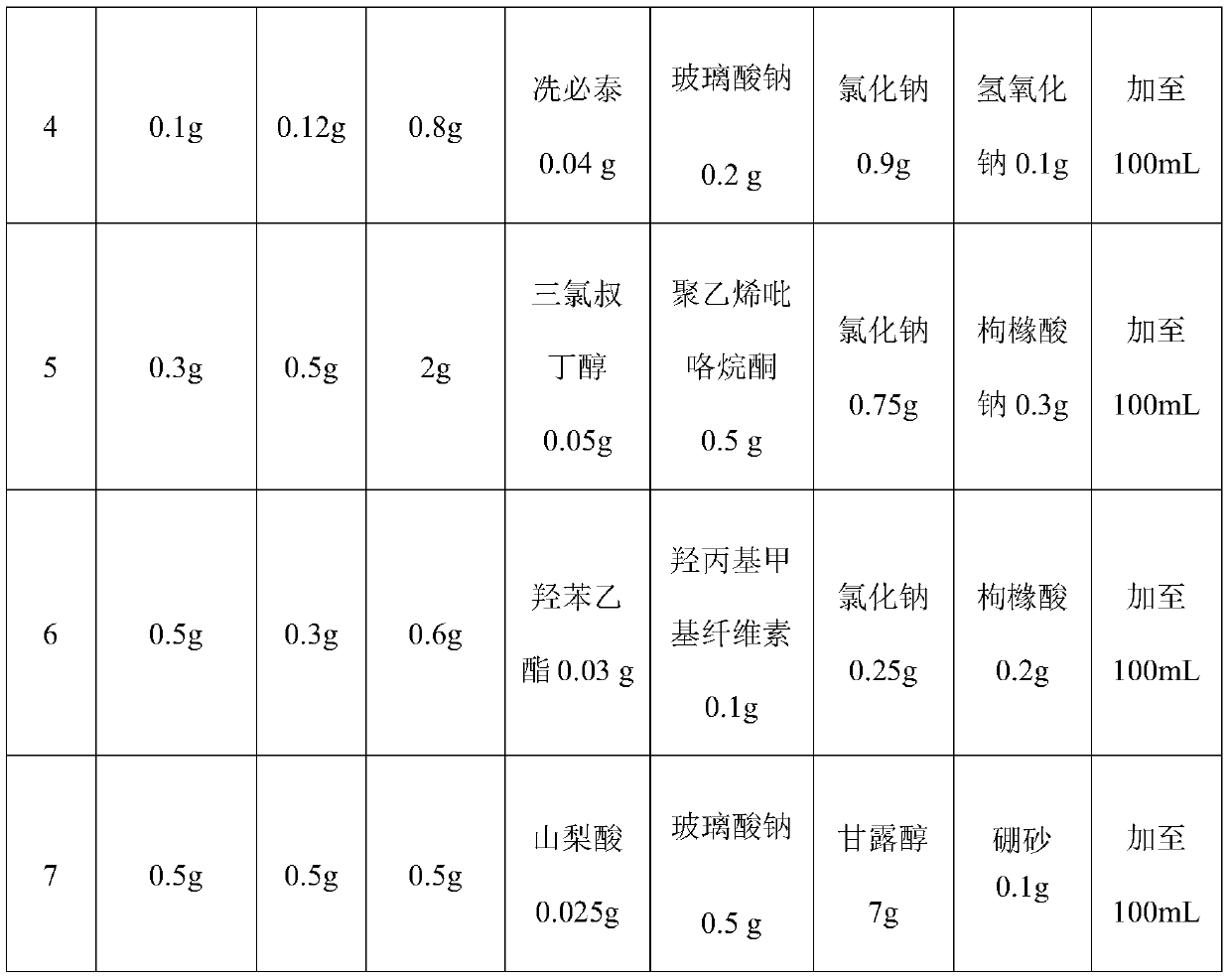
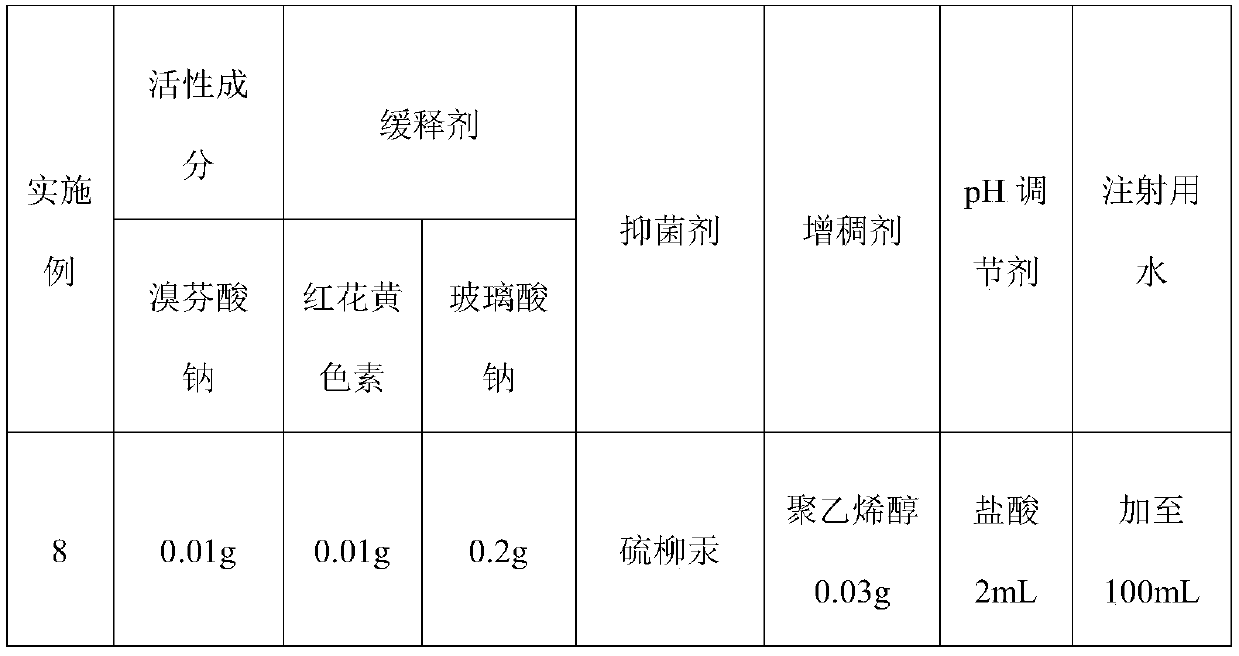
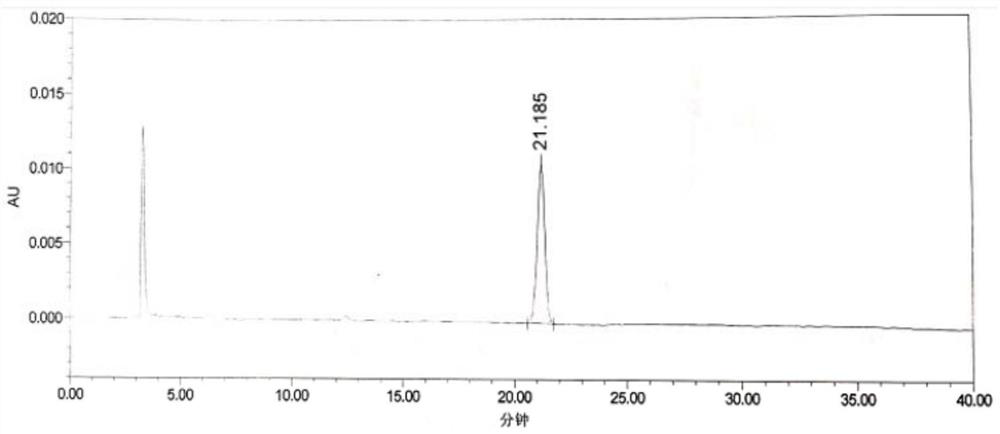
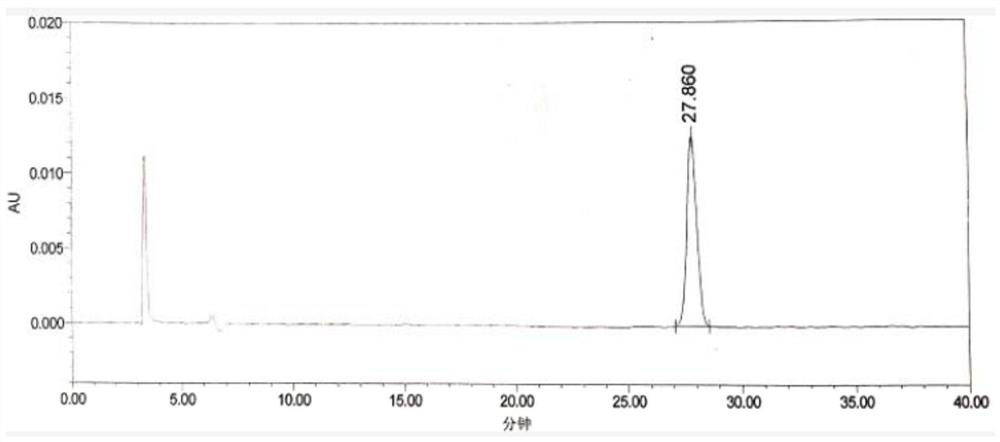
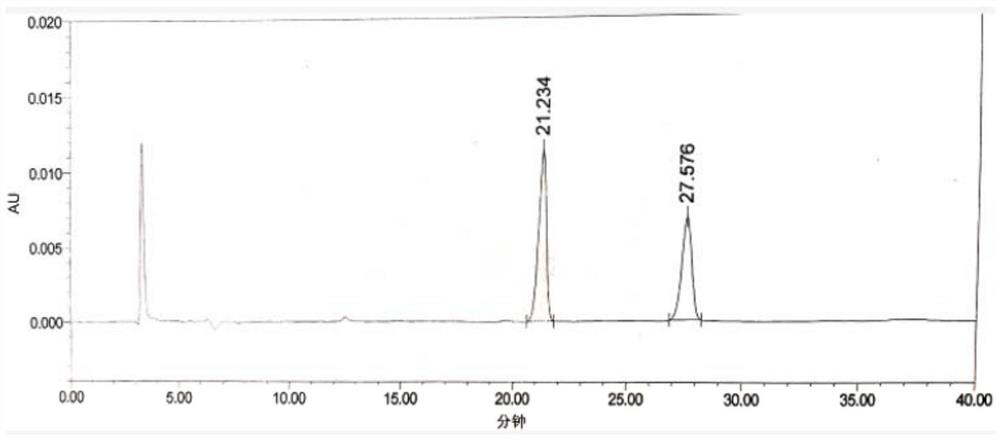
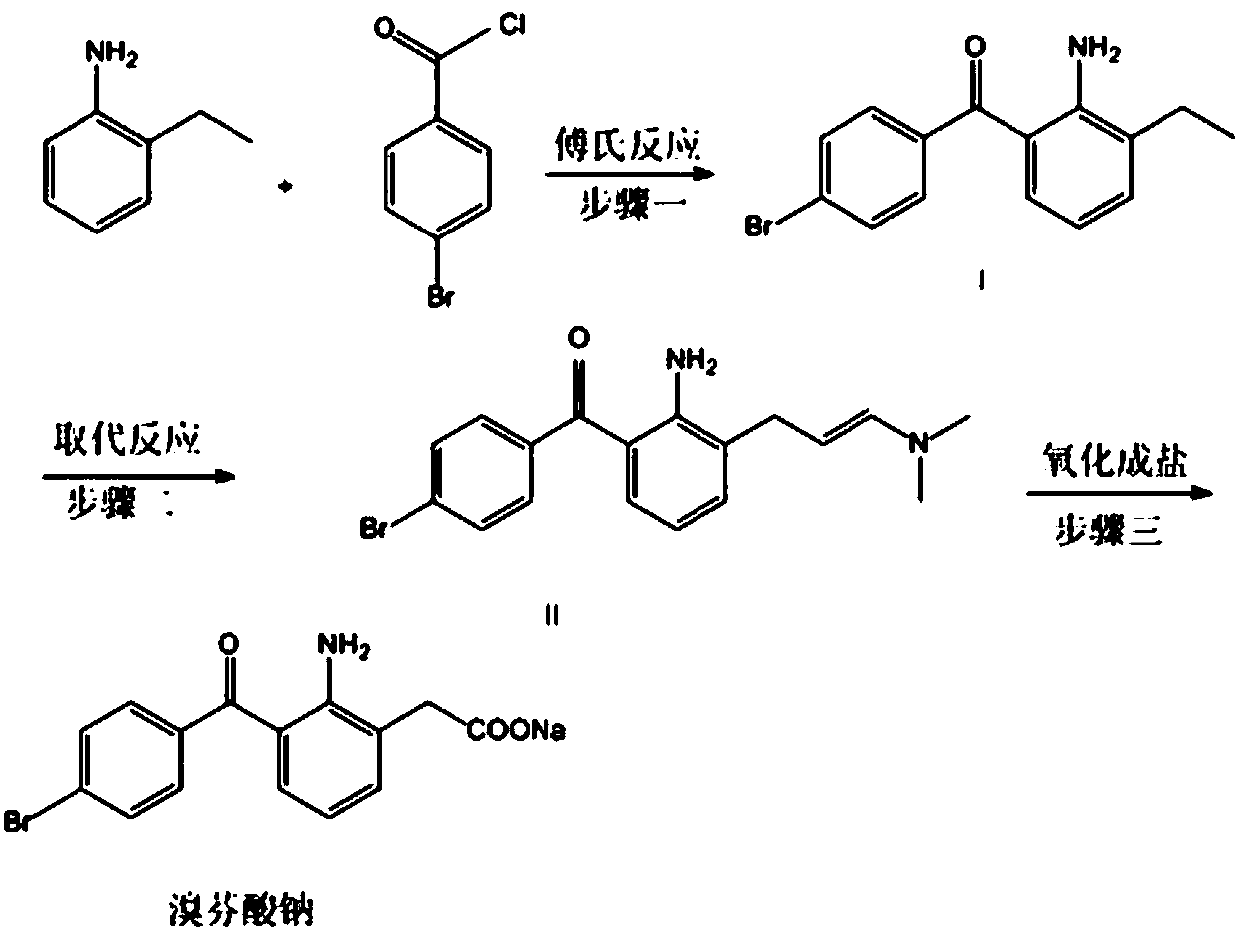
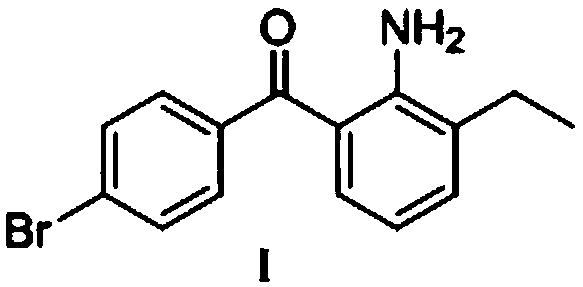
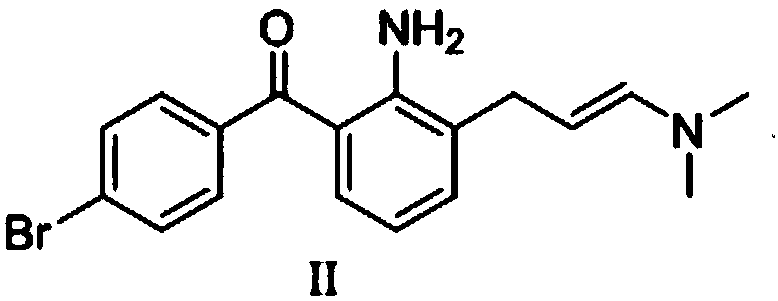
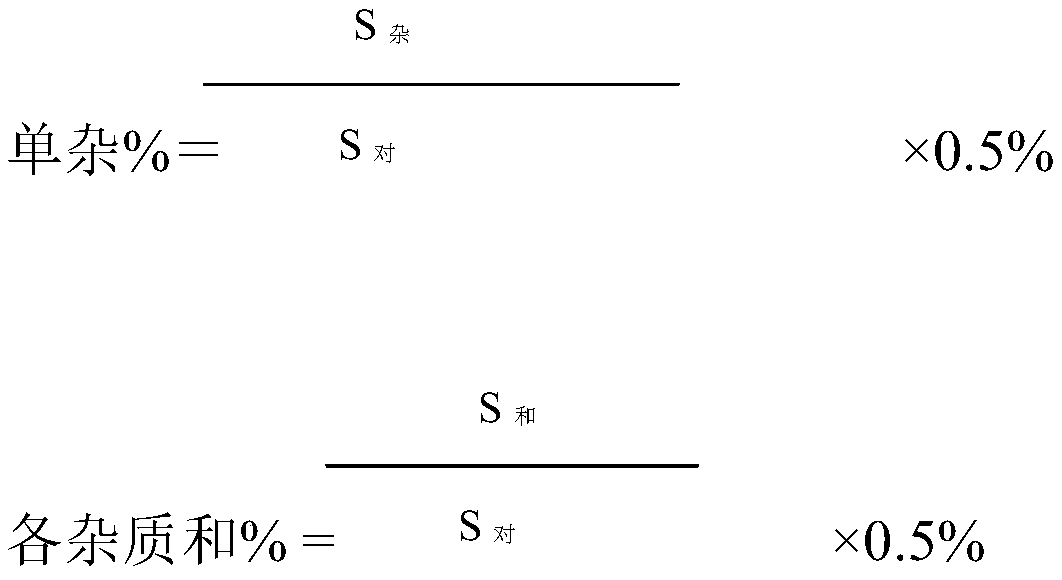
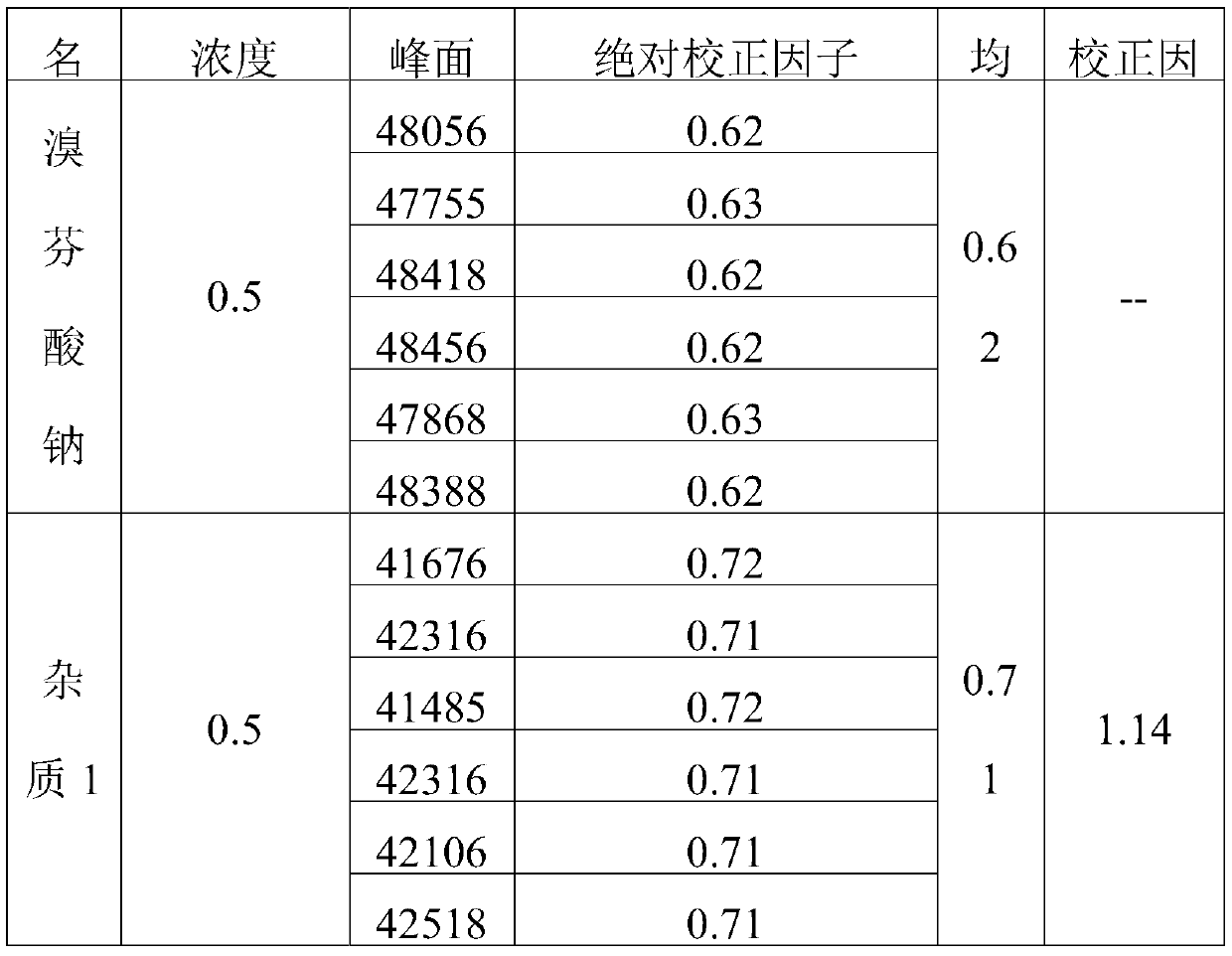
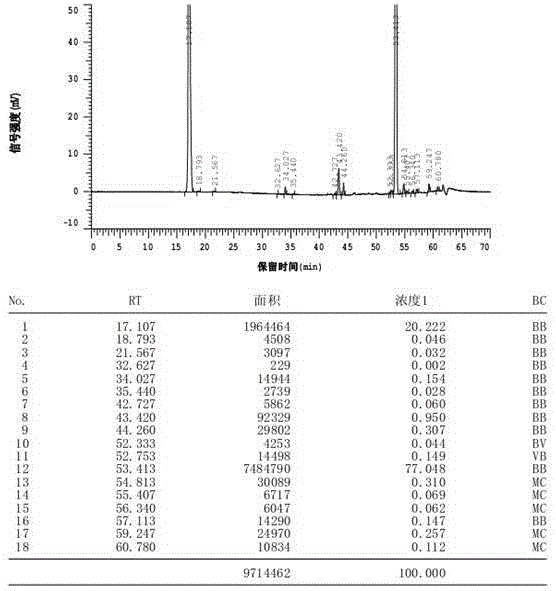
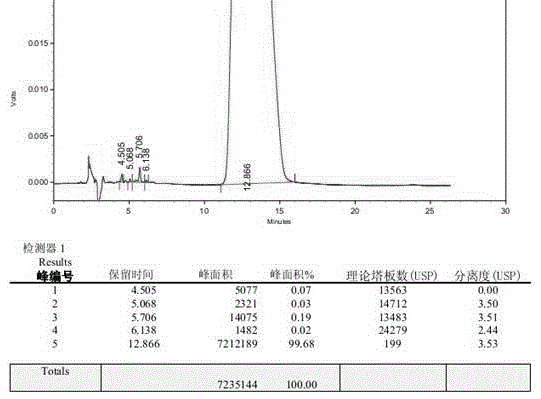
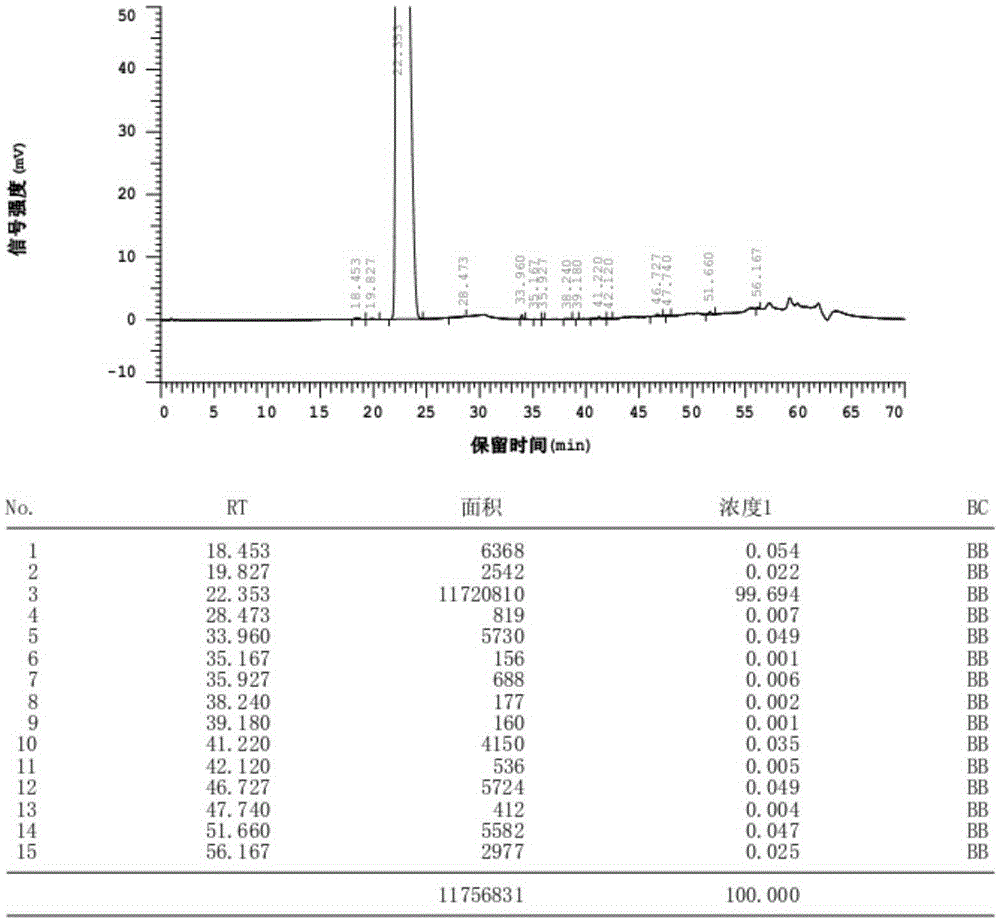
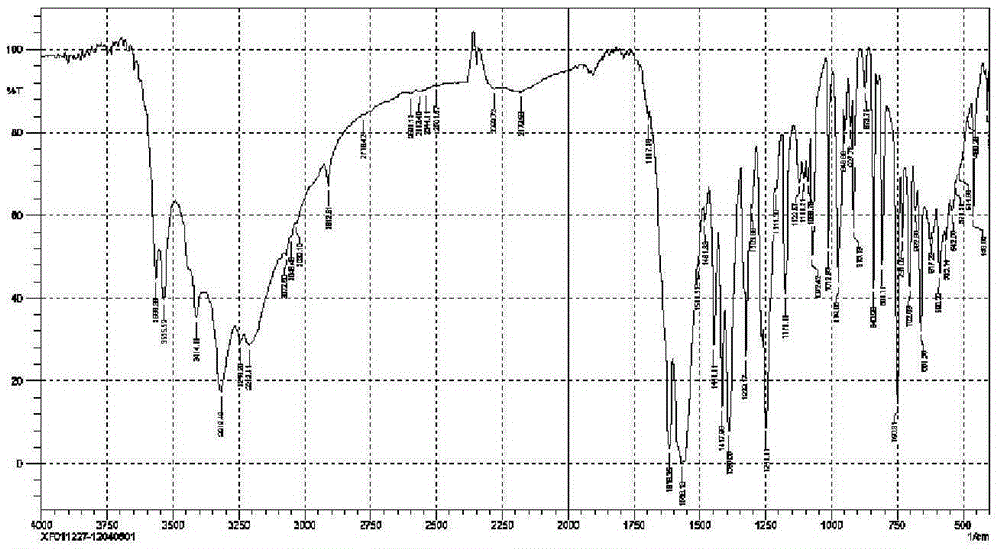
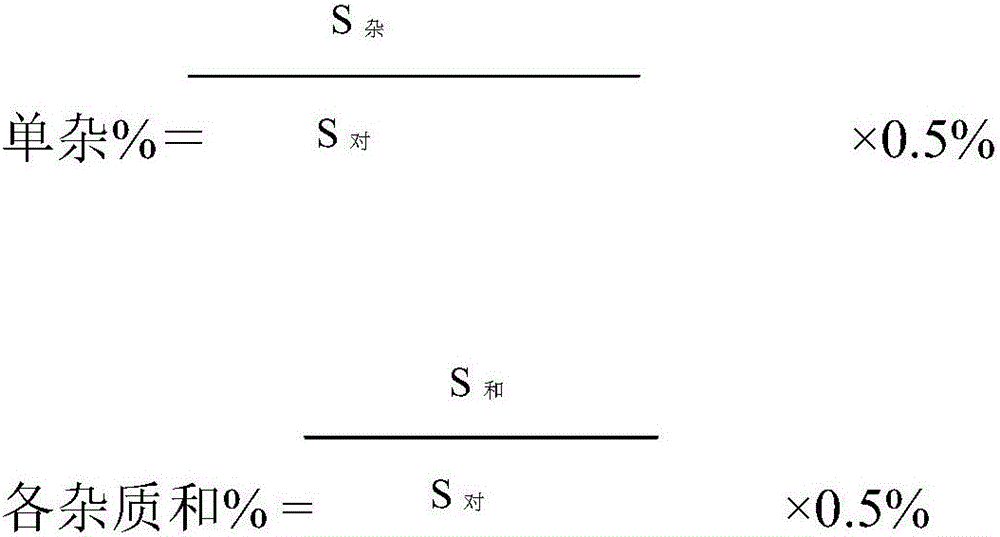Patents
Literature
30 results about "Bromfenac sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
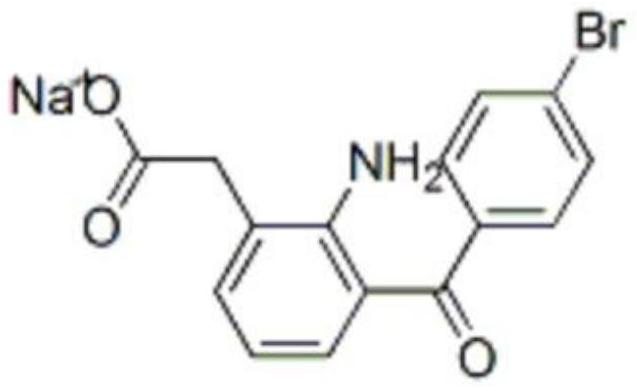
Bromfenac sodium preparation method
ActiveCN106397235AHigh purityShort synthetic routeOrganic compound preparationAmino-carboxyl compound preparationBoron trichlorideBromfenac
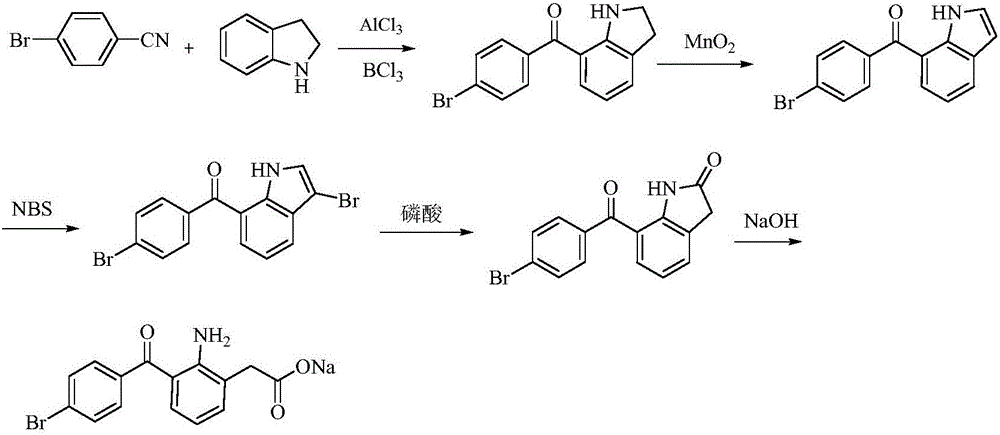
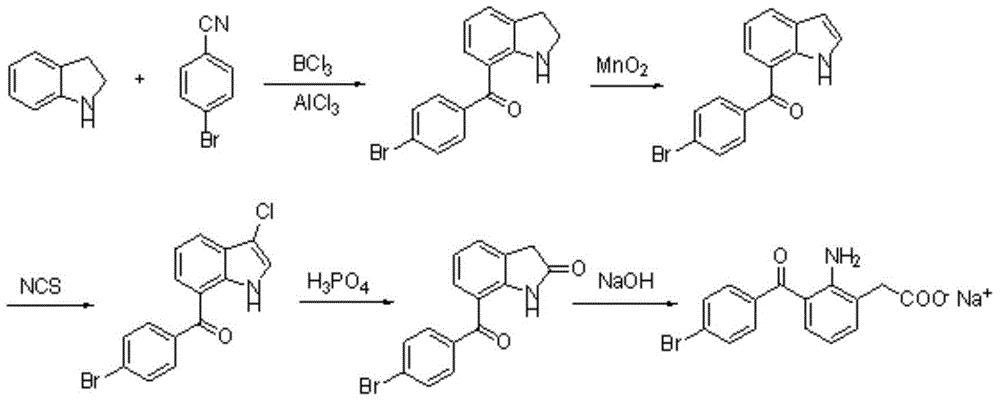
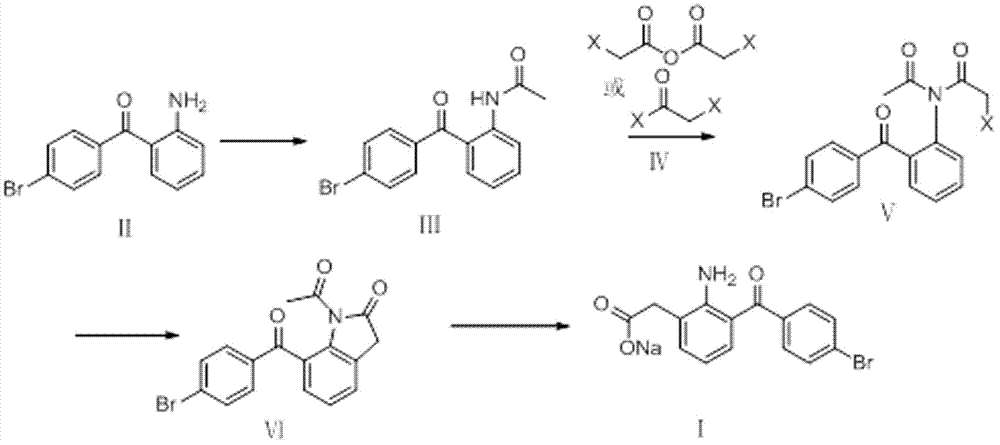
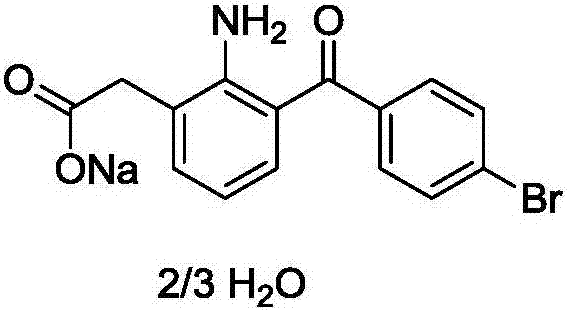
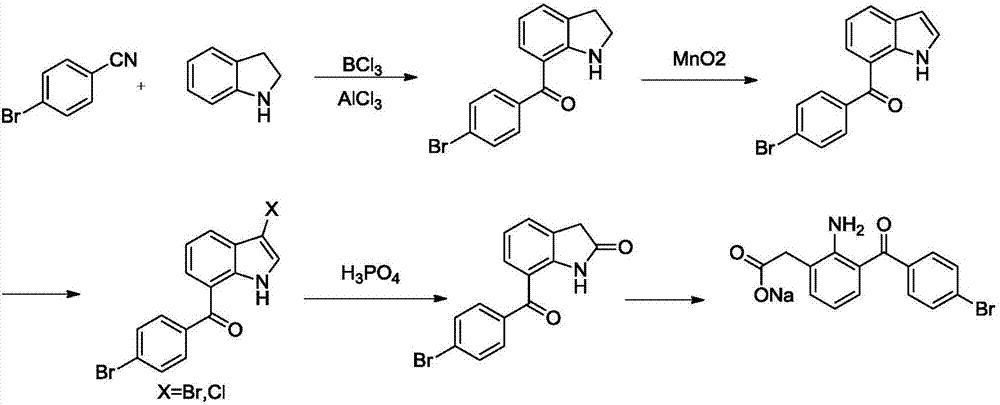
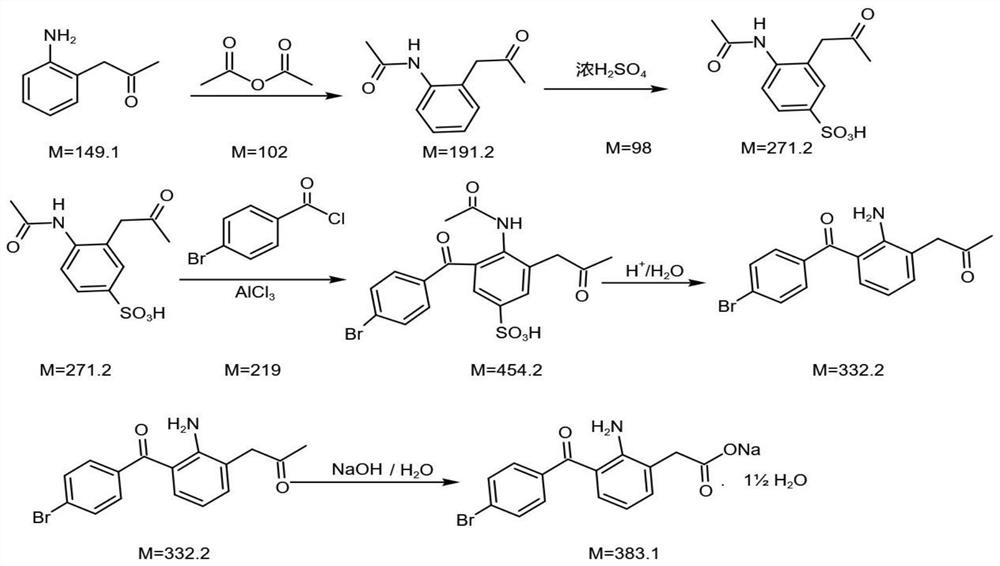
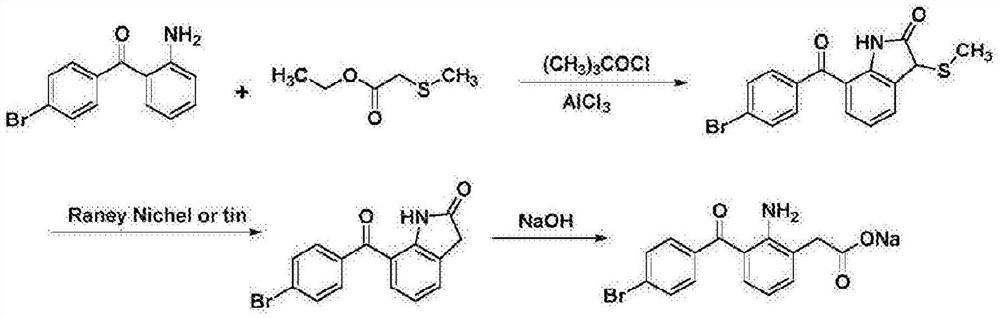
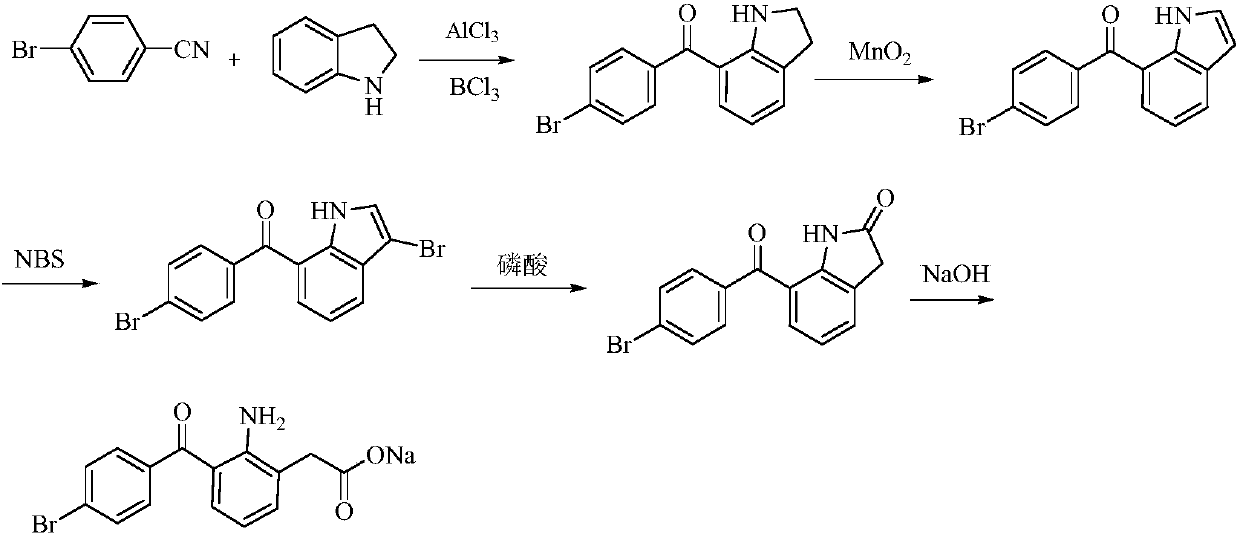
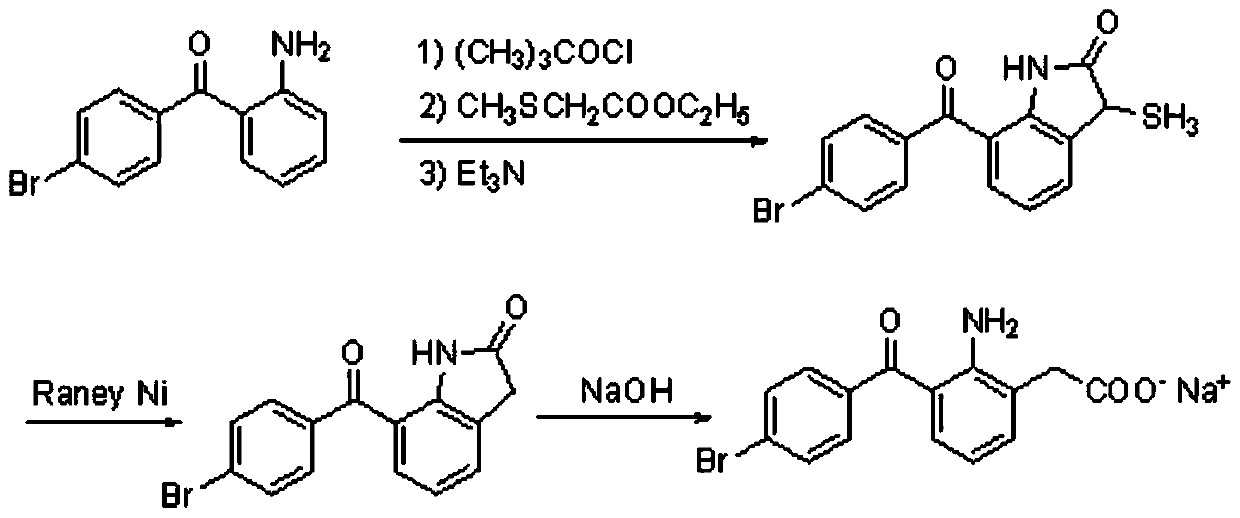
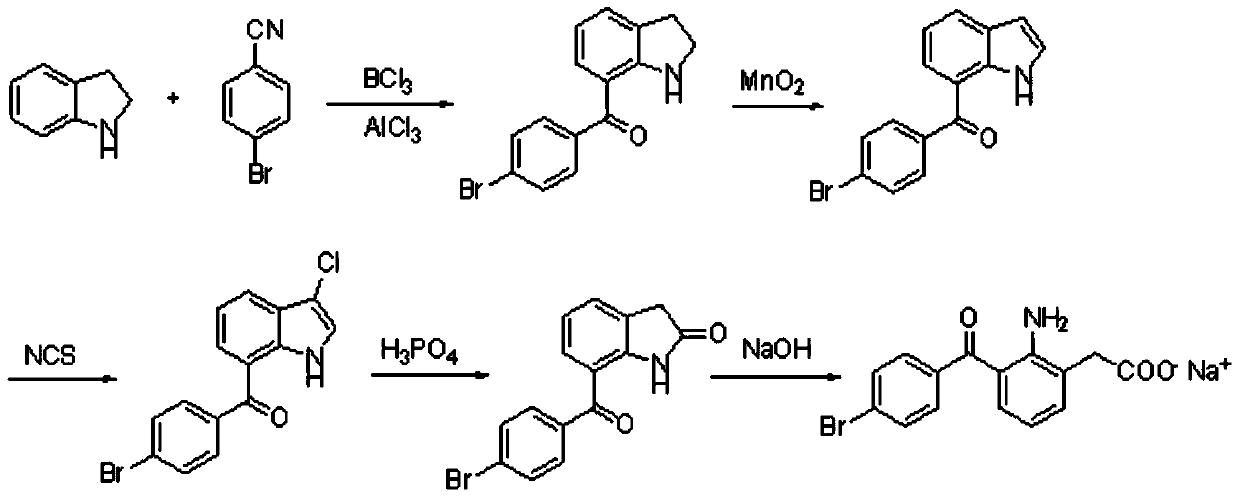
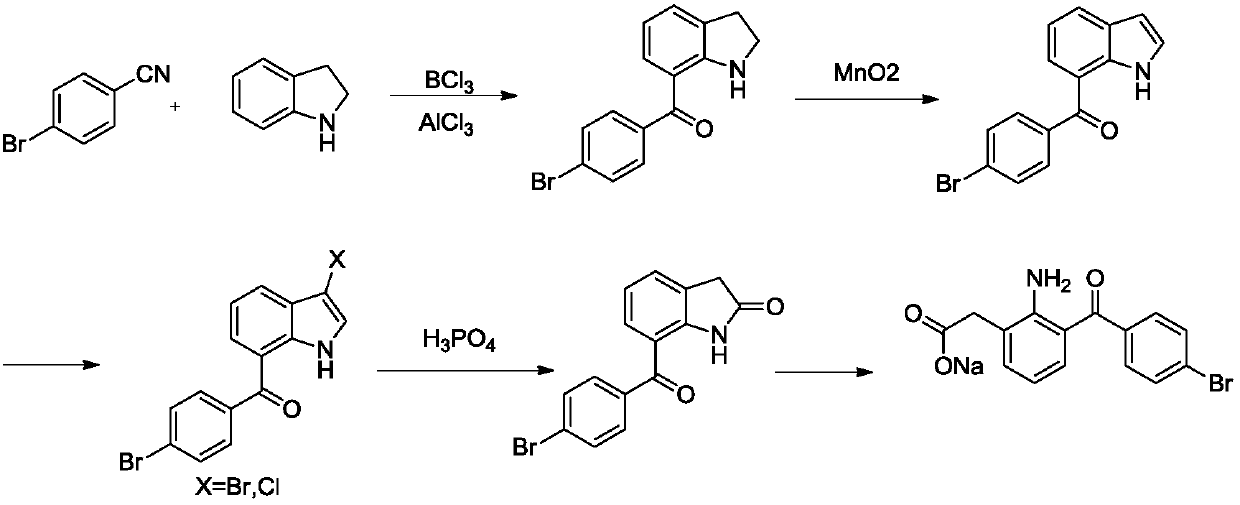
The invention relates to a bromfenac sodium preparation method. The preparation method comprises specific steps as follows: indole reacts under the action of DMSO (dimethylsulfoxide) to produce 3-bromoindole; 3-bromoindole is added to 2-methoxyethanol, acid is added for hydrolysis, and 2-indolinone is obtained; boron trichloride is added to methylbenzene, a methylbenzene mixed solution of p-bromobenzonitrile and 2-indolinone is added dropwise, aluminum chloride is then added, acid is added, a reaction is performed, and 7-(4-bromobenzoyl)-1,3-dihydro-indol-2-one is obtained; hydrolysis is performed with an alkaline solution, acid is added for neutralization, and bromfenac is obtained; ethanol is added to bromfenac, bromfenac and a sodium hydroxide solution form salt, the salt is cooled and subjected to recrystallization, and bromfenac sodium is obtained. Compared with the prior art, the synthetic route is short, high-purity bromfenac sodium can be prepared, the quality meets the latest standards of pharmacopoeia, industrial production is facilitated, and the method can provide powerful guarantee for industrial production of bromfenac sodium and intermediates of bromfenac sodium.
Owner:山东辰欣佛都药业股份有限公司
Preparation method and important intermediate of bromfenac sodium
ActiveCN104974057AReduce pollutionHigh purityOrganic compound preparationAmino-carboxyl compound preparationBiochemical engineeringSodium bromfenac
The invention provides a preparation and refining method of bromfenac sodium. The method has the advantages that the reaction conditions are mild, the operation is simple, and the obtained product is high in purity, high in yield and low in cost and can be industrially produced easily.
Owner:TIANJIN JINYAO GRP
Gel for eye containing bromfenac sodium hydrate and preparation thereof
InactiveCN101322683AOrganic active ingredientsSenses disorderTherapeutic effectBULK ACTIVE INGREDIENT
The invention relates to an ophthalmic gel containing the active ingredient of bromfenac sodium hydrate and a preparation method thereof; the bromfenac sodium hydrate ophthalmic gel contains the active ingredient of bromfenac sodium hydrate, macromolecular hydrogel substrate, osmotic pressure regulator, bacteriostat, antioxidant, metal ion chelating agent, pH regulator and water for injection; the ophthalmic gel has convenient application and quick effect; particularly, the gel possesses certain viscosity and can stay in the eyes for a long time, thus overcoming the defect that eye drops can only stay in the eyes for a short time after being diluted by tears and achieving more effective therapeutic effect.
Owner:ZHEJIANG SHENGBOKANG PHARMA CO LTD
Bromfenac sodium hydrate thermosensitive in-situ gel eye drops and preparation method thereof
InactiveCN105213303ASolve the problem of short residence time and low bioavailabilityImprove complianceOrganic active ingredientsSenses disorderActive componentBioavailability
The invention discloses bromfenac sodium hydrate thermosensitive in-situ gel eye drops which comprise, by weight, 0.05%-0.15% of active component bromfenac sodium hydrates, 1%-30% of themosensitive gel materials and auxiliary materials acceptable in pharmacy; the pH value of the eye drops is regulated to 6.5-9.0, and the osmotic pressure is set at 286-310 mOsmol / k. The invention further provides a preparation method of the bromfenac sodium hydrate thermosensitive in-situ gel eye drops. Compared with the prior art, the bromfenac sodium hydrate thermosensitive in-situ gel eye drops and the preparation method thereof have the advantages that the problems that traditional eye drops are short in stay time in the eyes and low in bioavailability are solved, the defects that the viscosity of traditional eye gel is high and transfer in the producing and using process is difficult are overcome, and the compliance of a patient is improved.
Owner:广州仁恒医药科技股份有限公司
Method for synthesizing bromfenac sodium
ActiveCN106957237ALow costReaction is easy to controlOrganic compound preparationCarboxylic acid amides preparationHalogenBenzoyl chloride
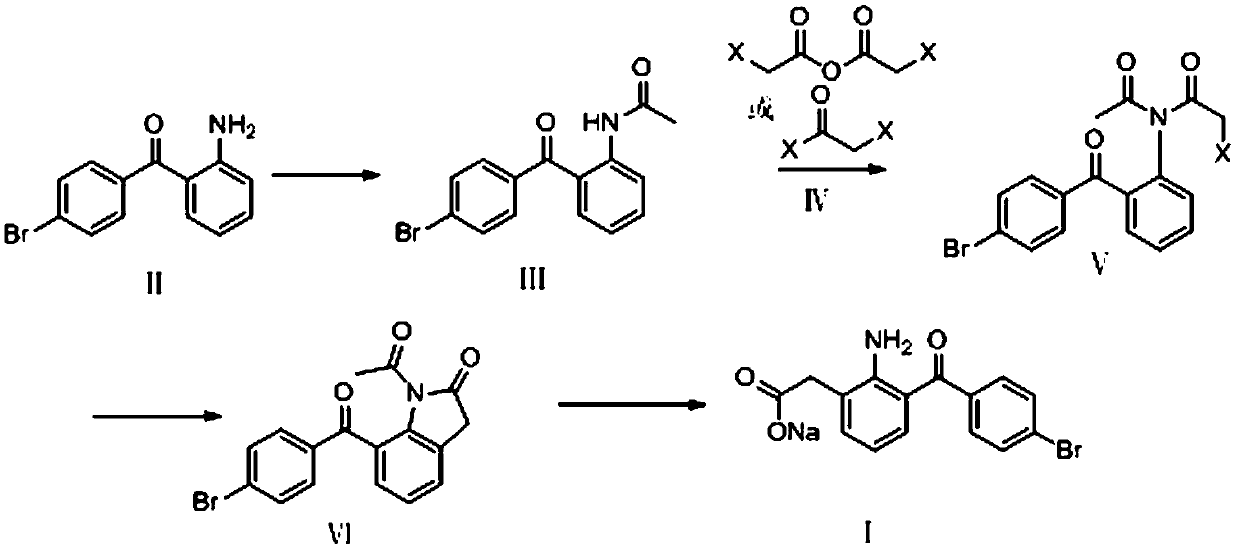
The invention discloses a method for synthesizing bromfenac sodium. The method comprises the steps of (1) carrying out acetylation reaction on 2-amino-4'-bromo benzophenone as a raw material to obtain a formula III: N-(2-(4'-bromo benzoyl)phenyl)acetamide; (2) reacting the N-(2-(4'-bromo benzoyl)phenyl)acetamide in a formula IV with a halogen acetylation reagent to obtain a formula V: N-acetyl-N-(2-(4'-bromo benzoyl)phenyl)-2-haloacetamide; (3) carrying out friedel-crafts reaction on the N-acetyl-N-(2-(4'-bromo benzoyl)phenyl)-2-haloacetamide to obtain a formula IV: 1-acetyl-7-(4-bromo-benzoyl chloride) indoline-2-ketone; and (4) finally hydrolyzing the 1-acetyl-7-(4-bromo-benzoyl chloride) indoline-2-ketone to obtain a target product. The method is low in cost, reaction is easy to control, post-treatment is simple, the overall yield is high, the method is economical and environmentally friendly, and a novel method for synthesizing the bromfenac sodium is provided.
Owner:SHANGHAI PUKANG PHARMA
Determination method for related substances of Bromfenac sodium eye drops
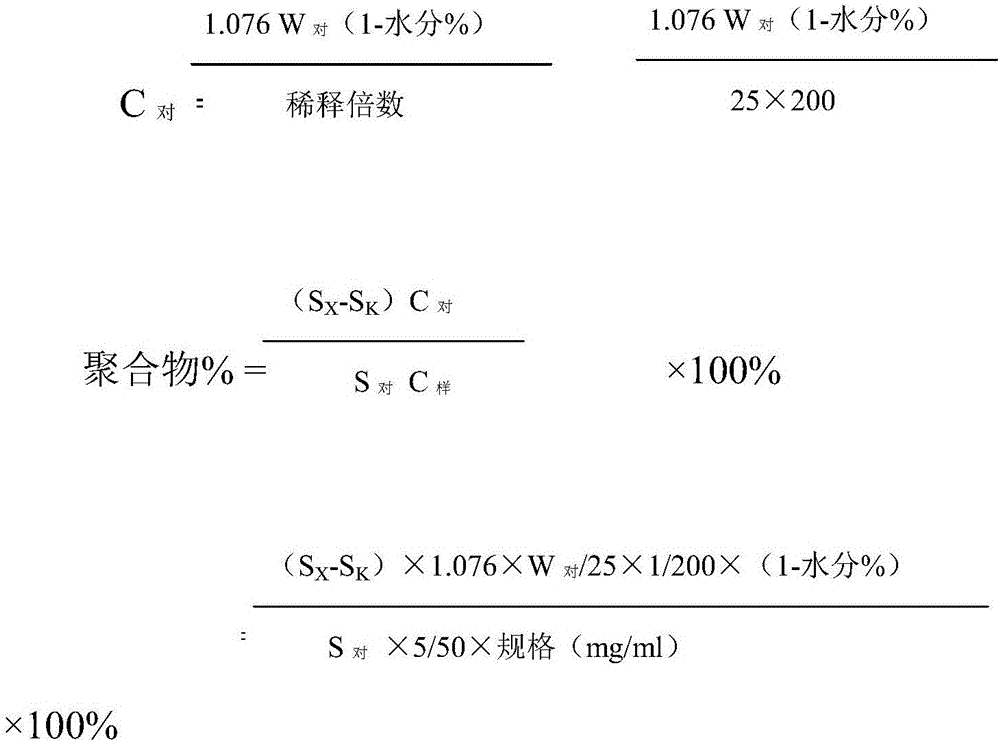
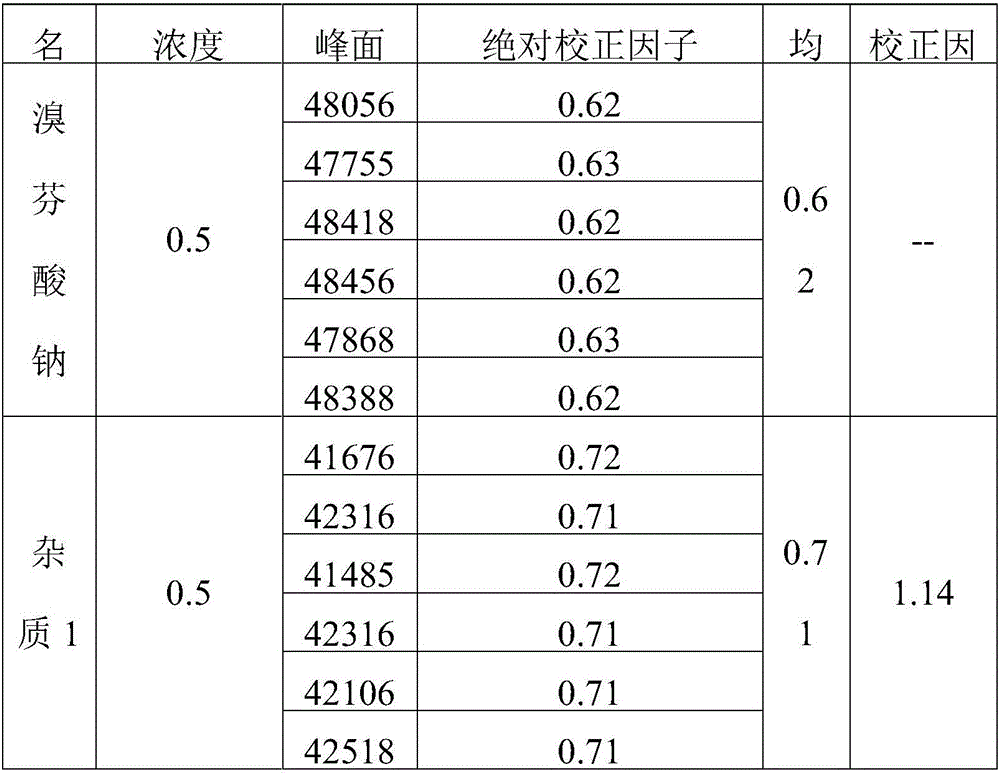
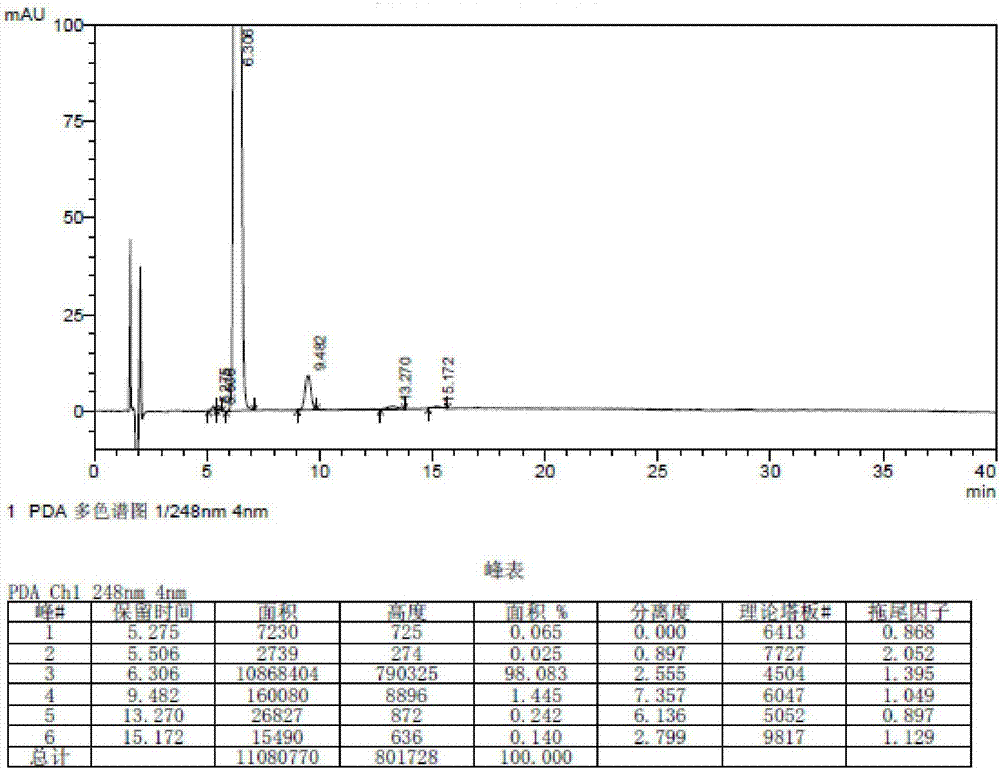
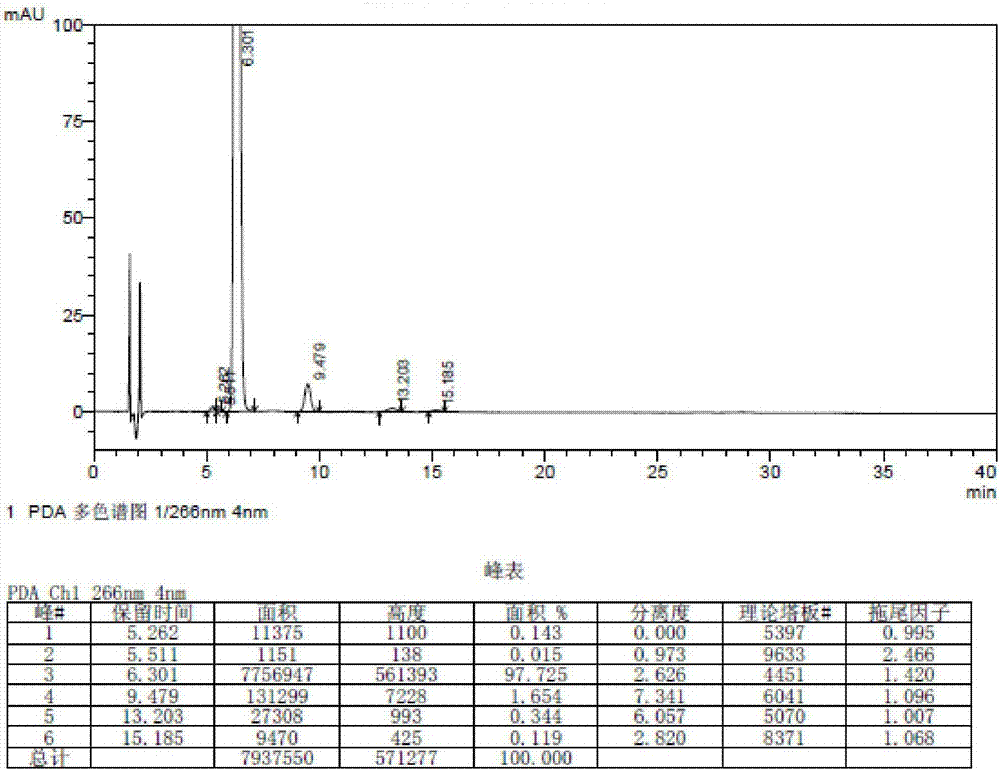
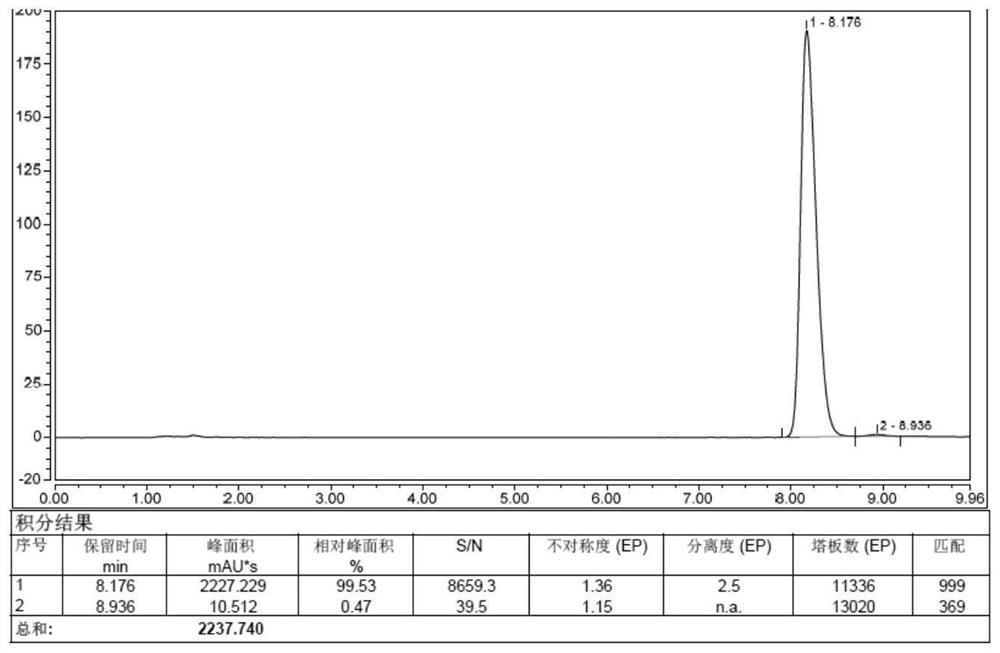
Belonging to the technical field of drug detection, the invention in particular relates to a determination method for related substances of Bromfenac sodium eye drops. The determination method includes the specific steps of: adopting an octadecyl silane bonded silica gel column or Lycopodine N-oxide M bonded silica gel column, and taking a phosphate buffer solution-acetonitrile as the mobile phase to conduct gradient elution, and setting the detection wavelength at 250-280nm, the column temperature at 10-30DEG C, the flow rate at 0.8-3ml / min, and a sample size of 20-70 microliter. Compared with the prior art, the determination method for related substances of Bromfenac sodium eye drops provided by the invention realizes separation and analysis of Bromfenac sodium and its related substances, and has the characteristics of good separation degree, strong specificity and high sensitivity, and can quantitate the three known impurities by self-control method without correction factor. The determination method has important practical significance for the quality control of Bromfenac sodium eye drops.
Owner:山东辰欣佛都药业股份有限公司
Novel synthesis method of bromfenac sodium
PendingCN113698308AEasy to purchaseLow costOrganic compound preparationCarboxylic acid amides preparationO-Phosphoric AcidSodium bromfenac
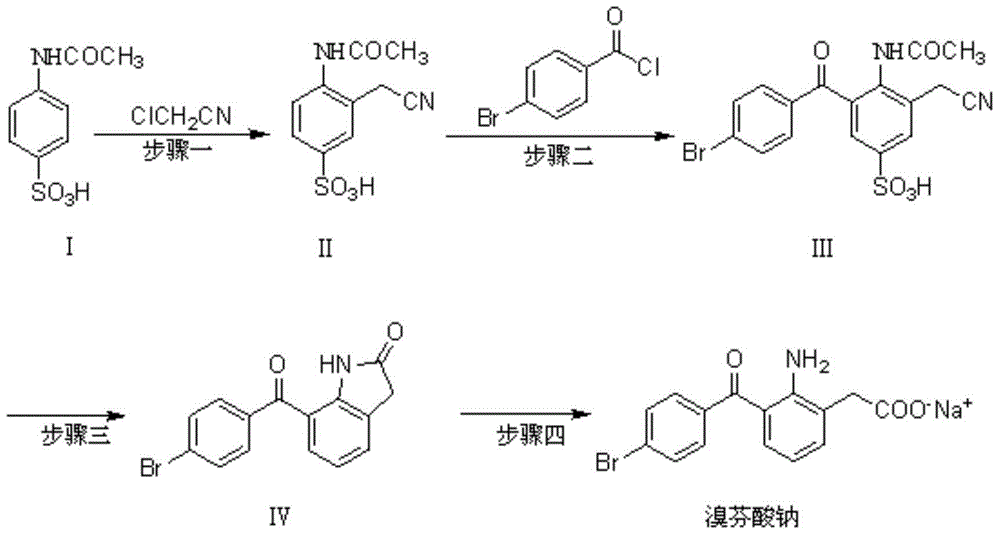
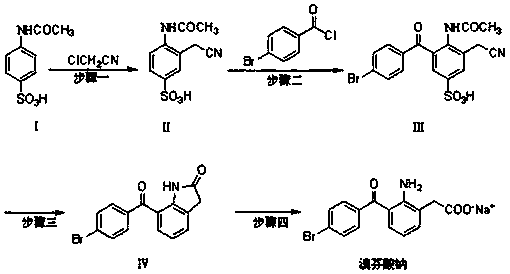
The invention discloses a novel synthesis method of bromfenac sodium, which belongs to the technical field of drug synthesis, and is characterized in that the preparation method comprises the following steps of taking o-aminophenylacetic acid as an initial raw material, and obtaining an intermediate I through acylation reaction, performing sulfonation reaction on the intermediate I to obtain an intermediate II, carrying out substitution reaction on the intermediate II and p-bromobenzoyl chloride to obtain an intermediate III, hydrolyzing the intermediate III to obtain bromfenac, and reacting bromfenac with sodium hydroxide to obtain the final product bromfenac sodium. The method has the beneficial effects that the generation of impurities containing indole rings caused by a synthesis method in the prior art is avoided; the problem that in the prior art, phosphoric acid or glacial acetic acid is used for producing acid salt, so that the pH value of a final finished product exceeds the standard is solved, the quality problem that the water content is too low due to the proportion of materials for preparing the bromfenac sodium finished product is solved, and meanwhile, the synthesis method is simple, easy to control and suitable for industrial production.
Owner:山东辰龙药业有限公司
Gel for eye containing bromfenac sodium hydrate and preparation thereof
InactiveCN101322683BOrganic active ingredientsSenses disorderTherapeutic effectBULK ACTIVE INGREDIENT
The invention relates to an ophthalmic gel containing the active ingredient of bromfenac sodium hydrate and a preparation method thereof; the bromfenac sodium hydrate ophthalmic gel contains the active ingredient of bromfenac sodium hydrate, macromolecular hydrogel substrate, osmotic pressure regulator, bacteriostat, antioxidant, metal ion chelating agent, pH regulator and water for injection; the ophthalmic gel has convenient application and quick effect; particularly, the gel possesses certain viscosity and can stay in the eyes for a long time, thus overcoming the defect that eye drops can only stay in the eyes for a short time after being diluted by tears and achieving more effective therapeutic effect.
Owner:ZHEJIANG SHENGBOKANG PHARMA CO LTD
A kind of preparation method of bromfenac sodium
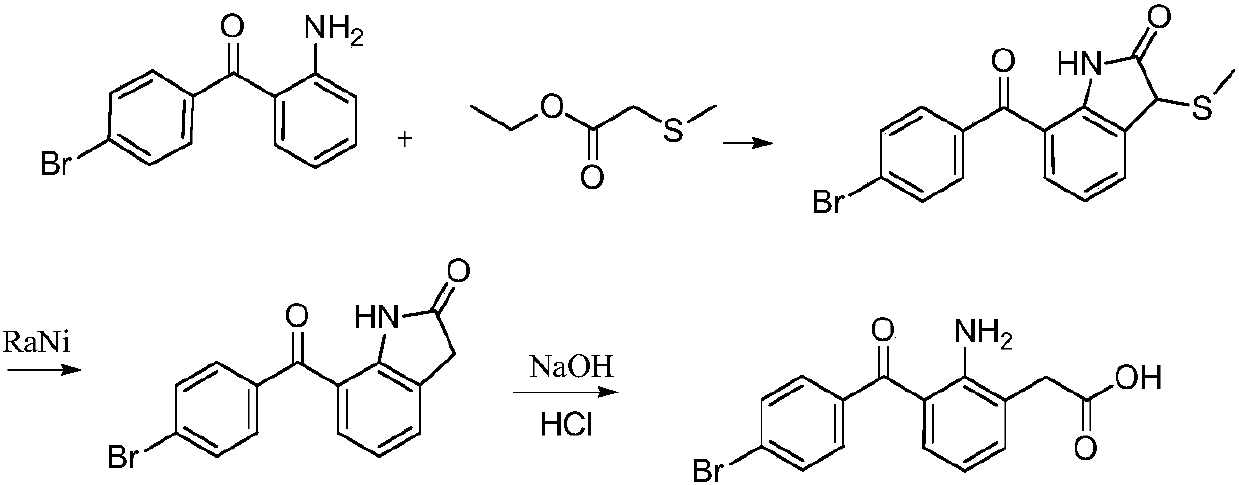
ActiveCN104177272BHigh reaction yieldImprove response qualityOrganic compound preparationAmino-carboxyl compound preparationN dimethylformamidePhosphoric acid
The invention relates to a preparation method of bromfenac sodium. The preparation method comprises the following steps of (a) reacting a compound represented by a formula (V) with an electrophilic substitutional reagent in the presence of N,N-dimethylformamide or dimethyl sulfoxide to obtain a compound represented by a formula (IV), (b) adding the compound represented by the formula (IV) into 2-methoxyethanol, adding phosphoric acid and carrying out acid hydrolysis to obtain a compound represented by a formula (III), (c) hydrolyzing the compound represented by the formula (III) with sodium hydroxide, extracting with dichloromethane and adding acetic acid to neutralize to obtain a compound represented by a formula (II) and (d) in the presence of an organic alcohol solvent, adding a sodium hydroxide solution, hydrolyzing the compound represented by the formula (II) to form a salt, adding the organic alcohol solvent, cooling and crystallizing to obtain bromfenac sodium. According to the preparation method disclosed by the invention, the reaction yield of the intermediate (IV) and the quality of the product are improved, high-purity bromfenac sodium can be obtained just by virtue of crystallizing with the organic alcohol solvent, the good environmental benefit is achieved and the generation of bromfenac sodium polymer impurity is reduced.
Owner:GUANGDONG XIANQIANG PHARMA +1
Polymorphs of bromfenac sodium and methods for preparing bromfenac sodium polymorphs
Different polymorphs of bromfenac sodium may be prepared and interconverted using crystallization / recrystallization, drying and / or hydration techniques.
Owner:MACFARLAN SMITH
Preparation method of bromfenac sodium dimer impurity
ActiveCN104262346APromote generationRealize refinement and purificationOrganic chemistryAcetic acidHydrogen
The invention belongs to the technical field of medicine, and provides a preparation method of a bromfenac sodium dimer impurity, which comprises the following steps: hydrolyzing 7-(4-bromobenzoyl)-1,3-dihydro-indolyl-2-one to obtain bromfenac, adding bromfenac into an alkali metal hydroxide solution, crystallizing to obtain a bromfenac sodium dimer impurity crude product, and adding the bromfenac sodium dimer impurity crude product into ethyl acetate to obtain the bromfenac sodium dimer impurity. The method can precipitate the impurity in the bromfenac sodium dimer impurity crude product by salification, simply and efficiently implements refinement and purification of the bromfenac sodium dimer impurity, enhances the purity to 99% above, is simple and convenient, and has the advantages of favorable environmental benefit and high yield.
Owner:GUANGDONG ZHONGSHENG PHARMA
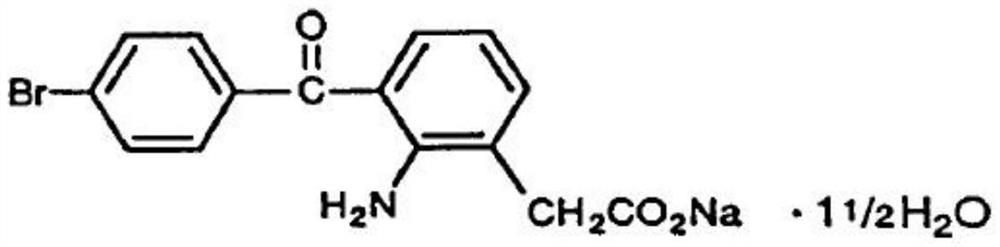
Preparation method of bromfenac sodium sesquihydrate
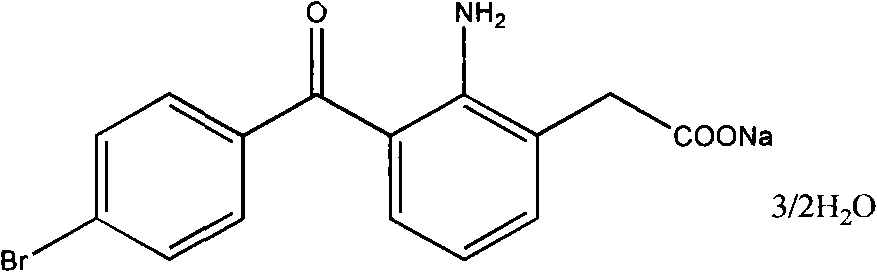
ActiveCN104151182AImprove stabilityReduce usageOrganic compound preparationAmino-carboxyl compound preparationAlcoholAnti solvent
The invention aims at providing a preparation method of bromfenac sodium sesquihydrate. The preparation method comprises the following steps: (1) adding bromfenac sodium and an antioxidant into a mixed solvent of water and an organic alcohol, heating, dissolving and adding a pH adjustor to adjust the pH to 7.0-10.5; (2) adding an anti-solvent into a solution obtained in the step (1), cooling the solution to 30 DEG C to 35 DEG C, adding bromfenac sodium monohydrate, bromfenac sodium sesquihydrate or a mixture thereof as a seed crystal, and stirring; and (3) cooling, crystallizing, collecting crystals and drying to obtain the bromfenac sodium sesquihydrate. The preparation method avoids use of ether agents and is mild in reaction condition and good in environmental benefit. The prepared bromfenac sodium sesquihydrate is high in purity.
Owner:GUANGDONG ZHONGSHENG PHARMA
Sustained-release bromfenac sodium ophthalmic preparation
ActiveCN110538138AImprove securityLess irritatingOrganic active ingredientsSenses disorderOcular bioavailabilitySide effect
Owner:合肥华威药业有限公司
Separation and detection method of isomer impurities in 3-halogenated-7-(4-bromobenzoyl)-1H-indole and application
The invention provides a separation and detection method of isomer impurities in 3-halogenated-7-(4-bromobenzoyl)-1H-indole and application, and relates to the technical field of analytical chemistry. The method adopts high performance liquid chromatography, and the chromatographic conditions comprise: adopting a normal-phase chiral chromatographic column; an n-hexane-isopropanol mixed solution is used as a mobile phase; isocratic elution is performed; and the isomer impurity is 3-halogenated-7-(3-bromobenzoyl)-1-hydrogen-indole, and the structural formula of the isomer impurity is shown in the description. System methodology verifies that the method is good in system adaptability, high in specificity, good in durability and high in sensitivity, can be used for measuring and separating raw materials of the raw material medicine bromfenac sodium and isomer impurities of the raw materials, and improves the use safety and effectiveness of the bromfenac sodium eye drops.
Owner:TIANJIN PHARMA GROUP CORP
Preparation method of bromfenac sodium
PendingCN109988075AReduce usageSimple processOrganic compound preparationAmino-carboxyl compound preparationPhosphoric acidChloride
Owner:TIANJIN PHARMA GROUP CORP
Method for synthesizing bromfenac sodium impurity standard substance
The invention discloses a method for synthesizing bromfenac sodium impurity standard substance. 7-(4-bromobenzoyl)-dihydro-2H-indol-2-one is used as the raw material, and subjected to brominating and hydrolyzing to obtain 7-(4-bromobenzoyl)-dihydro-2H-indol-2,3-one pure product. The content of the pure product is calibrated by adopting a conventional analysis way. The method is cheap and easily available in raw materials, simple in process and short in preparation period, and the content of the calibrated product is greater than 98.5%. The bromfenac sodium impurity provided by the invention can be used as an impurity standard substance and can be applied to qualitative and quantitative research and detection of the sodium bromate raw material and the preparation impurities thereof.
Owner:HEFEI JIUNUO MEDICAL TECH
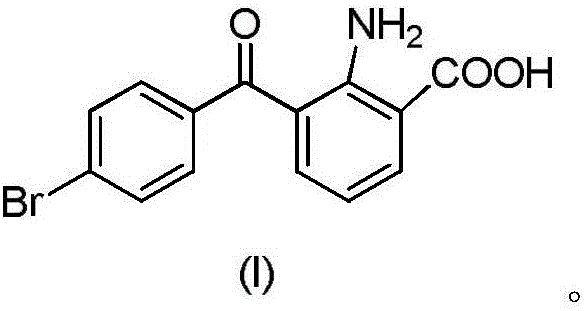
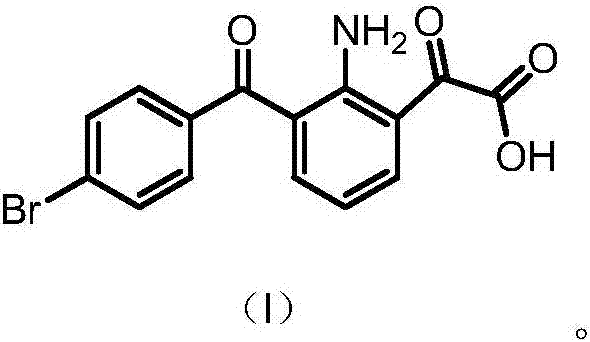
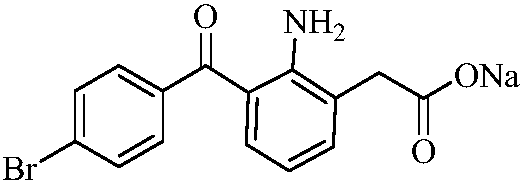
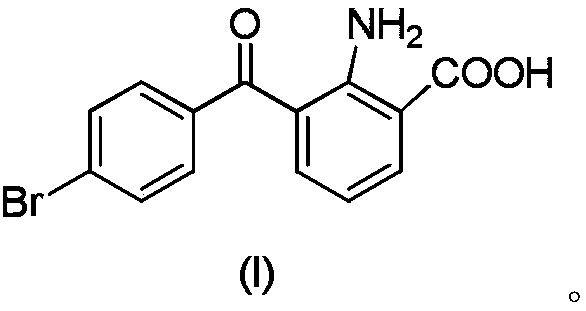
Synthesis method of bromfenac sodium impurity standard substance 2-amino-3-(4-bromo benzoyl) benzoic acid
ActiveCN106278918ASimple preparation processShort synthesis cycleOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidSynthesis methods
The invention discloses a synthesis method of a bromfenac sodium impurity standard substance 2-amino-3-(4-bromo benzoyl) benzoic acid. 7-(4-Bromobenzoyl)indoline-2,3-dione is taken as a material, the operations of decarboxylation and refining are carried out to prepare a pure product of 2-amino-3-(4-bromo benzoyl) benzoic acid, and the content of the pure product is calibrated through a conventional analysis means. The impurity preparation method provided by the invention is simple and convenient in process and short in preparation period, and calibration shows that the product content is higher than 99.0%. The bromfenac sodium impurity provided by the invention can serve as the impurity standard substance, and is applied to qualitative and quantitative researches and detection of the bromfenac sodium material and the preparation impurity.
Owner:HEFEI JIUNUO MEDICAL TECH
A kind of synthetic method of bromfenac sodium degradation impurity standard substance
ActiveCN106278917BSimple preparation processShort synthesis cycleOrganic compound preparationAmino-carboxyl compound preparationAcetic acidKetone
Owner:HEFEI JIUNUO MEDICAL TECH
Synthesis method of bromfenac sodium
PendingCN114736131AThe reaction conditions are mild and controllableSimple post-processingCarbamic acid derivatives preparationOrganic compound preparationBenzoic acidPtru catalyst
The invention discloses a synthesis method of bromfenac sodium. The preparation method comprises the following steps: firstly, by taking o-aminobenzoic acid as a raw material, adding a solvent, alkali and a fluorenylmethoxycarbonyl chloride isopropyl ether solution to react, and treating after the reaction to obtain 2-(9-fluorenylmethoxycarbonyl amino) benzoic acid; adding a solvent and strongly acidic cationic resin into the obtained product for reaction, and treating after the reaction to obtain 2-(9-fluorenylmethoxycarbonyl amino) ethyl benzoate; then a solvent, 4-bromobenzoyl chloride and a catalyst are added for a reaction, and 3-(4-bromo-benzoyl)-2-(9-fluorenylmethoxycarbonyl amino)-ethyl benzoate is obtained through treatment after the reaction; adding a solvent and a sodium hydroxide aqueous solution into the obtained product for reaction and treatment to obtain a bromfenac sodium crude product; and finally, refining the obtained bromfenac sodium crude product to obtain a bromfenac sodium product. The preparation method has the advantages of easily available raw materials, mild reaction conditions and high purity of the prepared finished product, and is suitable for industrial production.
Owner:郑州灏瑞医药科技有限公司
Pharmaceutical composition of bromfenac sodium
ActiveCN111743858AAvoid it happening againGuaranteed antibacterial propertiesOrganic active ingredientsSenses disorderSodium bromfenacAntioxidant
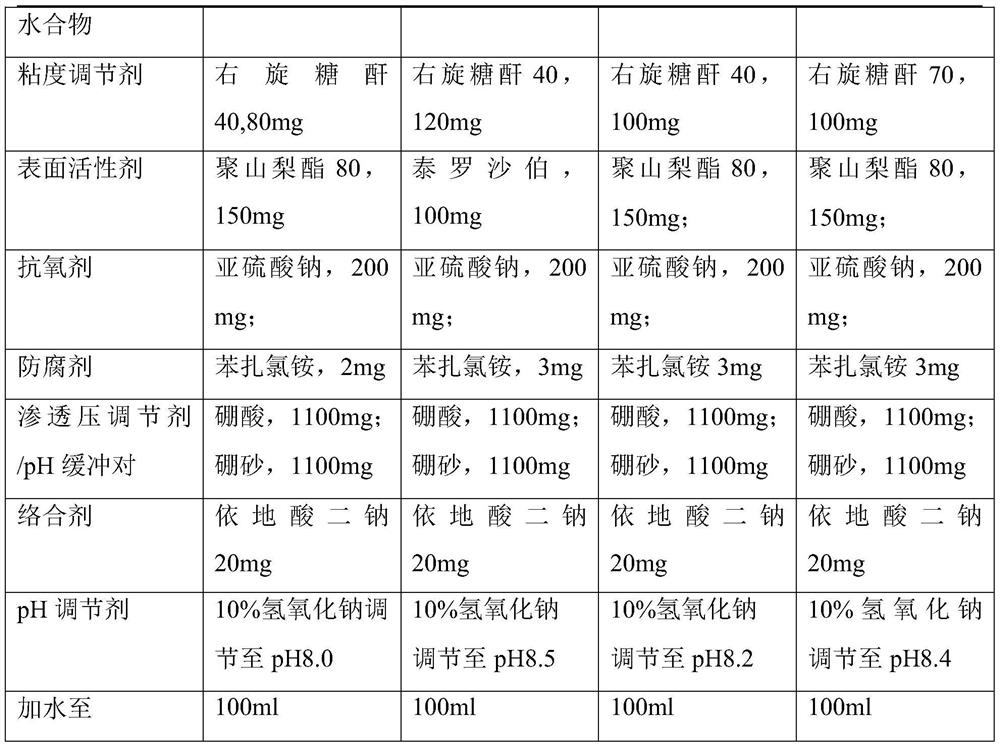
The invention provides a pharmaceutical composition of bromfenac sodium, which comprises the following components in percentage by weight: 0.01-0.5% of bromfenac sodium sesquihydrate and pharmacologically acceptable salt thereof; 0.01%-1% by weight of a viscosity modifier dextran; 0.01%-1% by weight of a surfactant; and 0.01%-0.5% by weight of an antioxidant; and optional other auxiliary materialsacceptable in pharmacology. The bromfenac sodium eye drops have the beneficial effects that when the bromfenac sodium eye drops contain dextran 40 or dextran 70, impurities U-II can be effectively prevented from being generated, and the condition that the addition of dextran can also ensure the antibacterial effect of benzalkonium chloride, prolong the intraocular residence time and improve the drug effect can be accidentally found.
Owner:TIANJIN PHARMA GROUP CORP
A kind of preparation method of bromfenac sodium
ActiveCN106397235BHigh purityShort synthetic routeOrganic compound preparationAmino-carboxyl compound preparationBoron trichlorideBromfenac
The invention relates to a bromfenac sodium preparation method. The preparation method comprises specific steps as follows: indole reacts under the action of DMSO (dimethylsulfoxide) to produce 3-bromoindole; 3-bromoindole is added to 2-methoxyethanol, acid is added for hydrolysis, and 2-indolinone is obtained; boron trichloride is added to methylbenzene, a methylbenzene mixed solution of p-bromobenzonitrile and 2-indolinone is added dropwise, aluminum chloride is then added, acid is added, a reaction is performed, and 7-(4-bromobenzoyl)-1,3-dihydro-indol-2-one is obtained; hydrolysis is performed with an alkaline solution, acid is added for neutralization, and bromfenac is obtained; ethanol is added to bromfenac, bromfenac and a sodium hydroxide solution form salt, the salt is cooled and subjected to recrystallization, and bromfenac sodium is obtained. Compared with the prior art, the synthetic route is short, high-purity bromfenac sodium can be prepared, the quality meets the latest standards of pharmacopoeia, industrial production is facilitated, and the method can provide powerful guarantee for industrial production of bromfenac sodium and intermediates of bromfenac sodium.
Owner:山东辰欣佛都药业股份有限公司
A kind of preparation method of bromfenac sodium
ActiveCN109608351BLow cost of harmless disposalEasy to operateOrganic compound preparationAmino-carboxyl compound preparationChemical industryPhenacyl
The invention discloses a preparation method of bromfenac sodium, relates to the technical field of medicine and chemical industry, comprising the following steps: preparing 7-(4-bromobenzoyl)-1,3-dihydro-2H-indole-2- Put the ketone and sodium hydroxide into the monohydric alcohol-deionized water system, stir and react in a closed manner at 90-110°C for 3-5 hours, cool down, add hydrochloric acid dropwise to adjust the pH, add decolorizer, reflux under normal pressure to decolorize, filter while hot, and analyze Crystallize and dry to obtain sodium bromophenolate. In the present invention, the mixed solvent of water-soluble organic solvents / water-insoluble organic solvents such as substituted toluene / ethanol, dichloromethane / ethanol etc. in the prior art is replaced with monohydric alcohol-deionized water system as reaction solvent, by changing the synthesis of bromfenac sodium The solvent system in the reaction simplifies the process operation, improves the product yield, and integrates the decolorization process into the post-reaction treatment process, which improves the product purity while decolorizing and saves production costs. The product purity can reach 100% as determined by HPLC. In addition, the reaction solvent can be recycled, and the cost of harmless disposal of the solvent is low.
Owner:HEFEI JIUNUO MEDICAL TECH
A kind of preparation method of bromfenac sodium intermediate
ActiveCN110172036BHigh purityEmission reductionOrganic chemistry methodsSodium bromfenacPhysical chemistry
The invention belongs to the field of medicinal chemistry, and in particular relates to a preparation method of bromfenac sodium and an intermediate thereof. The method adopts a new solvent system to prepare the intermediate, avoids the generation of brick-red by-products, greatly reduces the impurity content in the intermediate, and improves the purity and yield of the intermediate.
Owner:QILU PHARMA CO LTD
A kind of preparation method and important intermediate of bromfenac sodium
ActiveCN104974057BReduce pollutionHigh purityOrganic compound preparationAmino-carboxyl compound preparationSodium bromfenacProcess engineering
The invention provides a method for preparing and refining bromfenac sodium which is mild in reaction conditions, simple in operation, high in purity, high in yield, low in cost and easy for industrial production.
Owner:TIANJIN JINYAO GRP
A kind of method of synthesizing bromfenac sodium
ActiveCN106957237BLow costReaction is easy to controlOrganic compound preparationCarboxylic acid amides preparationHalogenIndoline
The invention discloses a method for synthesizing bromfenac sodium. The method comprises the steps of (1) carrying out acetylation reaction on 2-amino-4'-bromo benzophenone as a raw material to obtain a formula III: N-(2-(4'-bromo benzoyl)phenyl)acetamide; (2) reacting the N-(2-(4'-bromo benzoyl)phenyl)acetamide in a formula IV with a halogen acetylation reagent to obtain a formula V: N-acetyl-N-(2-(4'-bromo benzoyl)phenyl)-2-haloacetamide; (3) carrying out friedel-crafts reaction on the N-acetyl-N-(2-(4'-bromo benzoyl)phenyl)-2-haloacetamide to obtain a formula IV: 1-acetyl-7-(4-bromo-benzoyl chloride) indoline-2-ketone; and (4) finally hydrolyzing the 1-acetyl-7-(4-bromo-benzoyl chloride) indoline-2-ketone to obtain a target product. The method is low in cost, reaction is easy to control, post-treatment is simple, the overall yield is high, the method is economical and environmentally friendly, and a novel method for synthesizing the bromfenac sodium is provided.
Owner:SHANGHAI PUKANG PHARMA
Method for preparing bromfenac sodium
ActiveCN109608351ALow cost of harmless disposalEasy to operateOrganic compound preparationAmino-carboxyl compound preparationChemical industryWater insoluble
The invention discloses a method for preparing bromfenac sodium and relates to the technical field of medicinal chemical industry. The method comprises the following steps: adding 7-(4-bromobenzoyl)-1,3-dihydro-2H-indolyl-2-one and sodium hydroxide into a monoalcohol-deionized water system, carrying out a closed stirred reaction for 3 to 5 hours at the temperature of 90 DEG C to 110 DEG C, carrying out cooling, dropwise adding hydrochloric acid to adjust pH, adding a decolorant, carrying out refluxing-stirring decolorization under atmospheric pressure, carrying out filtering while the solutionis hot, and carrying out crystallization and drying, thereby obtaining bromfenac sodium. According to the method, the monoalcohol-deionized water system serves as a reaction solvent and replaces mixed solvents of water-soluble organic solvents / water-insoluble organic solvents in the prior art such as toluene / ethanol and dichloromethane / ethanol; through changing a solvent system of a bromfenac sodium synthesis reaction, process operations are simplified, and the product yield is increased; a decolorization process is integrated into a reaction aftertreatment process, so that the product purityis improved while decolorization is carried out, the production cost is reduced, and the product purity can reach 100% through HPLC measurement; and the reaction solvent can be recycled, and the harmless treatment cost of the solvent is low.
Owner:HEFEI JIUNUO MEDICAL TECH
A kind of determination method of related substances of bromfenac sodium eye drops
Belonging to the technical field of drug detection, the invention in particular relates to a determination method for related substances of Bromfenac sodium eye drops. The determination method includes the specific steps of: adopting an octadecyl silane bonded silica gel column or Lycopodine N-oxide M bonded silica gel column, and taking a phosphate buffer solution-acetonitrile as the mobile phase to conduct gradient elution, and setting the detection wavelength at 250-280nm, the column temperature at 10-30DEG C, the flow rate at 0.8-3ml / min, and a sample size of 20-70 microliter. Compared with the prior art, the determination method for related substances of Bromfenac sodium eye drops provided by the invention realizes separation and analysis of Bromfenac sodium and its related substances, and has the characteristics of good separation degree, strong specificity and high sensitivity, and can quantitate the three known impurities by self-control method without correction factor. The determination method has important practical significance for the quality control of Bromfenac sodium eye drops.
Owner:山东辰欣佛都药业股份有限公司
A kind of preparation method of bromfenac sodium dimer impurity
ActiveCN104262346BPromote generationRealize refinement and purificationOrganic chemistryAcetic acidHydrogen
The invention belongs to the technical field of medicine, and provides a preparation method of a bromfenac sodium dimer impurity, which comprises the following steps: hydrolyzing 7-(4-bromobenzoyl)-1,3-dihydro-indolyl-2-one to obtain bromfenac, adding bromfenac into an alkali metal hydroxide solution, crystallizing to obtain a bromfenac sodium dimer impurity crude product, and adding the bromfenac sodium dimer impurity crude product into ethyl acetate to obtain the bromfenac sodium dimer impurity. The method can precipitate the impurity in the bromfenac sodium dimer impurity crude product by salification, simply and efficiently implements refinement and purification of the bromfenac sodium dimer impurity, enhances the purity to 99% above, is simple and convenient, and has the advantages of favorable environmental benefit and high yield.
Owner:GUANGDONG ZHONGSHENG PHARMA
A kind of synthetic method of bromfenac sodium impurity standard product 2-amino-3-(4-bromobenzoyl) benzoic acid
ActiveCN106278918BSimple preparation processShort synthesis cycleOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidSynthesis methods
The invention discloses a synthesis method of a bromfenac sodium impurity standard substance 2-amino-3-(4-bromo benzoyl) benzoic acid. 7-(4-Bromobenzoyl)indoline-2,3-dione is taken as a material, the operations of decarboxylation and refining are carried out to prepare a pure product of 2-amino-3-(4-bromo benzoyl) benzoic acid, and the content of the pure product is calibrated through a conventional analysis means. The impurity preparation method provided by the invention is simple and convenient in process and short in preparation period, and calibration shows that the product content is higher than 99.0%. The bromfenac sodium impurity provided by the invention can serve as the impurity standard substance, and is applied to qualitative and quantitative researches and detection of the bromfenac sodium material and the preparation impurity.
Owner:HEFEI JIUNUO MEDICAL TECH
A kind of preparation method of bromfenac sodium sesquihydrate
ActiveCN104151182BImprove stabilityReduce usageOrganic compound preparationAmino-carboxyl compound preparationAlcoholAnti solvent
The invention aims at providing a preparation method of bromfenac sodium sesquihydrate. The preparation method comprises the following steps: (1) adding bromfenac sodium and an antioxidant into a mixed solvent of water and an organic alcohol, heating, dissolving and adding a pH adjustor to adjust the pH to 7.0-10.5; (2) adding an anti-solvent into a solution obtained in the step (1), cooling the solution to 30 DEG C to 35 DEG C, adding bromfenac sodium monohydrate, bromfenac sodium sesquihydrate or a mixture thereof as a seed crystal, and stirring; and (3) cooling, crystallizing, collecting crystals and drying to obtain the bromfenac sodium sesquihydrate. The preparation method avoids use of ether agents and is mild in reaction condition and good in environmental benefit. The prepared bromfenac sodium sesquihydrate is high in purity.
Owner:GUANGDONG ZHONGSHENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com