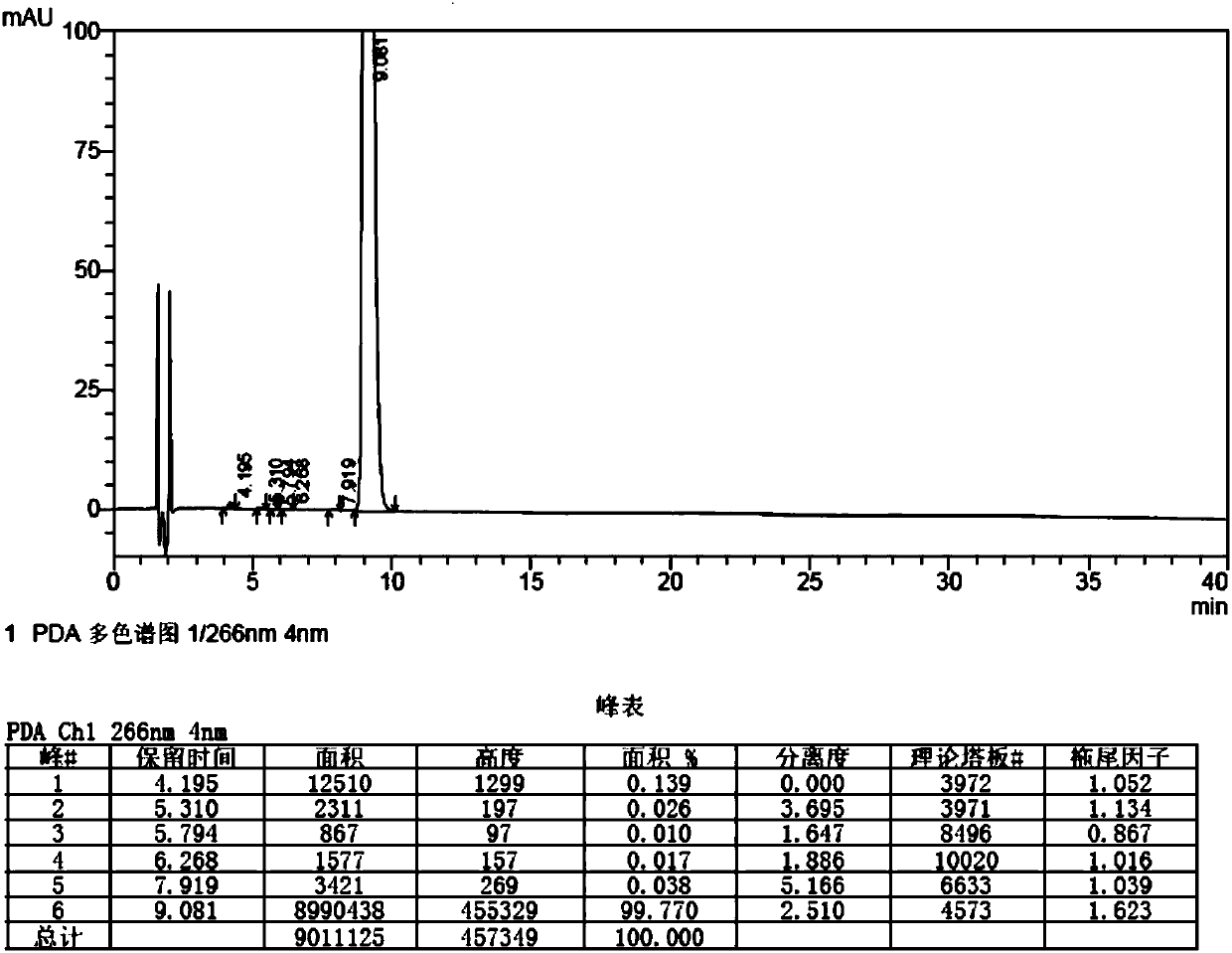
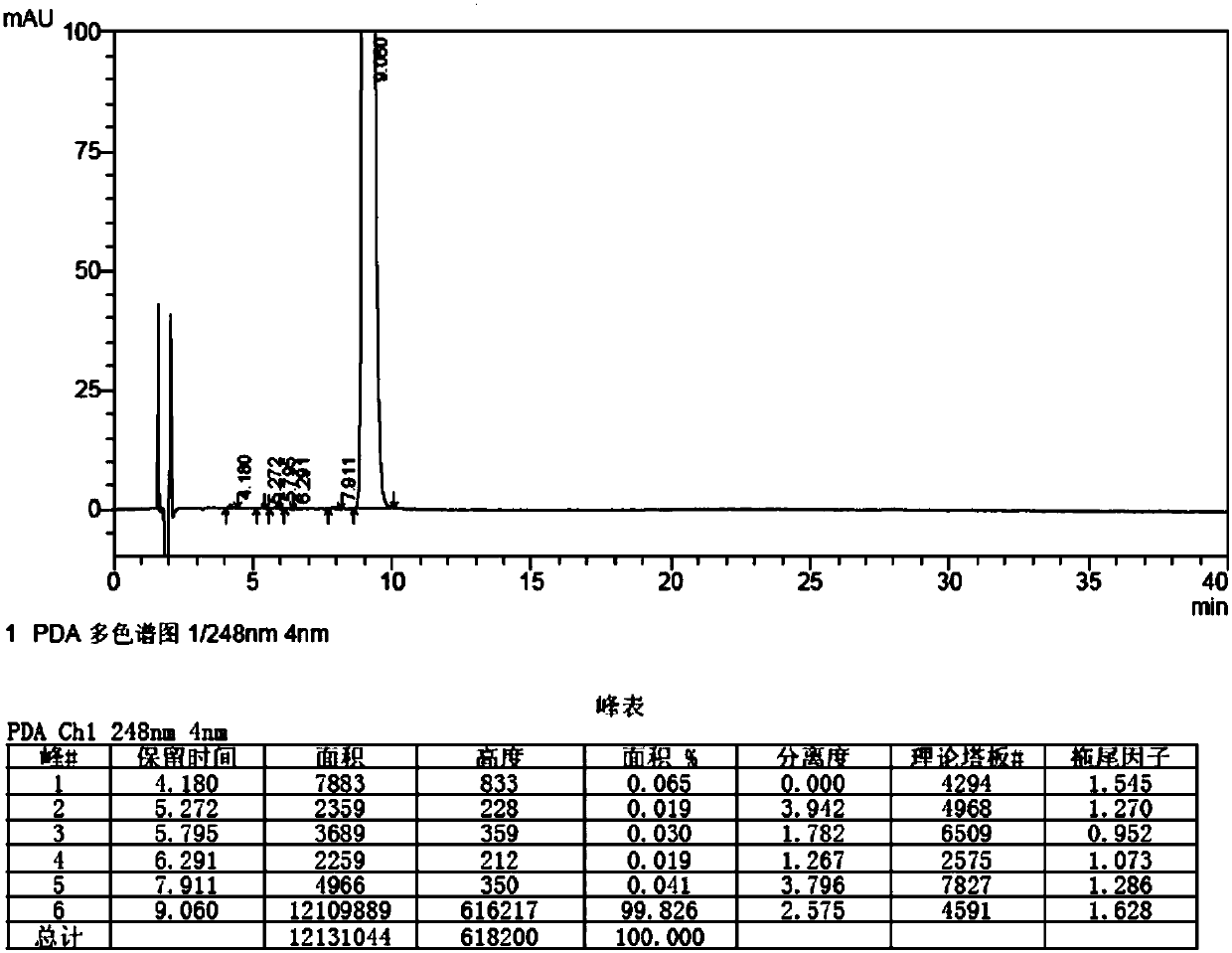
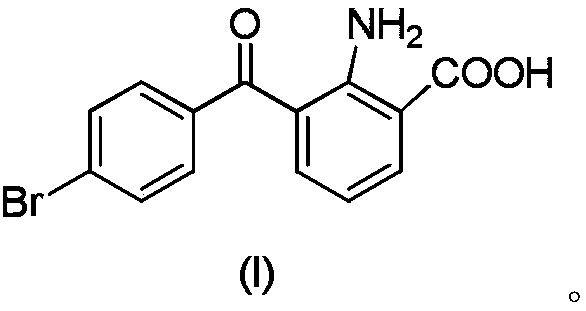
A kind of synthetic method of bromfenac sodium impurity standard product 2-amino-3-(4-bromobenzoyl) benzoic acid
A kind of technology of bromobenzoyl, bromfenac sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 7-(4-bromobenzoyl)indoline-2,3-dione
[0039] 7-(4-bromobenzoyl)indolin-2-one (CAS No. 91713-91-6) prepared by a general industrial method is used as a raw material.
[0040] Dissolve 25g (79mmol) of 7-(4-bromobenzoyl)indolin-2-one in 750ml of ethyl acetate with stirring, add 88.2g (395mmol) of copper bromide, and react under reflux at 78-85°C After 4 hours, the temperature was lowered to 20-30°C, the reaction solution was washed successively with water equal to the volume of ethyl acetate and saturated aqueous sodium chloride solution, the organic phase was concentrated to dryness at 50-60°C under reduced pressure, and methanol / water (volume ratio 4 : 1) 700ml of mixed solution, reflux at 70-80°C and stir for 3h, cool to 20-30°C, filter, wash the filter cake with appropriate amount of water and methanol successively, add 500ml of methanol to the solid, reflux at 65-70°C for 30min, Filtrate hot, cool the filtrate to -5~0°C, stir and crystalliz...
Embodiment 2
[0047] Embodiment 2: Preparation of 2-amino-3-(4-bromobenzoyl)benzoic acid
[0048]1. Put 12g (36.3mmol) of 7-(4-bromobenzoyl)indoline-2,3-dione into 180g (225mmol) of 5% sodium hydroxide aqueous solution, stir and dissolve at 50-60°C, drop Add 180ml (1.8mol) of 30% hydrogen peroxide, after the dropwise addition, stir and react at 50-60°C for 1 hour, stir and cool down to 20-30°C, filter, adjust the pH of the filtrate to 3-4 with 10% hydrochloric acid, filter, and water the filter cake Appropriate amount of washing was carried out, and the solid was dried under reduced pressure at 50-60°C for 4 hours to obtain 8.85 g of crude 2-amino-3-(4-bromobenzoyl)benzoic acid, with a yield of 76.1%.
[0049] 2. Add 450ml of ethyl acetate to 8.85g of crude 2-amino-3-(4-bromobenzoyl)benzoic acid, heat and stir at 75-85°C to dissolve, filter while hot, stir the filtrate to cool down to 0-5°C, Stir and crystallize for 2 hours, filter, and dry the solid under reduced pressure at 50-60°C for 4...
Embodiment 3
[0059] Embodiment 3: Preparation of 2-amino-3-(4-bromobenzoyl)benzoic acid
[0060] 1. Put 10.0g (30.3mmol) of 7-(4-bromobenzoyl)indoline-2,3-dione into 160g (200mmol) 5% sodium hydroxide aqueous solution, stir and dissolve at 50-60°C, Add 170ml (1.7mol) of 30% hydrogen peroxide dropwise, after the dropwise addition, stir and react at 50-60°C for 1 hour, stir and cool down to 20-30°C, filter, adjust the pH of the filtrate to 3-4 with 10% hydrochloric acid, filter, and filter the cake Wash with an appropriate amount of water, and dry the solid under reduced pressure at 50-60°C for 6 hours to obtain 7.5 g of crude 2-amino-3-(4-bromobenzoyl)benzoic acid, with a yield of 77.3%.
[0061] 2. Add 200ml of ethanol to 7.5g of crude 2-amino-3-(4-bromobenzoyl)benzoic acid, heat and stir at 75-85°C to dissolve, filter while hot, stir and cool the filtrate to 0-5°C, stir and analyze Crystallized for 3 hours, filtered, and the solid was dried under reduced pressure at 50-60°C for 6 hours t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com