Patents
Literature
55 results about "2-indolinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
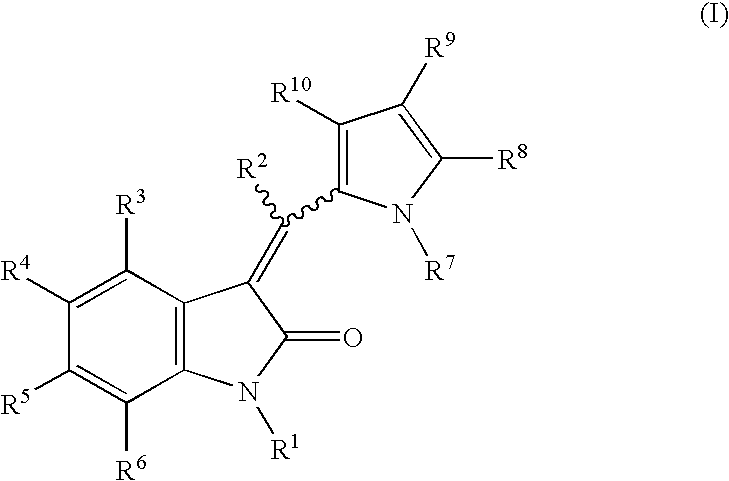
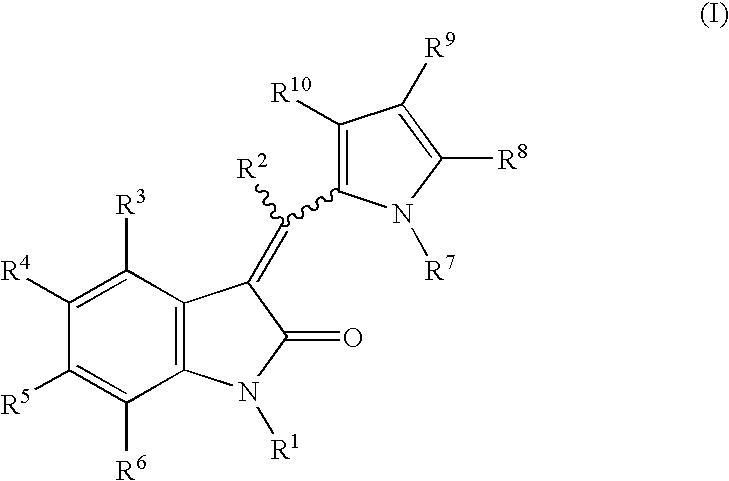
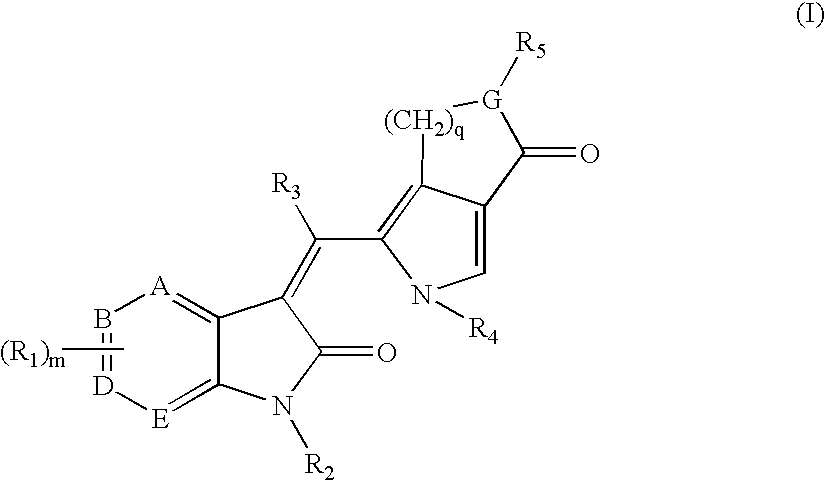
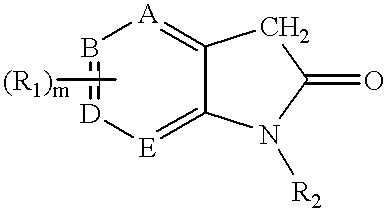
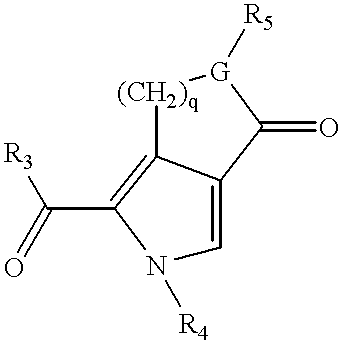
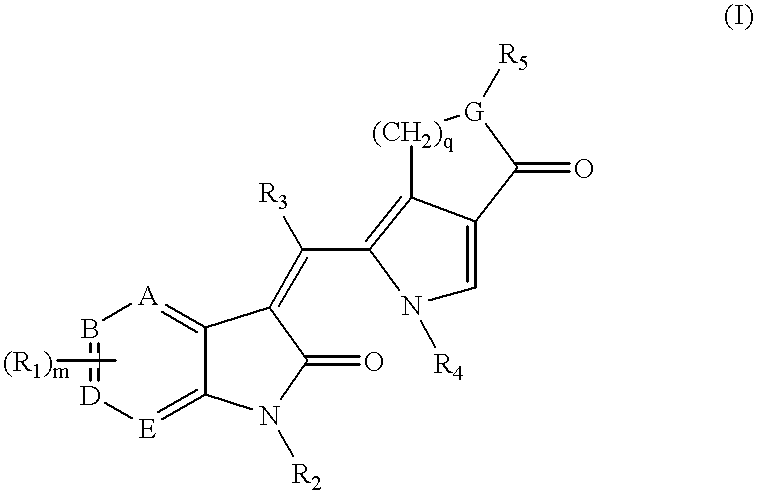
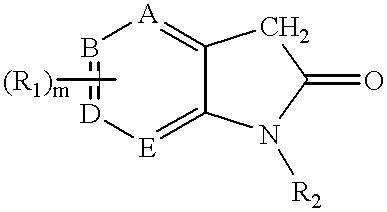
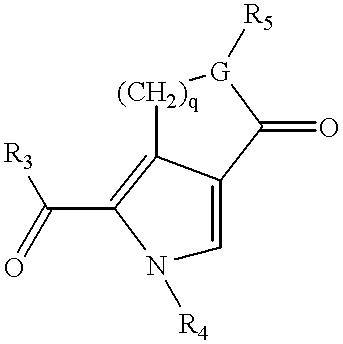
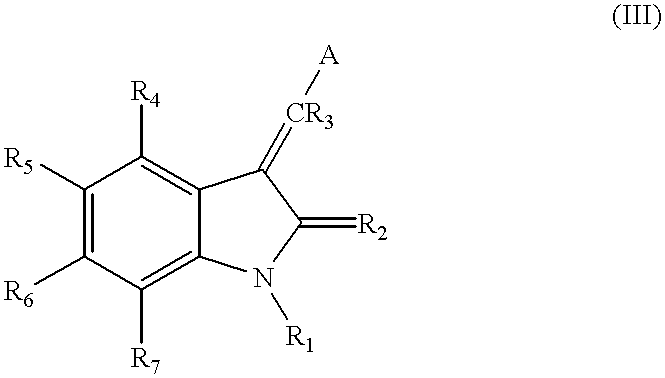
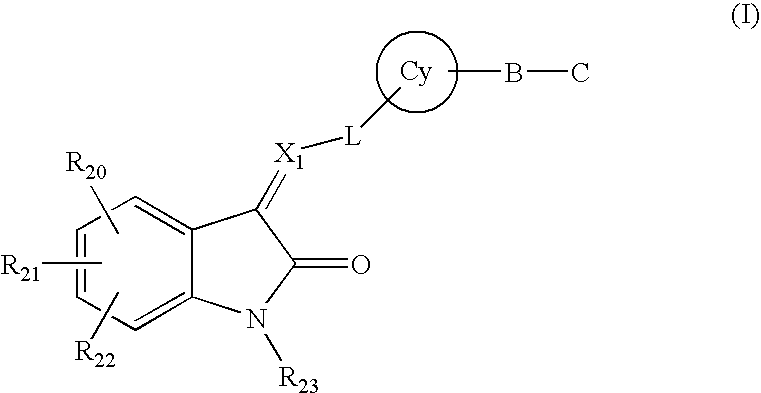
Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors
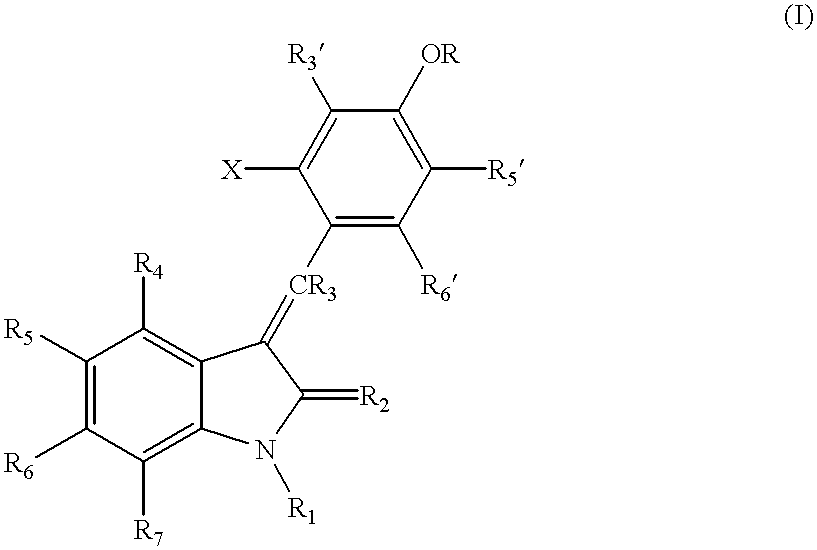
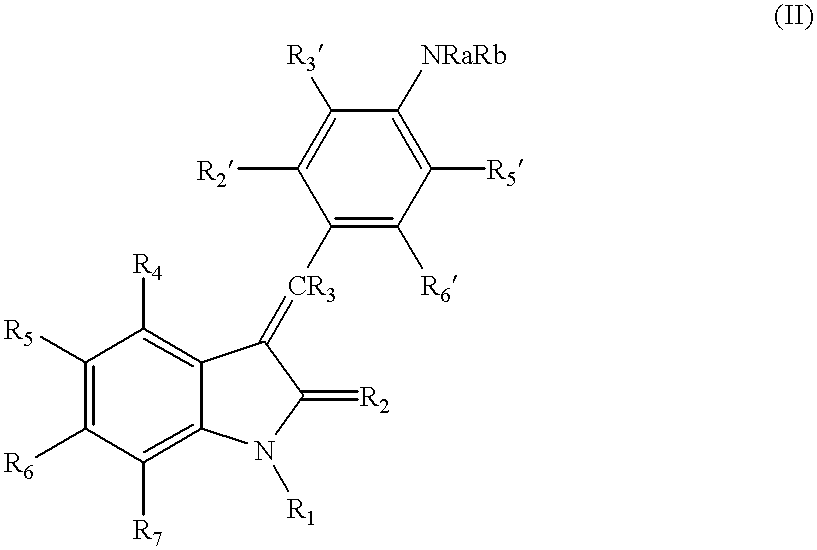
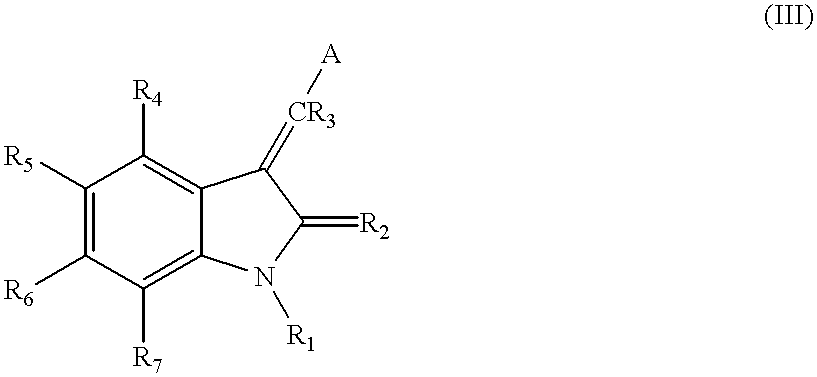
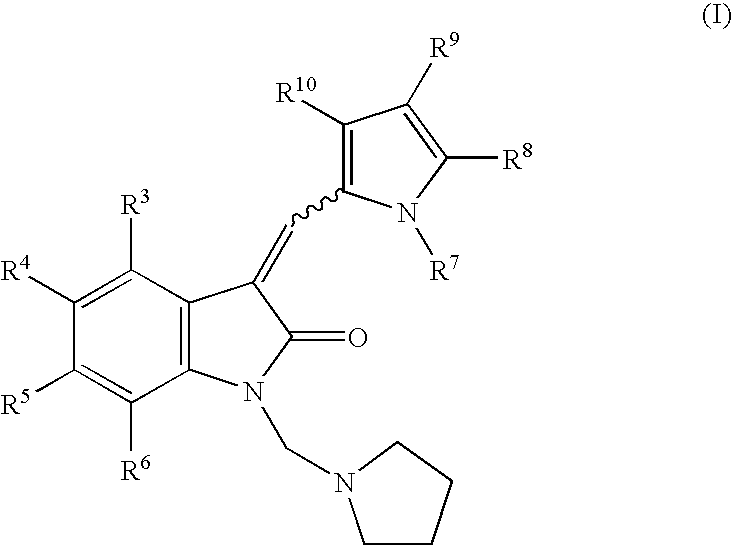
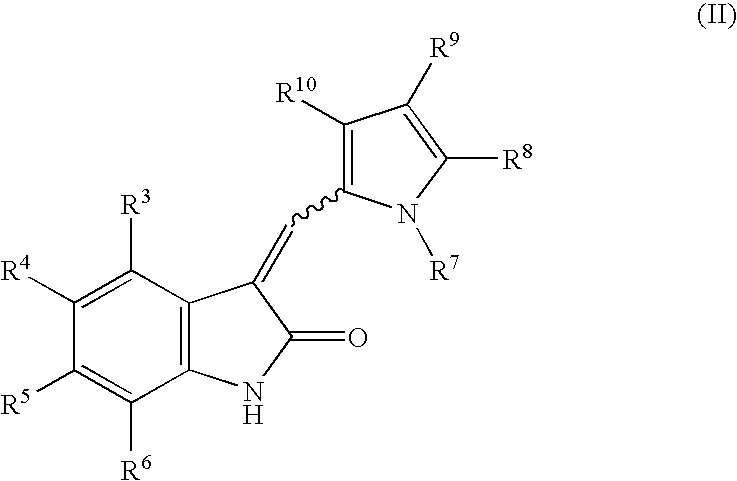
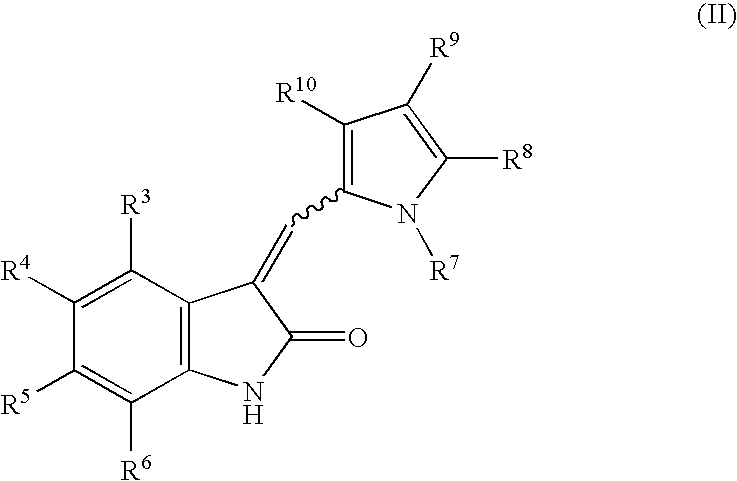
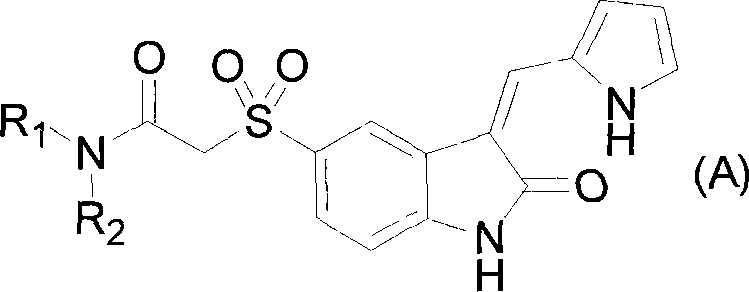
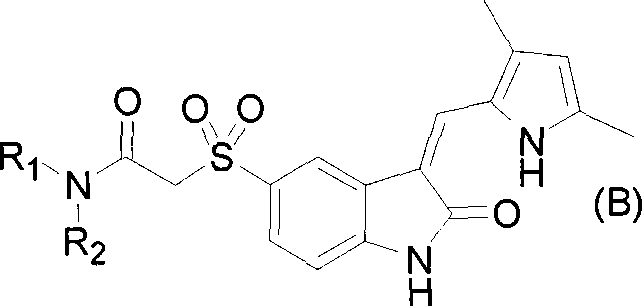
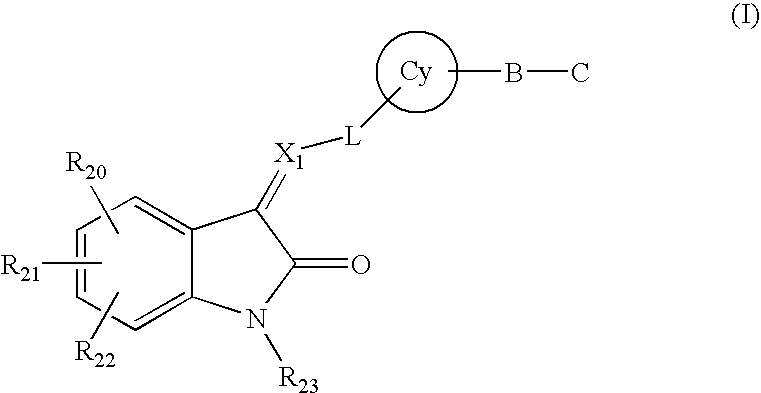
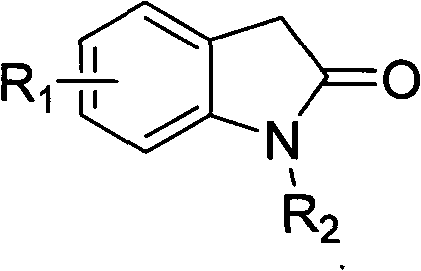
The present invention relates to pyrrolo[3,2-c]pyridine-4-one 2-indolinone compounds of Formula (I) and their pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8X, Y and have the meaning cited in the specification. Also disclosed are the pharmaceutical compositions containing the foregoing compounds, methods for the preparation and pharmaceutical use thereof, particularly as protein kinase inhibitors. Formula (I).
Owner:SHANGHAI HENGRUI PHARM CO LTD
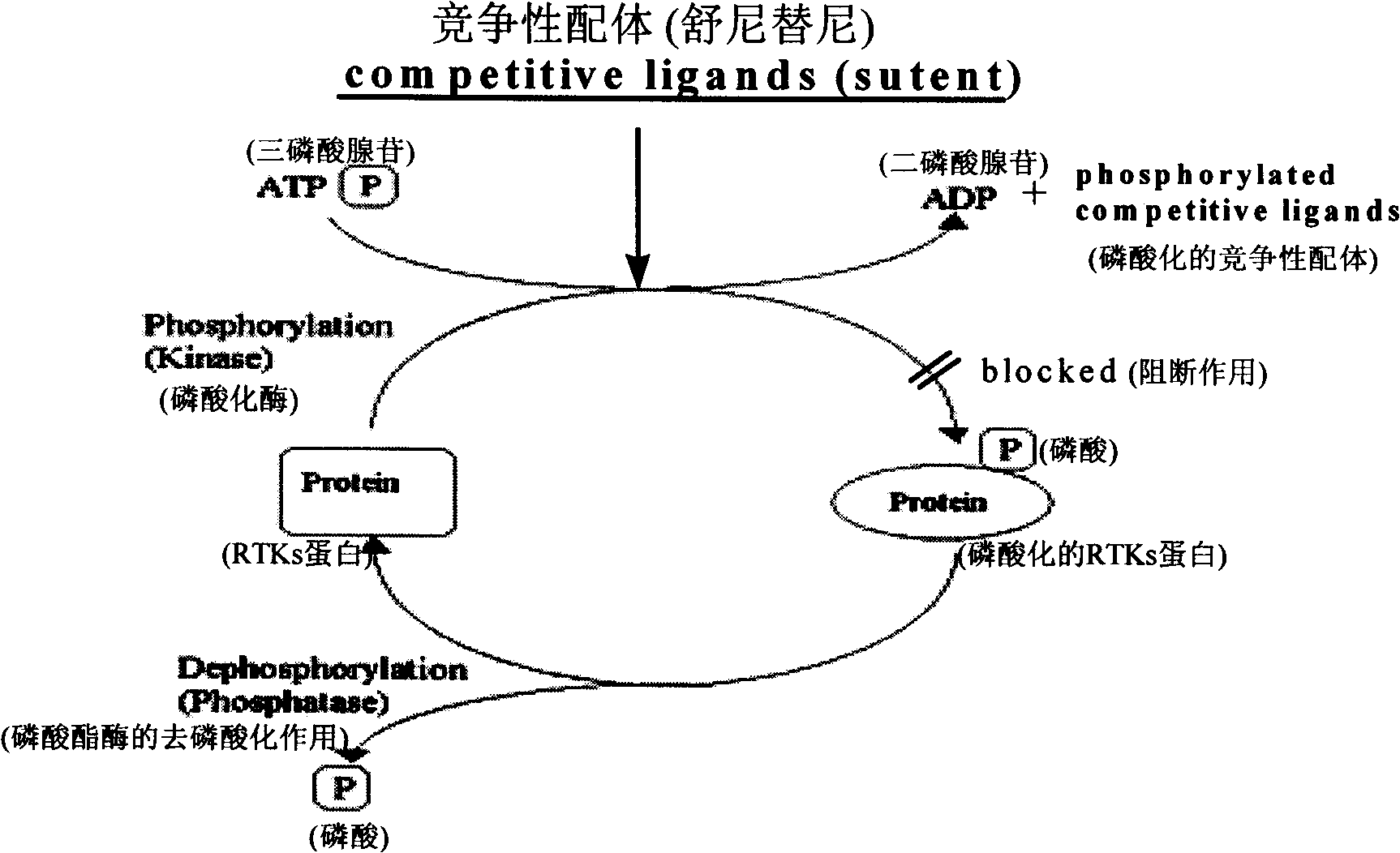
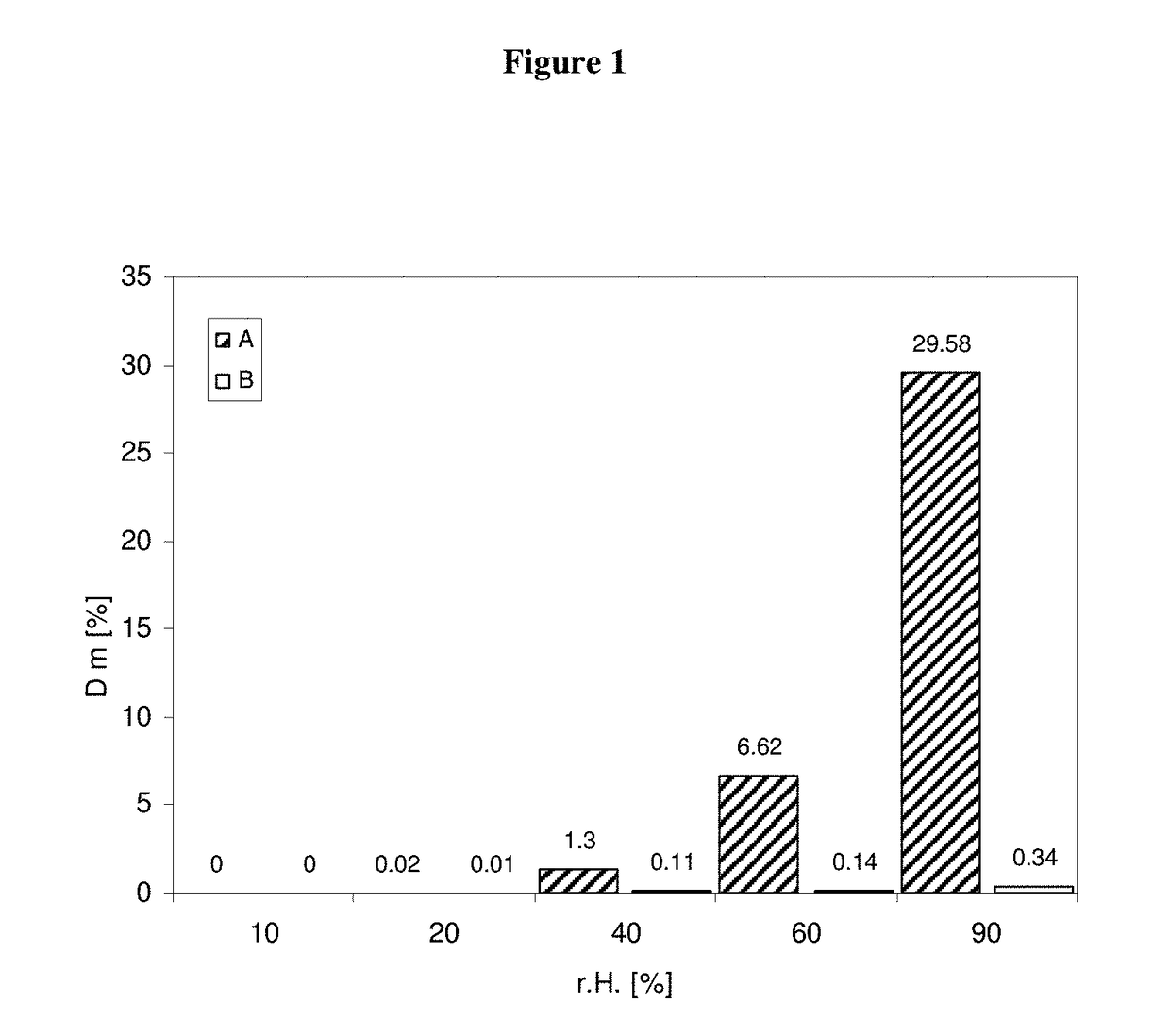
Formulations for pharmaceutical agents ionizable as free acids or free bases
InactiveUS6878733B1Sufficient tenacityHigh affinityBiocidePharmaceutical non-active ingredientsOral medicationMedicinal chemistry
The present invention features formulations of indolinones which compounds are ionizable as free acids or free bases. The formulation is suitable for parenteral or oral administration, wherein the formulation comprises an ionizable substituted indolinone, and a pharmaceutically acceptable carrier therefor. The term “ionizable substituted indolinone” includes pyrrole substituted 2-indolinones which, in addition to being otherwise optionally substituted on both the pyrrole and 2-indolinone portions of the compound, are necessarily substituted on the pyrrole moiety with one or more hydrocarbon chains which themselves are substituted with at least one polar group. The formulations and the compounds themselves are useful for the treatment of protein kinase related disorders as discussed herein.
Owner:SUGEN INC
3-(pyrolyllactone)-2-indolinone compounds as kinase inhibitors
The invention relates to certain indolinone compounds, their method of synthesis, and a combinatorial library consisting of the indolinone compounds of the invention. The invention also relates to methods of modulating the function of protein kinases using indolinone compounds of the invention and methods of treating diseases by modulating the function of protein kinases and related signal transduction pathways.
Owner:SUGEN INC
3-(pyrolyllactone)-2-indolinone compounds as kinase inhibitors
The invention relates to certain indolinone compounds, their method of synthesis, and a combinatorial library consisting of the indolinone compounds of the invention. The invention also relates to methods of modulating the function of protein kinases using indolinone compounds of the invention and methods of treating diseases by modulating the function of protein kinases and related signal transduction pathways.
Owner:SUGEN INC
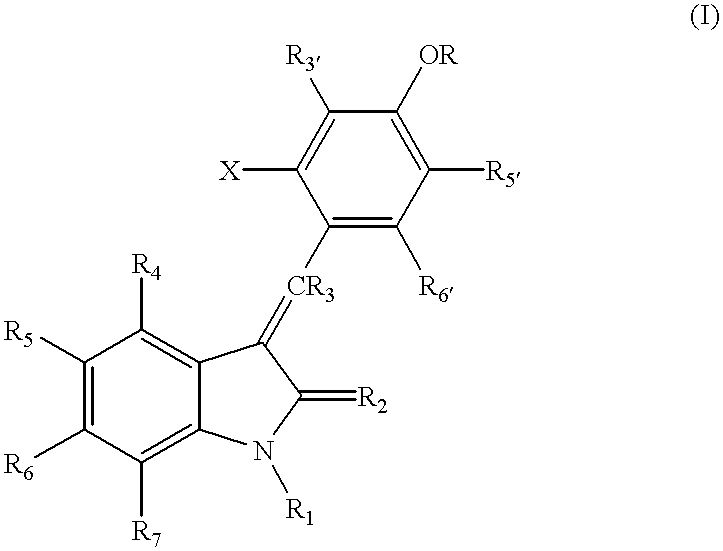
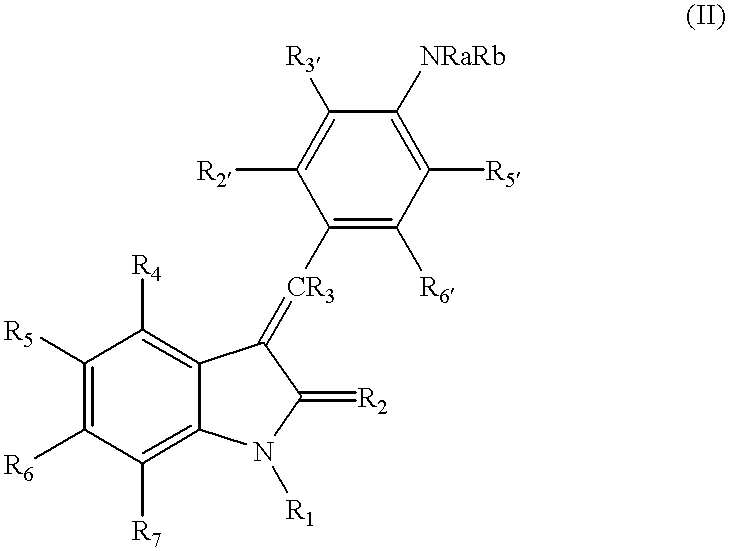
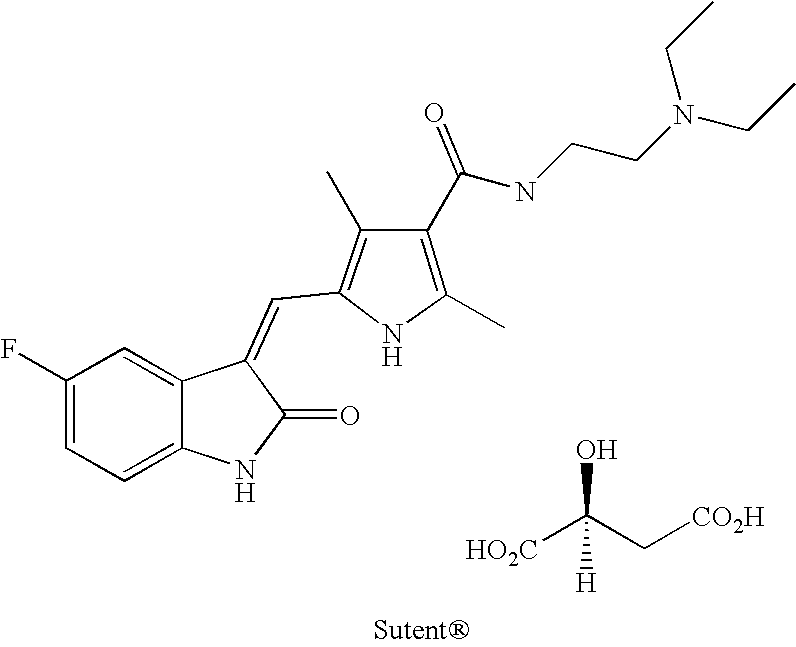
3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition
ActiveUS7119093B2High pharmacological potencyOrganic active ingredientsOrganic chemistryMedicine2-indolinone
Owner:BOEHRINGER INGELHEIM PHARMA KG
Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors
Owner:SHANGHAI HENGRUI PHARM CO LTD
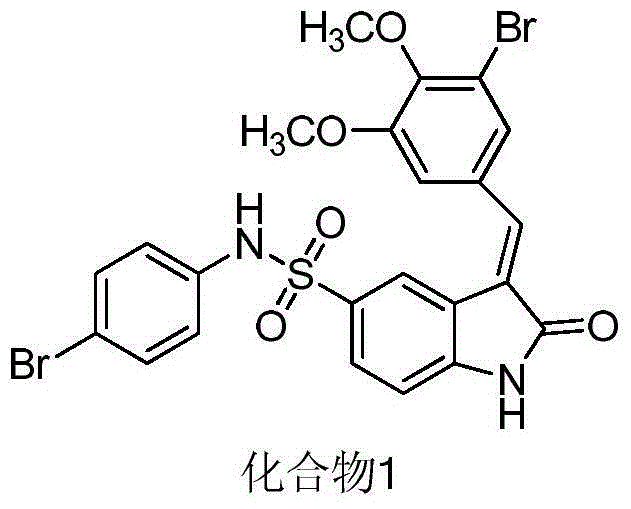
3-(4'-bromobenzylindenyl)-2-indolinone and analogues thereof for the treatment of disease
The present invention relates to organic molecules capable of modulating tyrosine kinase signal transduction in order to regulate, modulate and / or inhibit abnormal cell proliferation.
Owner:SUGEN INC
3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition
ActiveUS20040176392A1High pharmacological potencyOrganic active ingredientsOrganic chemistryMedicine2-indolinone
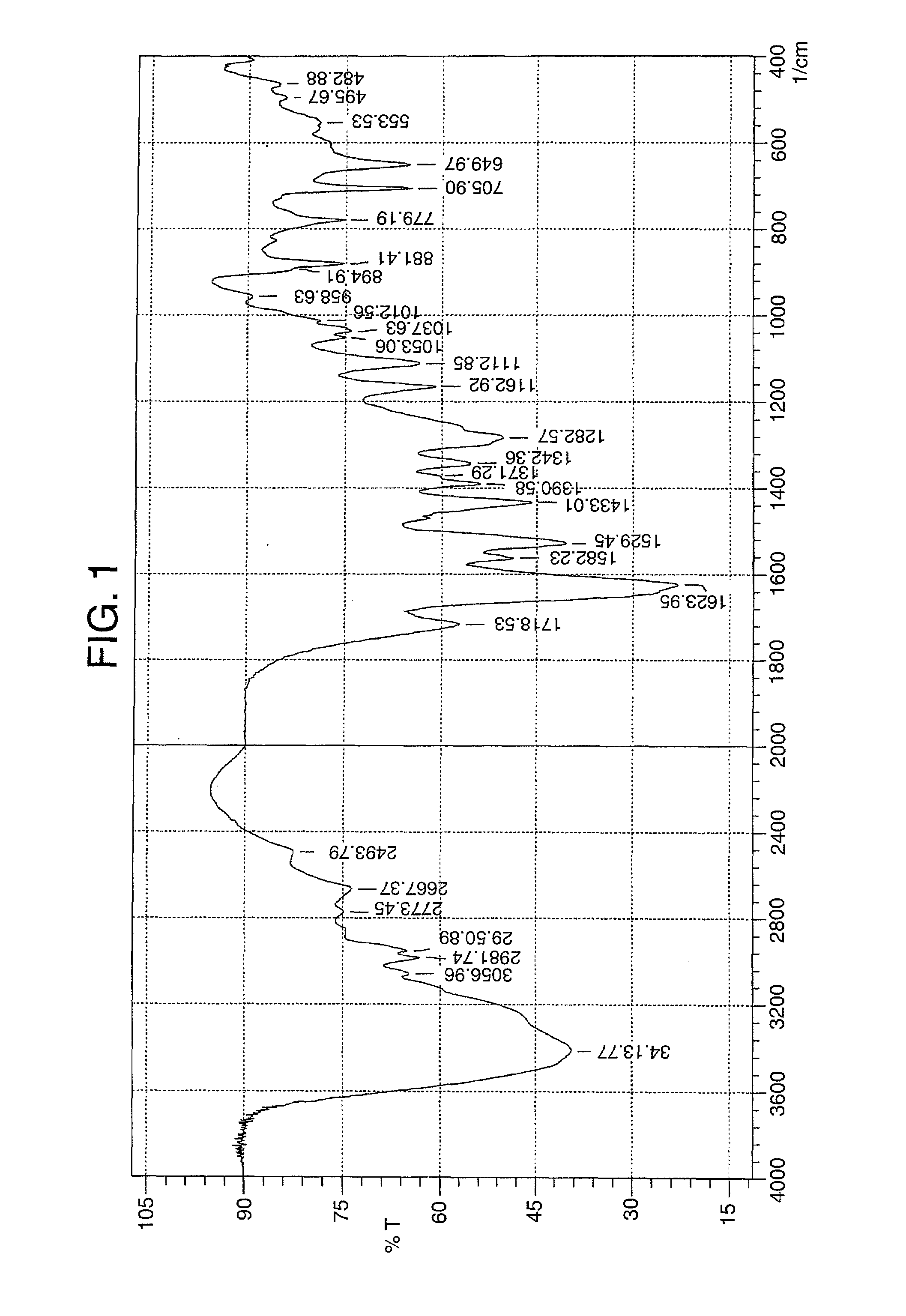
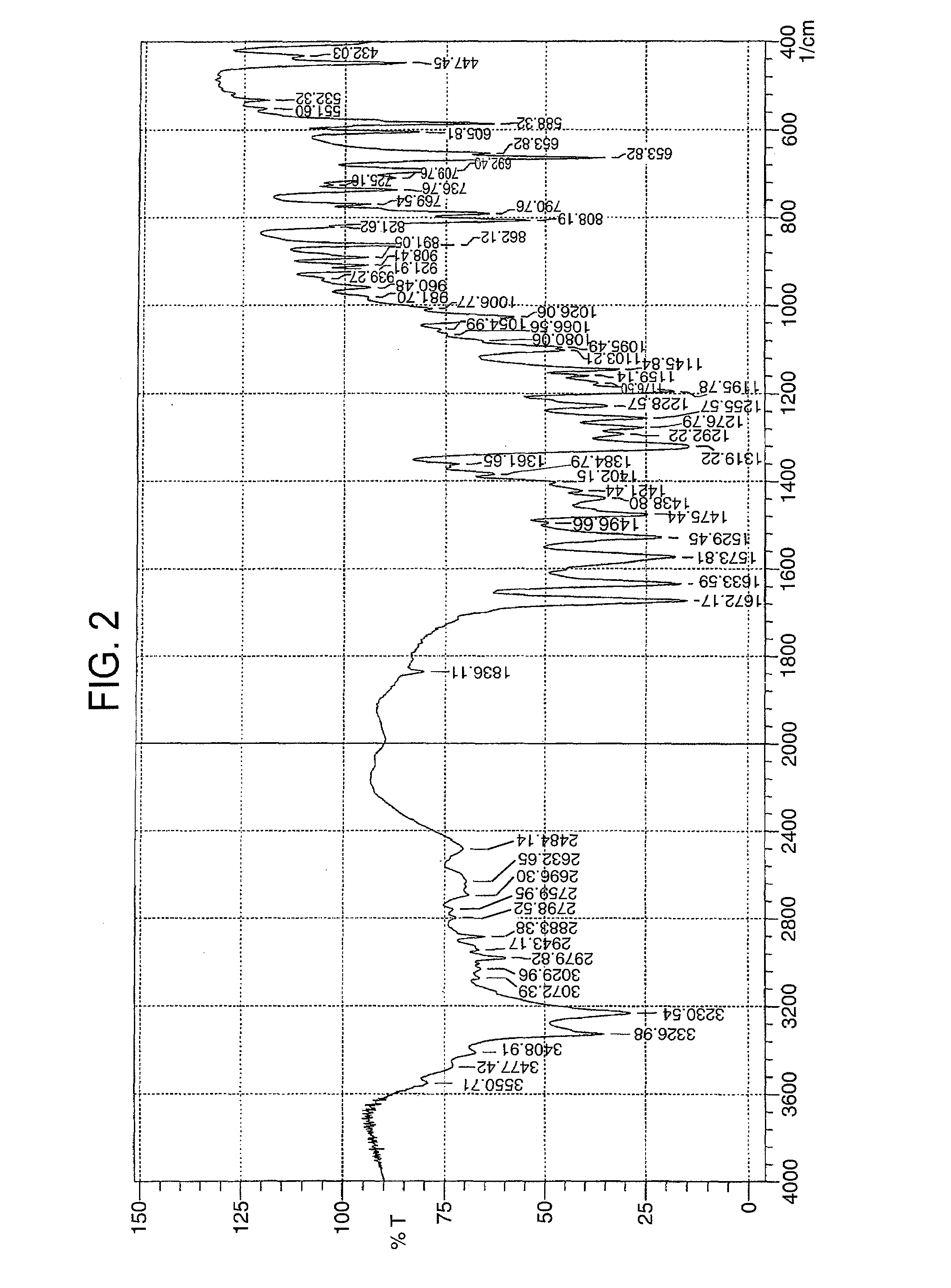
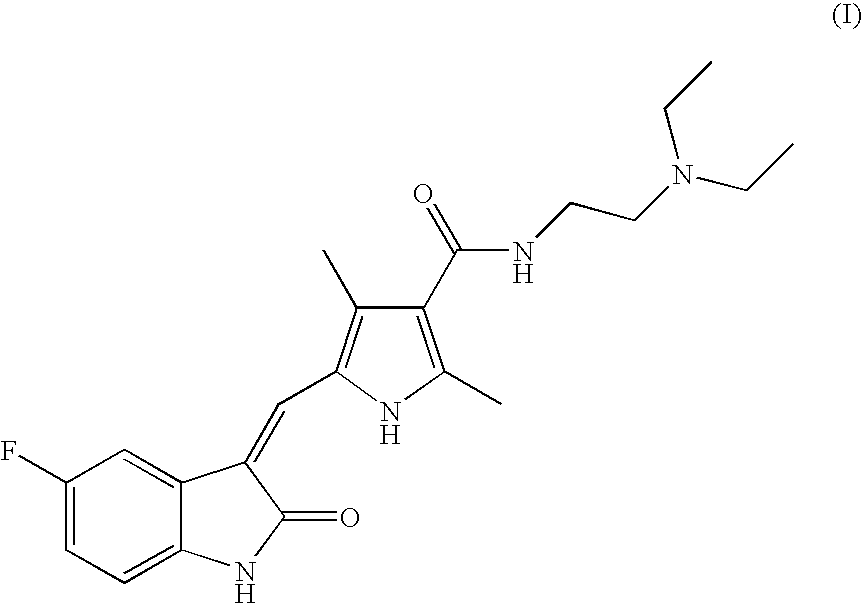
The present invention relates to the compound 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate of formula I and the use thereof as a pharmaceutical composition.
Owner:BOEHRINGER INGELHEIM PHARM KG
Substituted 2-indolinone as ptk inhibitors containing a zinc binding moiety
InactiveUS20080125478A1Effective for treating diseaseHigh activityBiocideOrganic chemistryDiseasePTK Inhibitors
The present invention relates to substituted 2-indolinone containing zinc-binding moiety based derivatives that have enhanced or unique properties as inhibitors of protein tyrosine kinase (PTK) receptors and their use in the treatment of PTK related diseases and disorders such as cancer. The said derivatives may further act as HDAC inhibitors.
Owner:CURIS INC
1,3-substituted-5-acetaminoindolone compounds and application thereof to anti-tumor drugs
ActiveCN103554008AHas anti-tumor biological activitySimple operation processOrganic active ingredientsOrganic chemistryHuman leukemiaKetone
The invention relates to 1,3-substituted-5-acetaminoindolone compounds and an application thereof to anti-tumor drugs. The compounds are 1-methyl-5-acetamino-2-indolinone, 1-(4-bromobenzyl)-5-acetamino-2-indolinone, 1-(4-methylbenzyl)-3-oxime-5-acetaminoindolone, 1-(4-methoxybenzyl)-3-oxime-5-acetaminoindolone and the like. The in-vitro tumor cell inhibitory activities of the 1,3-substituted-5-acetaminoindolone compounds synthesized in the invention are tested and the results show that such kind of compounds have certain inhibiting effects (IC50(100mu M)) on human leukemia cells (K562), human colon cancer cells (HT-29) and human liver cancer cells (HepG2), have anti-tumor activities and can be used for preparing anti-tumor drugs.
Owner:无锡珉琰管理咨询服务有限公司
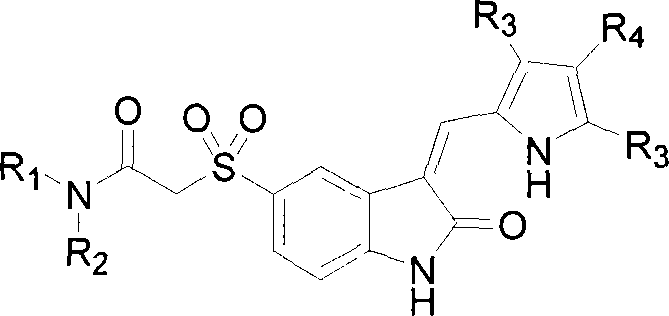
Heterocyclic substituted-3-heteroarylidenyl-2-indolinone derivative
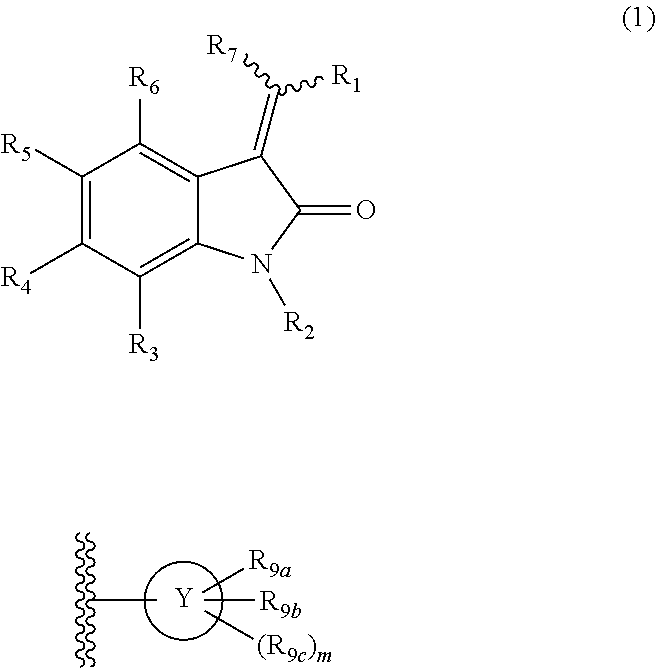
Disclosed is a compound represented by formula (1) or a pharmacologically acceptable salt thereof. (In the formula, R1 is optionally substituted heteroaryl etc.; R2 is hydrogen etc.; R3 and R4 are each independently hydrogen etc., R5 is the following group: (wherein Y is optionally substituted five membered heteroaryl etc., R9a is optionally substituted aryl etc., R9b and R9c are each dependently hydrogen etc., and m is the integral 0 etc.) etc.; R6 is hydrogen etc.; and R7 is hydrogen etc.
Owner:BOSTON BIOMEDICAL
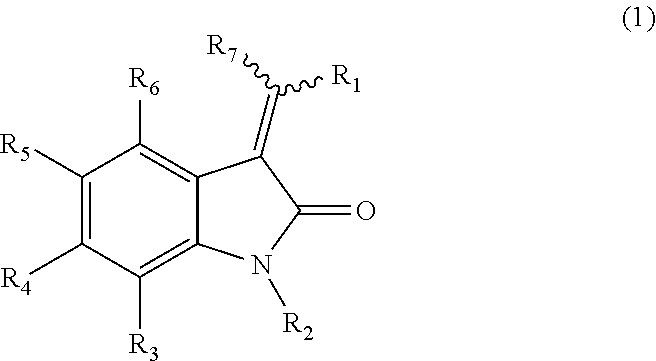
Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors
The present invention relates to pyrrolo[3,2-c]pyridine-4-one 2-indolinone compounds of Formula (I) and their pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8 X, Y and have the meaning cited in the specification. Also disclosed are the pharmaceutical compositions containing the foregoing compounds, methods for the preparation and pharmaceutical use thereof, particularly as protein kinase inhibitors. Formula (I).
Owner:SHANGHAI HENGRUI PHARM CO LTD
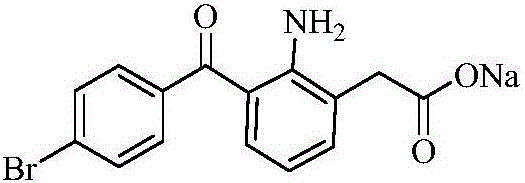
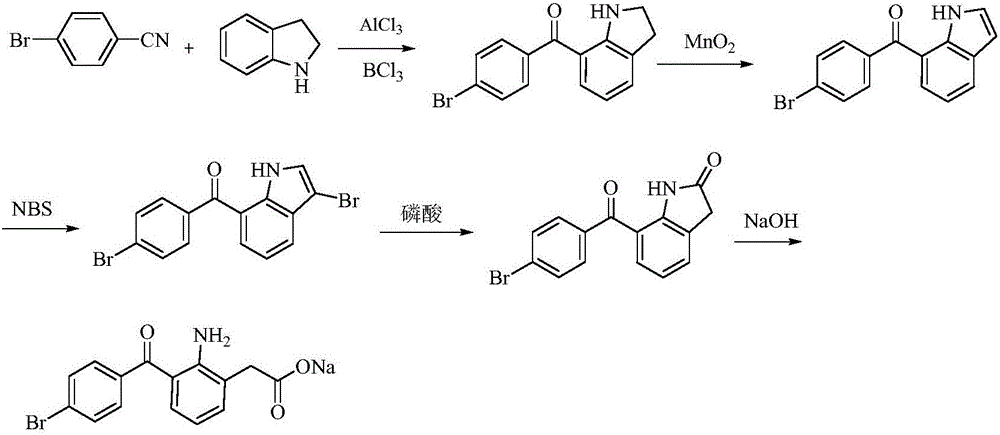
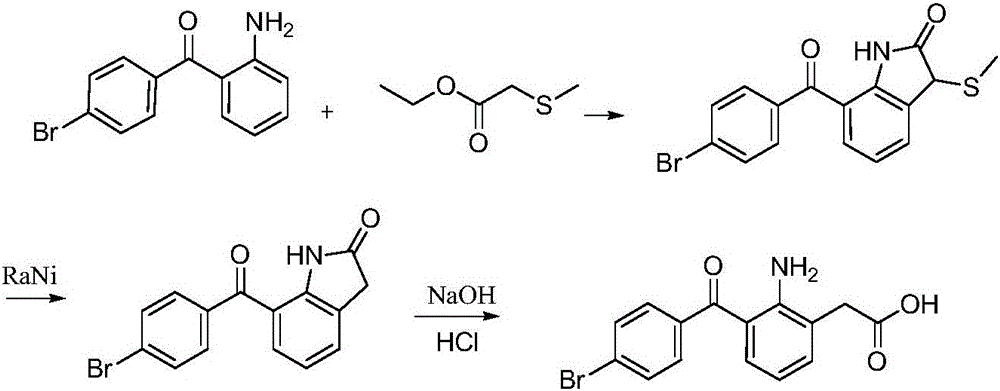
Bromfenac sodium preparation method
ActiveCN106397235AHigh purityShort synthetic routeOrganic compound preparationAmino-carboxyl compound preparationBoron trichlorideBromfenac
The invention relates to a bromfenac sodium preparation method. The preparation method comprises specific steps as follows: indole reacts under the action of DMSO (dimethylsulfoxide) to produce 3-bromoindole; 3-bromoindole is added to 2-methoxyethanol, acid is added for hydrolysis, and 2-indolinone is obtained; boron trichloride is added to methylbenzene, a methylbenzene mixed solution of p-bromobenzonitrile and 2-indolinone is added dropwise, aluminum chloride is then added, acid is added, a reaction is performed, and 7-(4-bromobenzoyl)-1,3-dihydro-indol-2-one is obtained; hydrolysis is performed with an alkaline solution, acid is added for neutralization, and bromfenac is obtained; ethanol is added to bromfenac, bromfenac and a sodium hydroxide solution form salt, the salt is cooled and subjected to recrystallization, and bromfenac sodium is obtained. Compared with the prior art, the synthetic route is short, high-purity bromfenac sodium can be prepared, the quality meets the latest standards of pharmacopoeia, industrial production is facilitated, and the method can provide powerful guarantee for industrial production of bromfenac sodium and intermediates of bromfenac sodium.
Owner:山东辰欣佛都药业股份有限公司
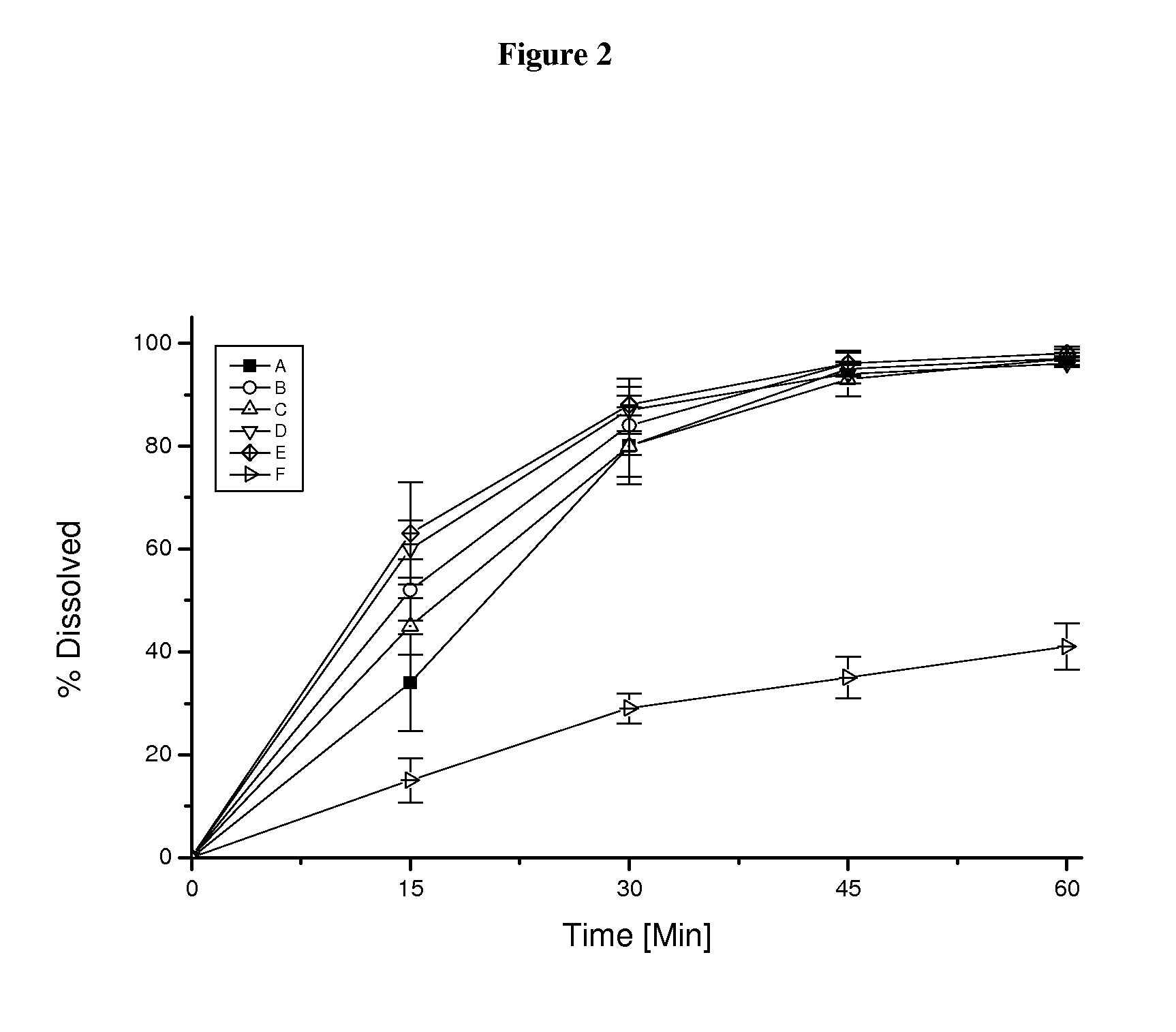
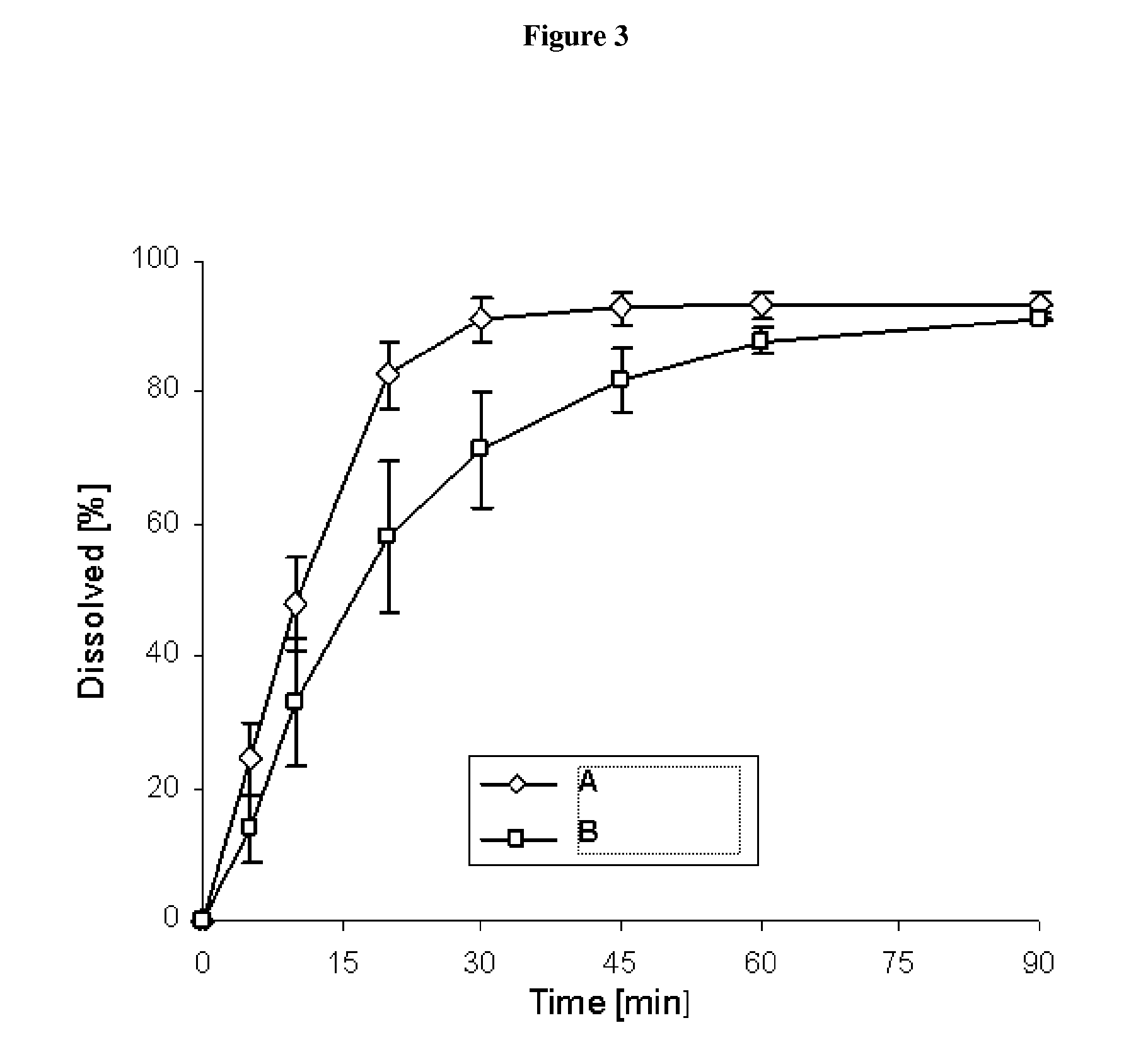
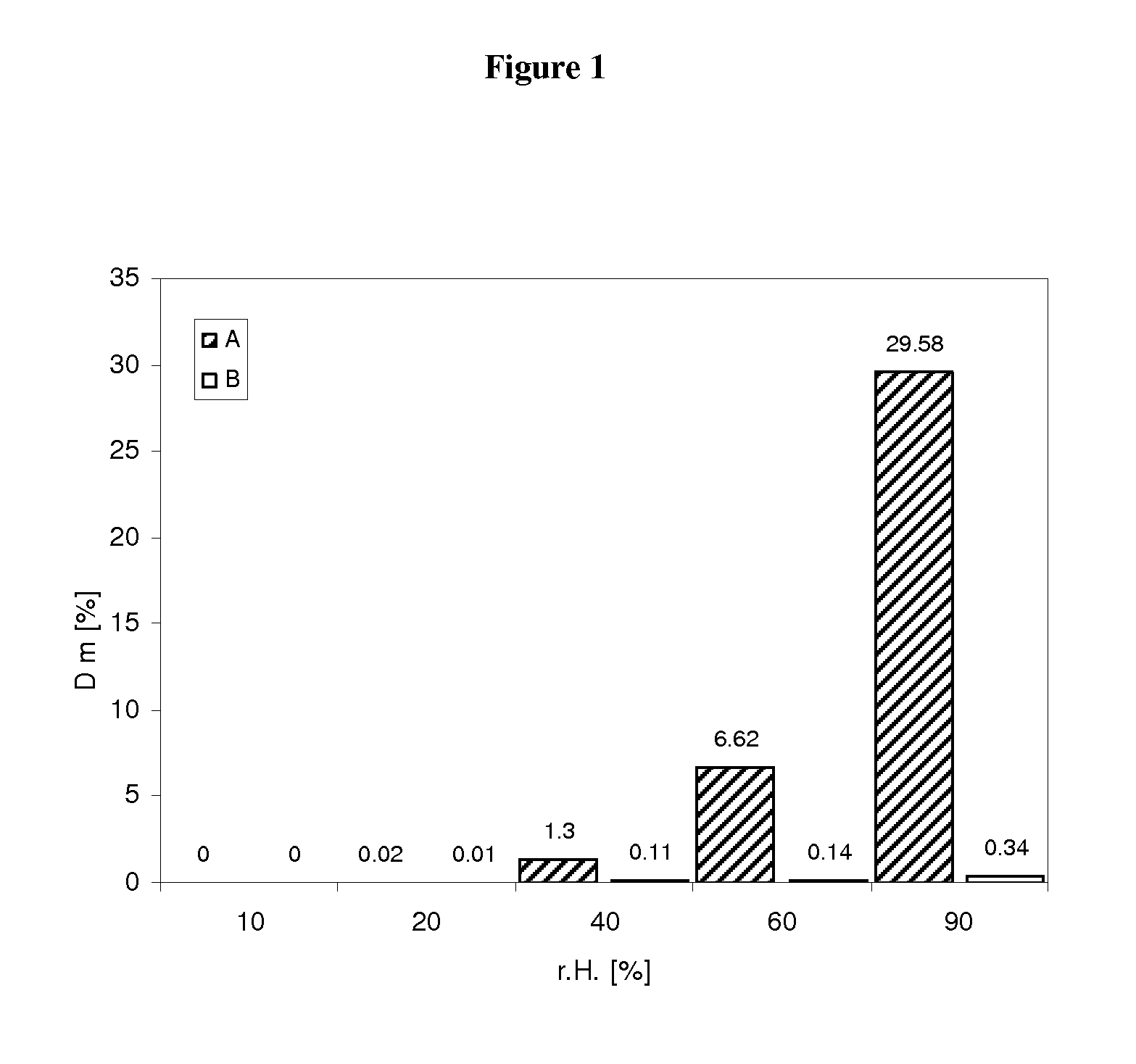
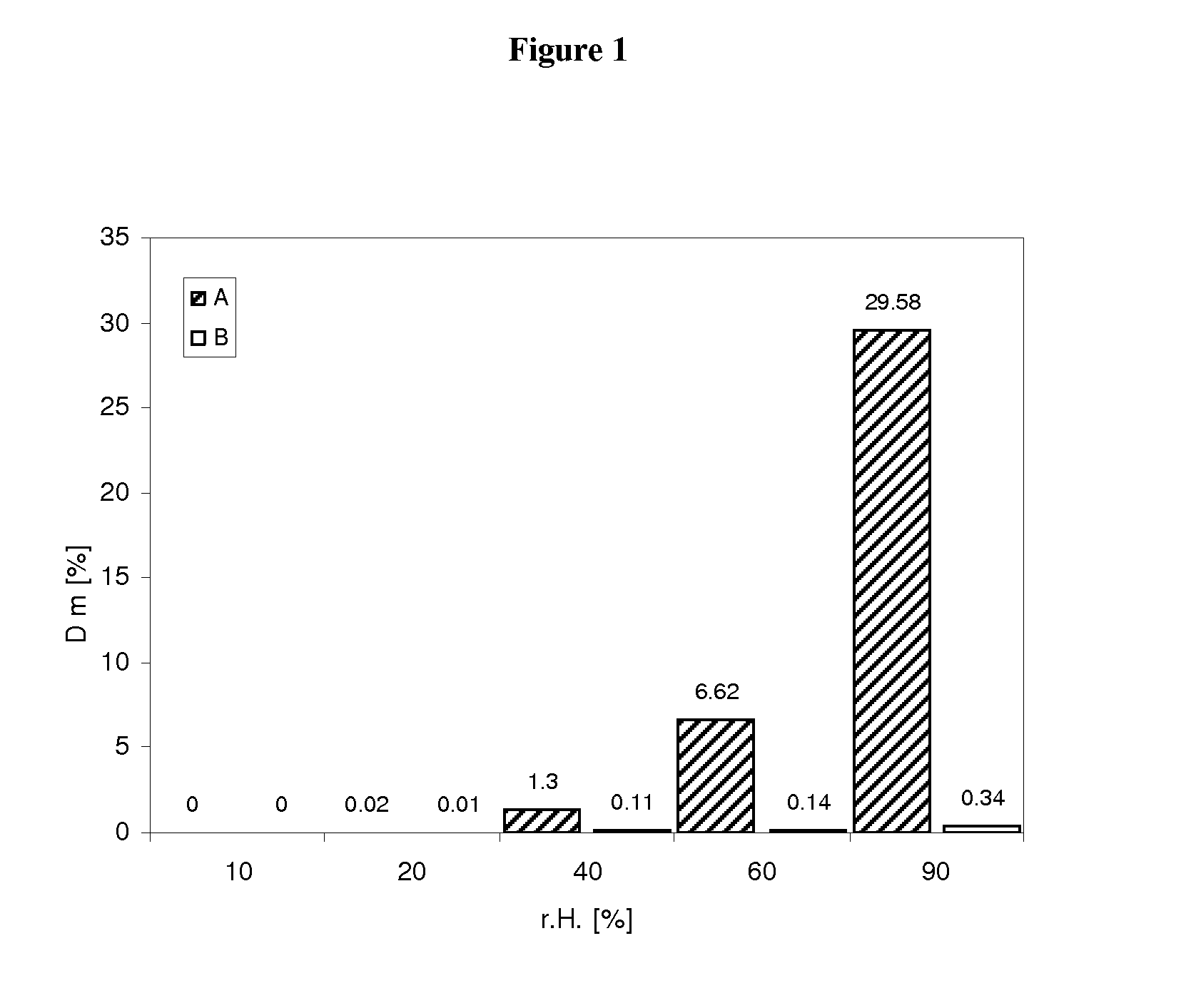
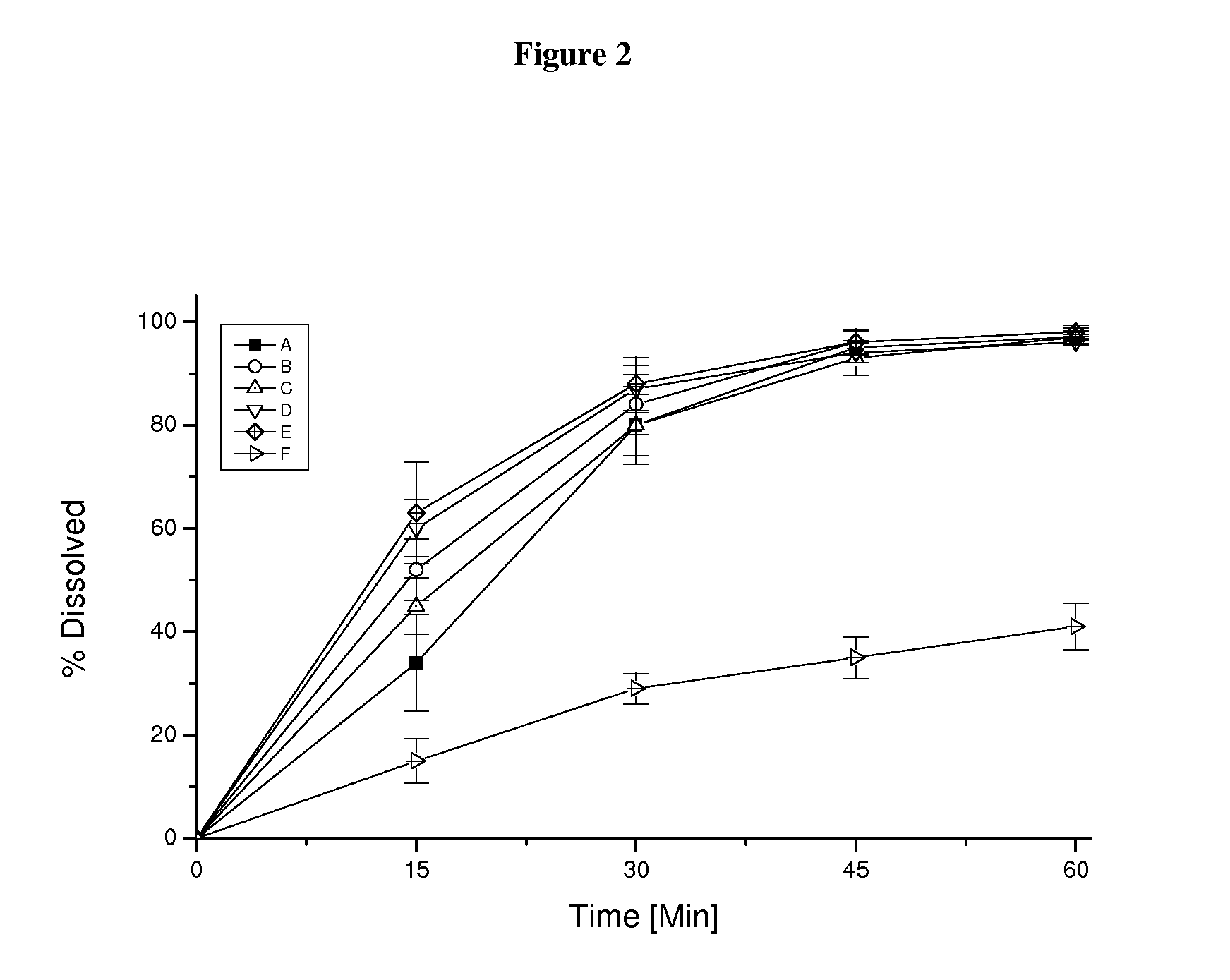
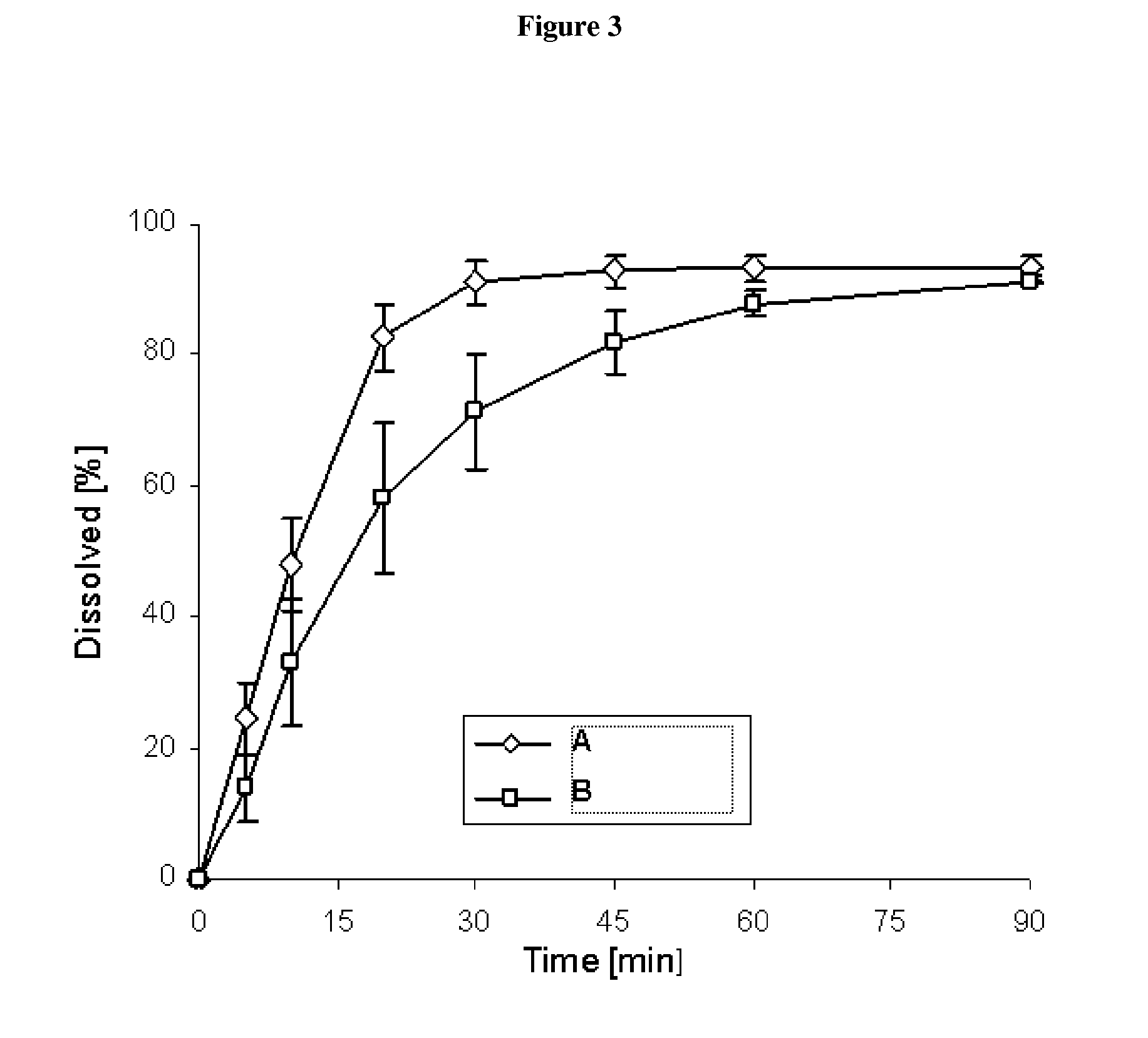
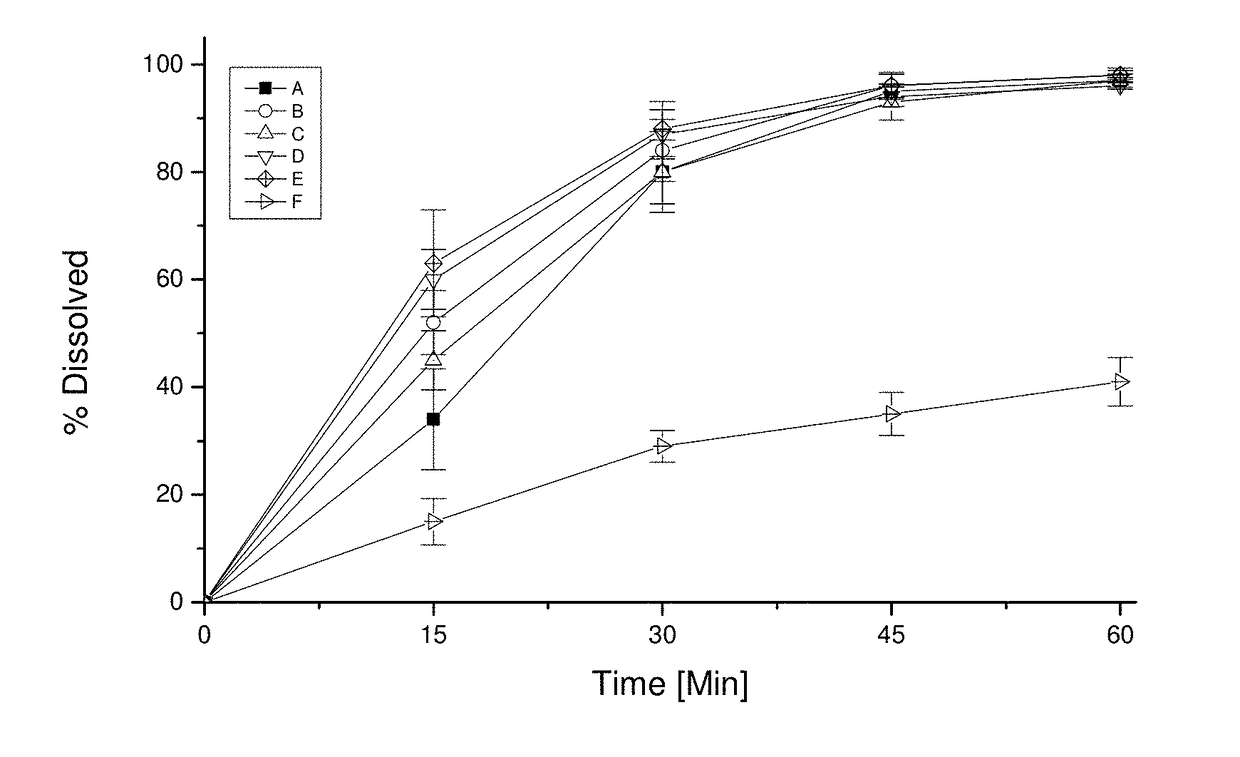
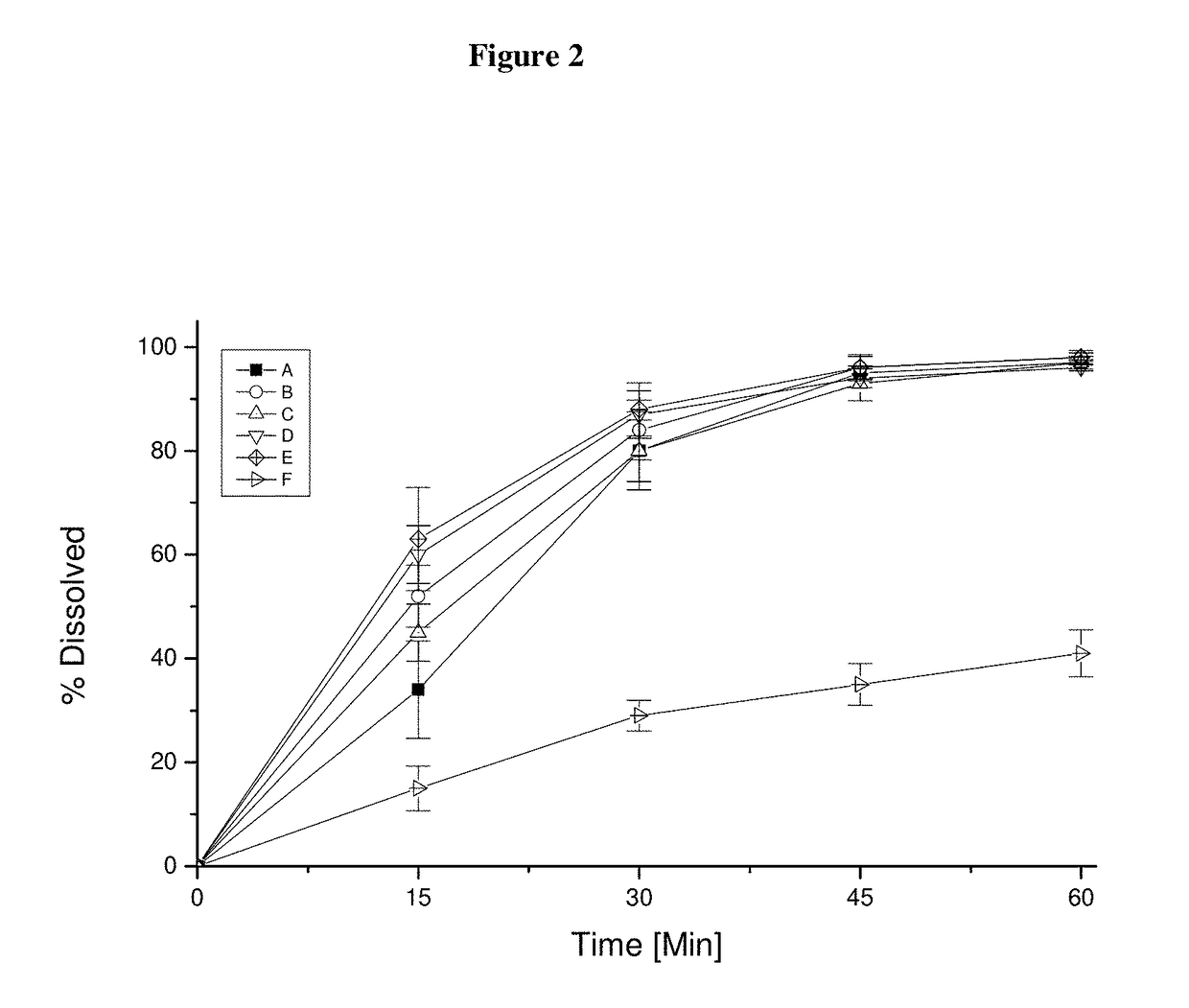
Pharmaceutical dosage form for immediate release of an indolinone derivative
The present invention relates to a pharmaceutical dosage form delivering an immediate release profile containing the active substance 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate.
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted 2-indoline ketone derivatives as well as preparation method and uses thereof
ActiveCN101255154AImprove solubilityHigh anticancer activityOrganic active ingredientsSugar derivativesSolubilityKetone
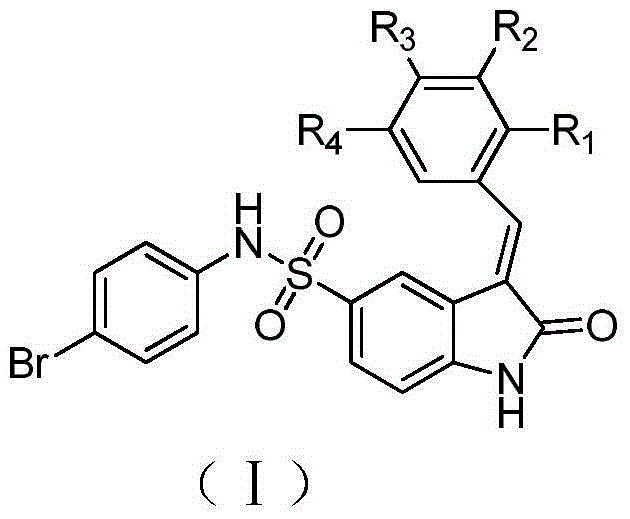
The invention relates to a substituted 2-indolinone derivate, a preparation method and the applications thereof in antineoplastic. The substituted 2-indolinone derivate of the invention has a chemical general structure shown in the drawing, wherein X represents one of halogen and hydrogen, or nitro and alkoxyl; R1 and R2 are any one selected from hydrogen, alkyl, cycloalkyl, aryl and heteroary respectively; A represents any one of glycoside and isomer thereof, hydroxy and alkyl, and hydroxy and alkoxyl group. The substituted substituted 2-indolinone derivate of the invention generates nucleoside analogue of sunitinib by saccharification reaction or alkylation reaction of 2-indolinone, greatly increases the solubility, the antitumor activity and the virus resistance.
Owner:长治市三宝生化药业有限公司
Pharmaceutical dosage form for immediate release of an indolinone derivative
Owner:BOEHRINGER INGELHEIM INT GMBH
Process For Preparing A 3-Pyrrole Substituted 2-Indolinone Malate Salt
The invention relates to the malic acid salt of N-[2-(diethylamino)ethyl]-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide, to the use thereof as an intermediate for preparing the malic acid salt of sunitinib, and to pharmaceutical compositions comprising said malic acid salt of sunitinib.
Owner:MEDICHEM
3-(4'-bromobenzylindenyl)-2-indolinone and analogues thereof for the treatment of disease
The present invention relates to organic molecules capable of modulating tyrosine kinase signal transduction in order to regulate, modulate and / or inhibit abnormal cell proliferation.
Owner:SUGEN INC
1-(Pyrrolidin-1-ylmethyl)-3-(pyrrol-2-ylmethylidene)-2-indolinone derivatives
The present invention is directed to 1-pyrrolidin-1-ylmethyl-3-(pyrrol-2-ylmethylidene)-2-indolinone derivatives that modulate the activity of protein kinases (“PKs”). Pharmaceutical compositions comprising these compounds, methods of treating diseases related to abnormal PK activity utilizing pharmaceutical compositions comprising these compounds and methods of preparing them are also disclosed.
Owner:PHARMACIA & UPJOHN CO
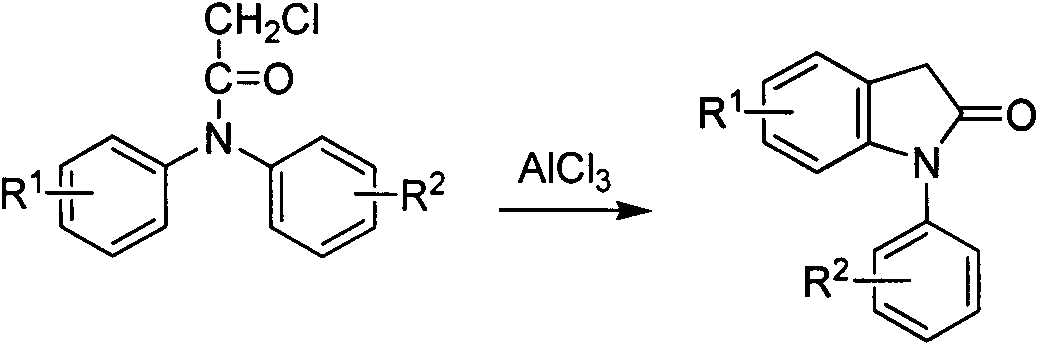
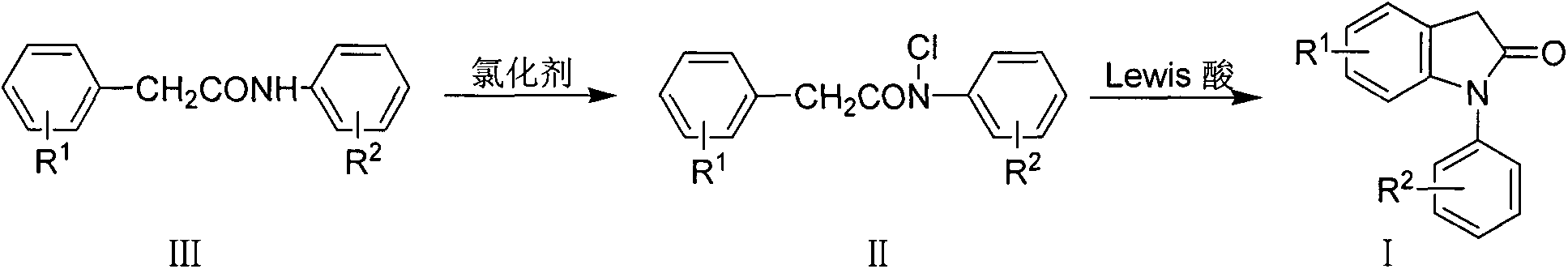
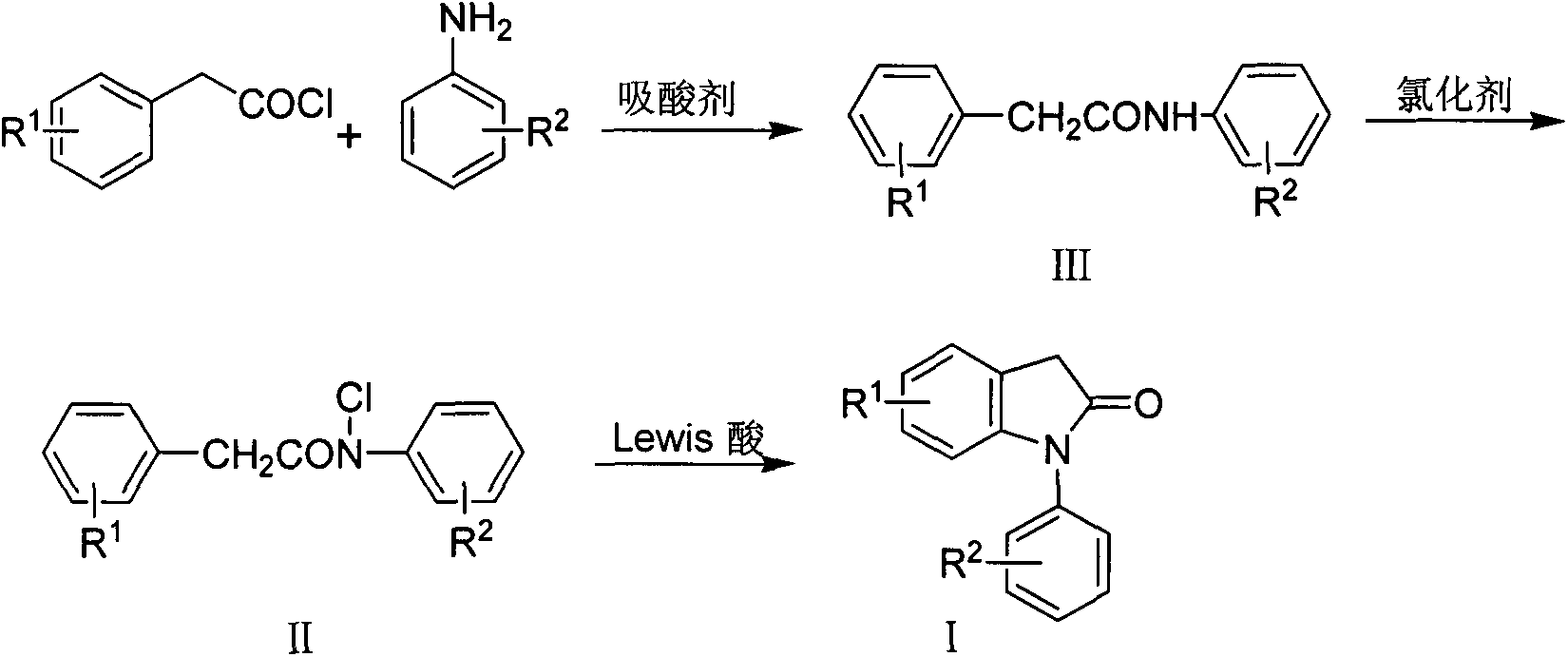
Preparation method for 1-aryl-2-indolinone derivatives
ActiveCN103288708ARaw materials are cheap and easy to getLow costOrganic chemistryArylOrganic solvent
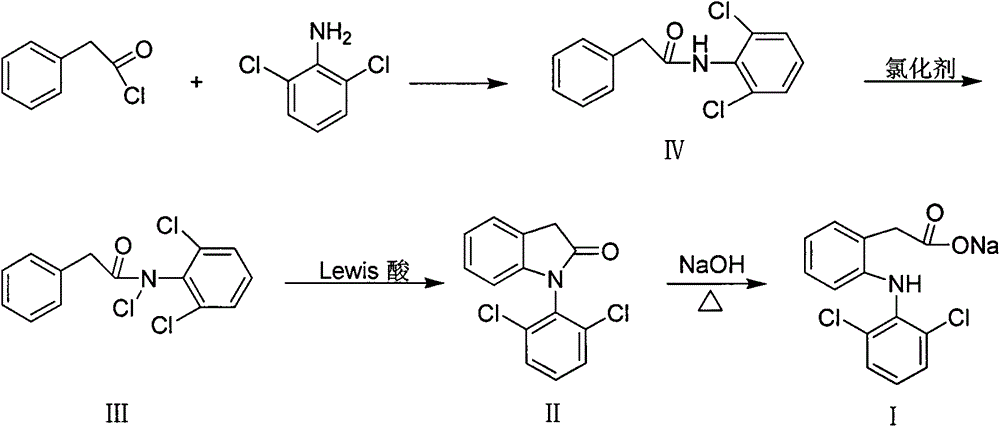
The invention discloses a method for synthesis of 1-aryl-2-indolinone derivatives. The method comprises the following steps: dissolving N-aryl-substituted phenylacetamide (III) in an organic solvent, adding a chlorination reagent and carrying out a reaction so as to obtain N-chloro-N-aryl-substituted phenylacetamid (II); and subjecting N-chloro-N-aryl-substituted phenylacetamid to a reaction with a certain amount of Lewis acid and a proper amount of an organic solvent at a certain temperature so as to obtain 1-aryl-2-indolinone (I). The method provided by the invention has the advantages of simple operation, easily available reagents, a low price, mild conditions and capability of synthesizing a plurality of 1-aryl-2-indolinone (I) compounds.
Owner:中国人民解放军防化学院
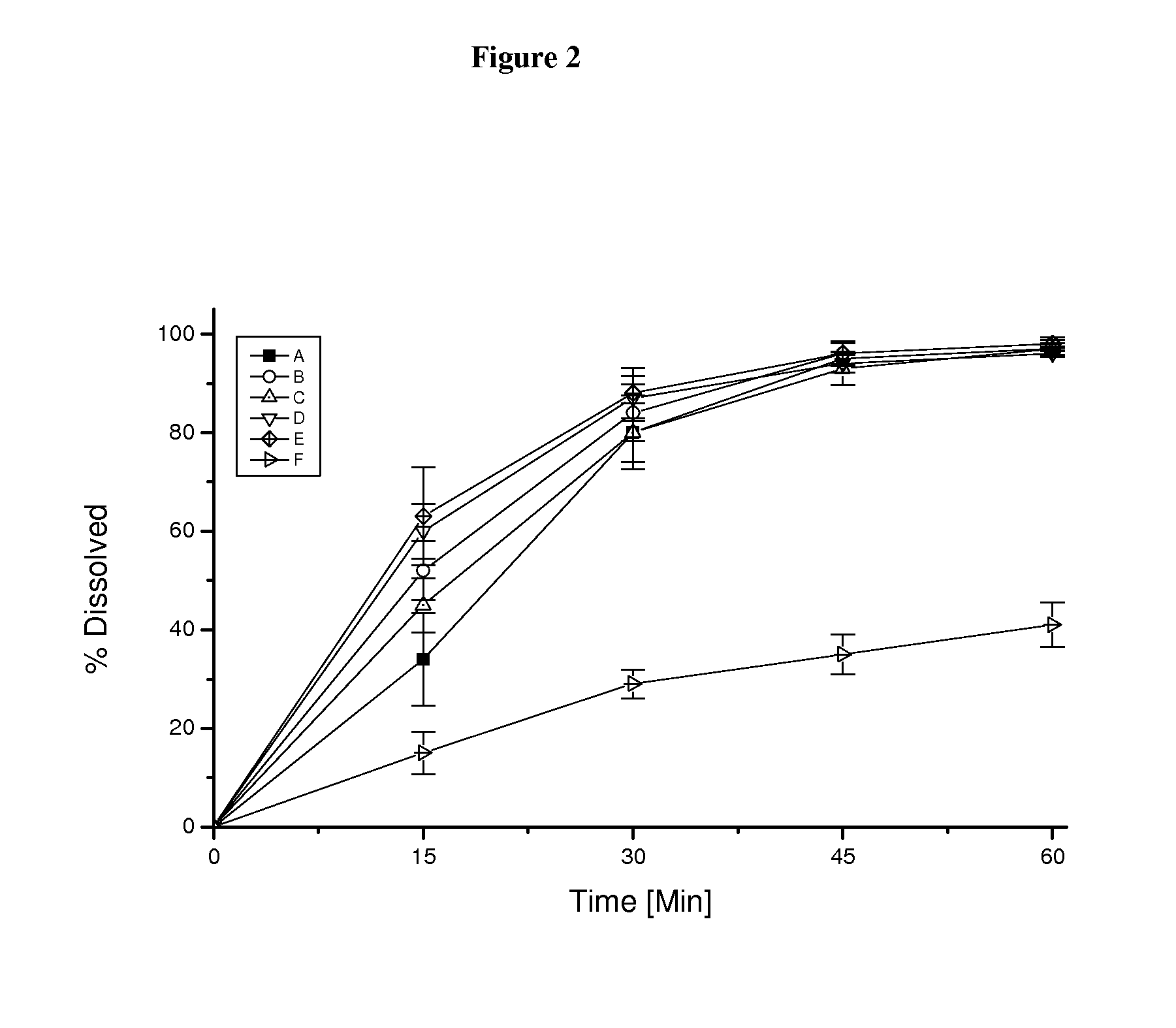
Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
The present invention relates to a suspension formulation containing the active substance 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate, to a capsule pharmaceutical dosage form containing said suspension formulation, to a process for preparing said suspension formulation, to a process for preparing said capsule comprising said suspension formulation and to the packaging material for the finished capsule.
Owner:BOEHRINGER INGELHEIM INT GMBH
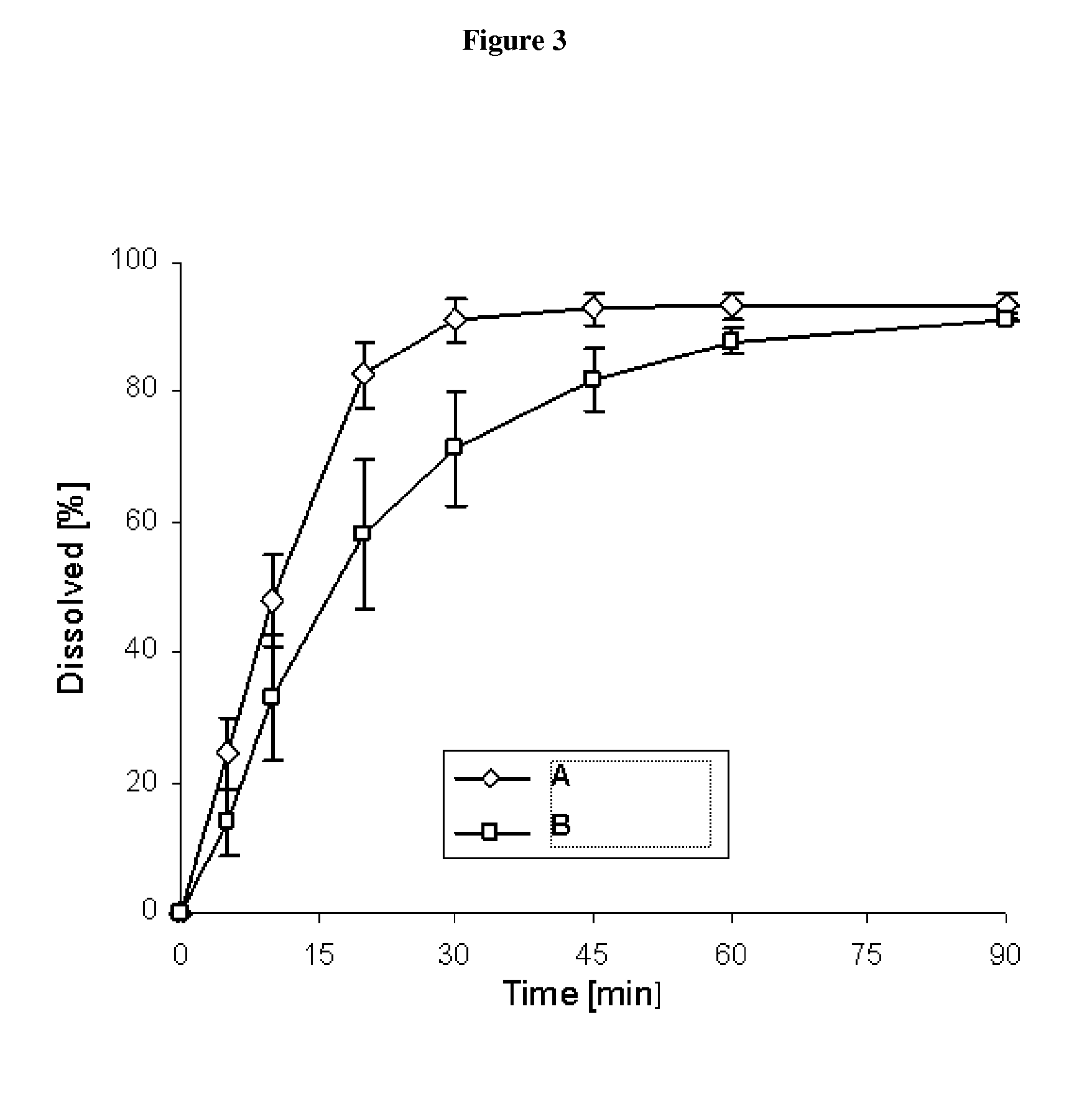
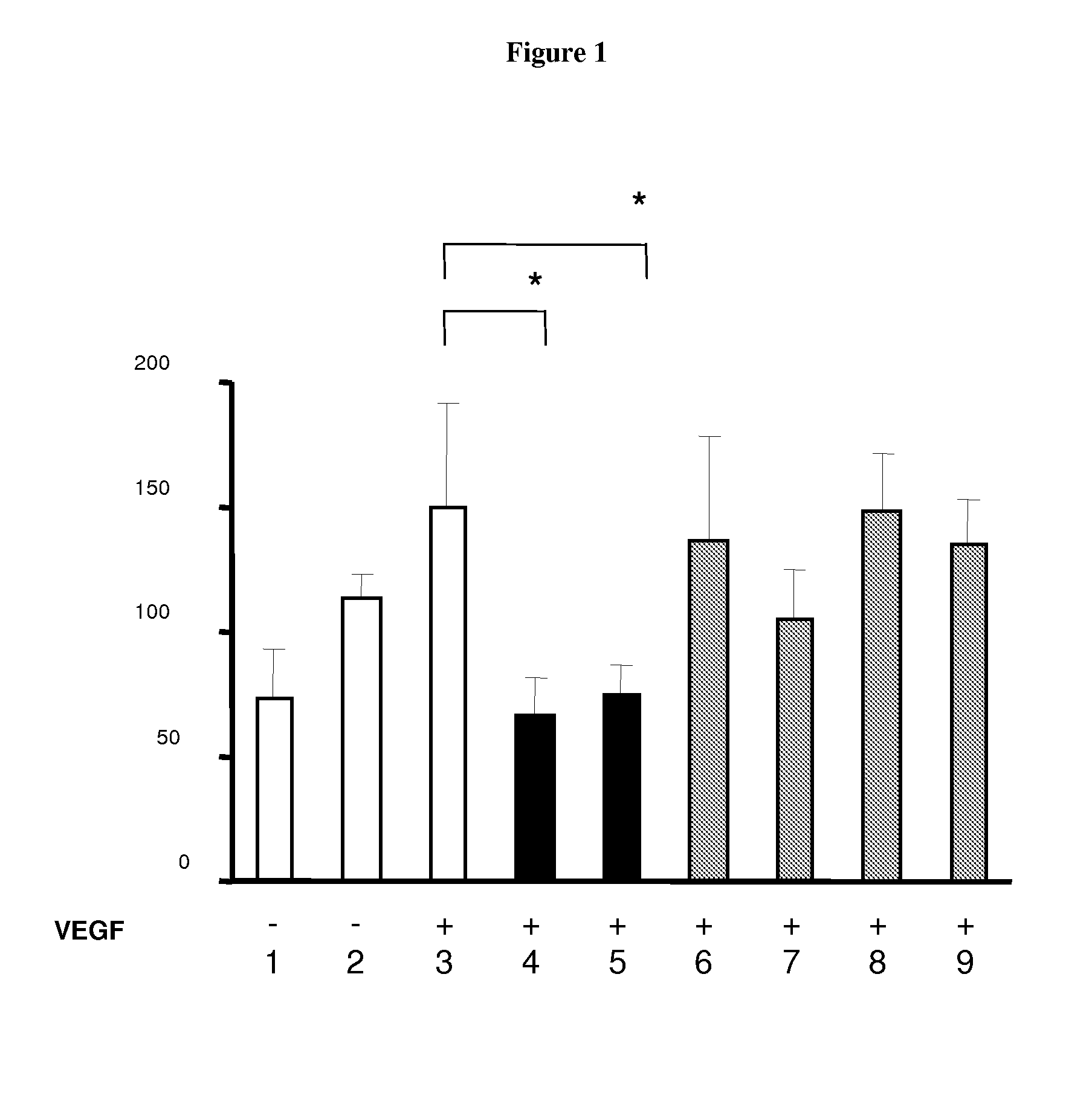
Method or system using biomarkers for the monitoring of a treatment
InactiveUS20100233705A1Few and less-troublesome side-effectsMicrobiological testing/measurementDisease diagnosisBULK ACTIVE INGREDIENTBiomarker (petroleum)
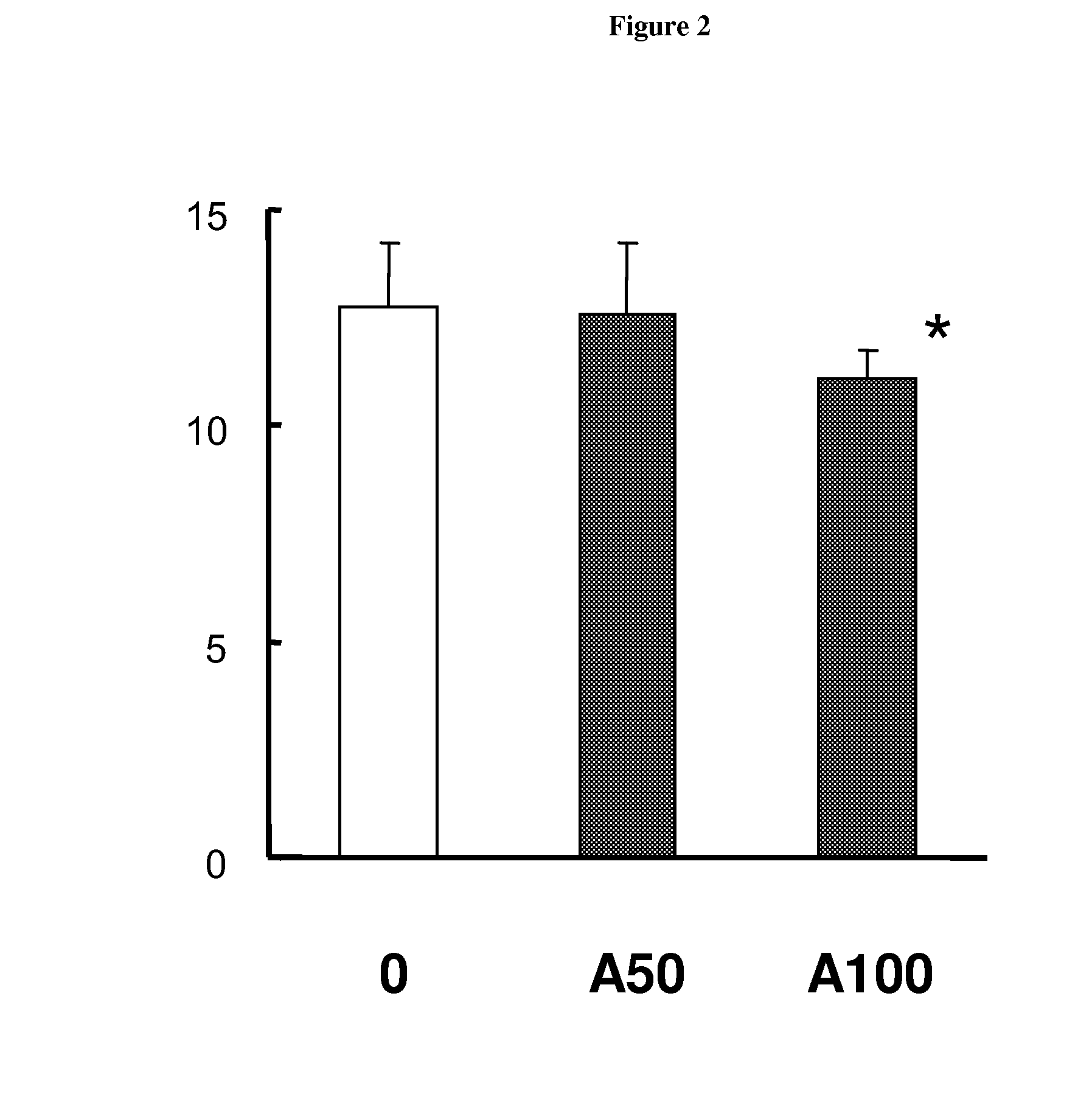
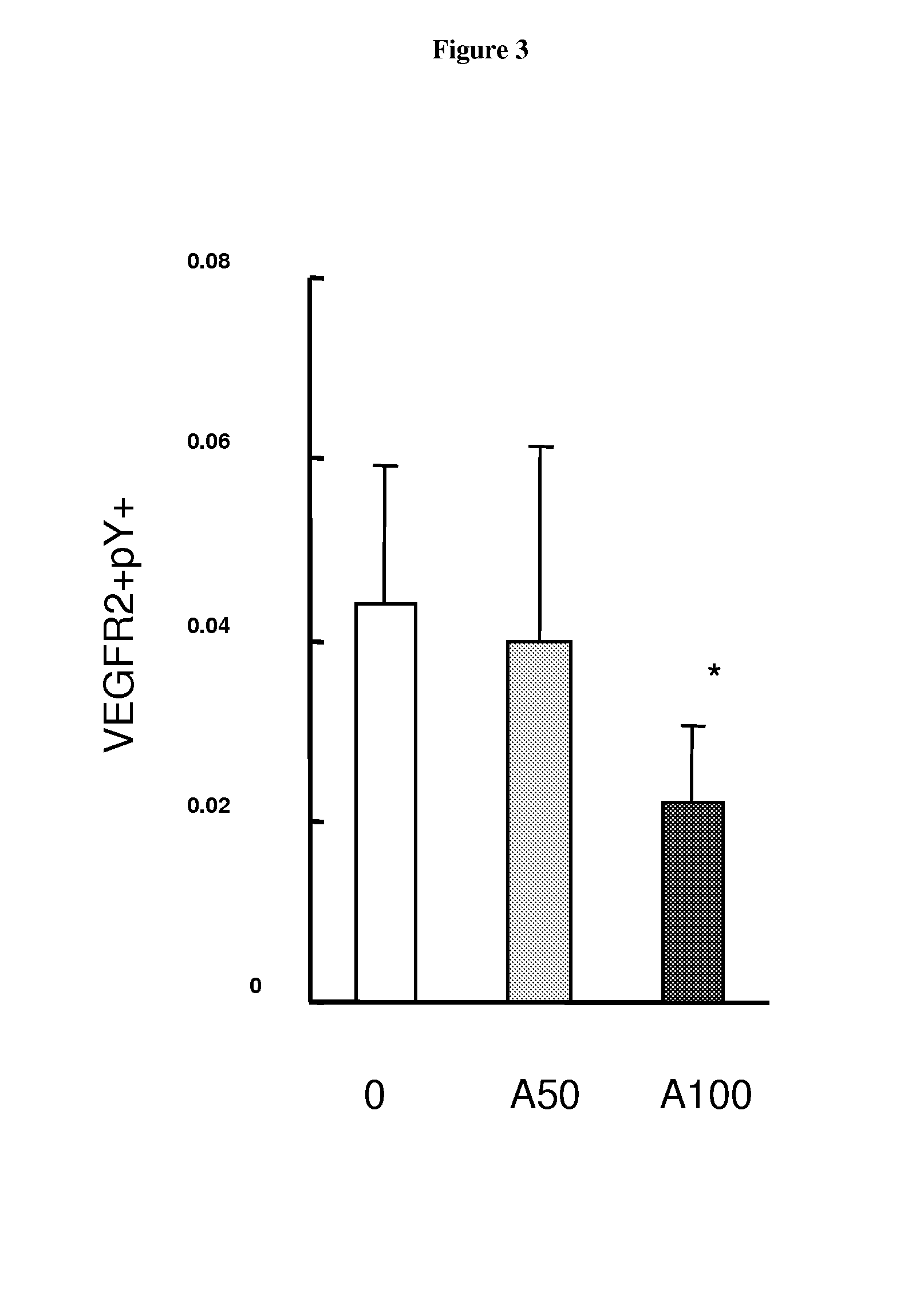
The present invention relates to biomarkers to monitor the activity of the compound 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone or a pharmaceutically acceptable salt thereof, and especially its monoethanesulphonate salt form, when used alone or optionally in combination with further pharmaceutically active ingredients and / or further treatments, such as for example radiotherapy.
Owner:BOEHRINGER INGELHEIM INT GMBH
Method for preparing diclofenac sodium
InactiveCN103145574ARaw materials are cheap and easy to getLow costOrganic compound preparationAmino-carboxyl compound preparationOrganic solventDiclofenac Sodium
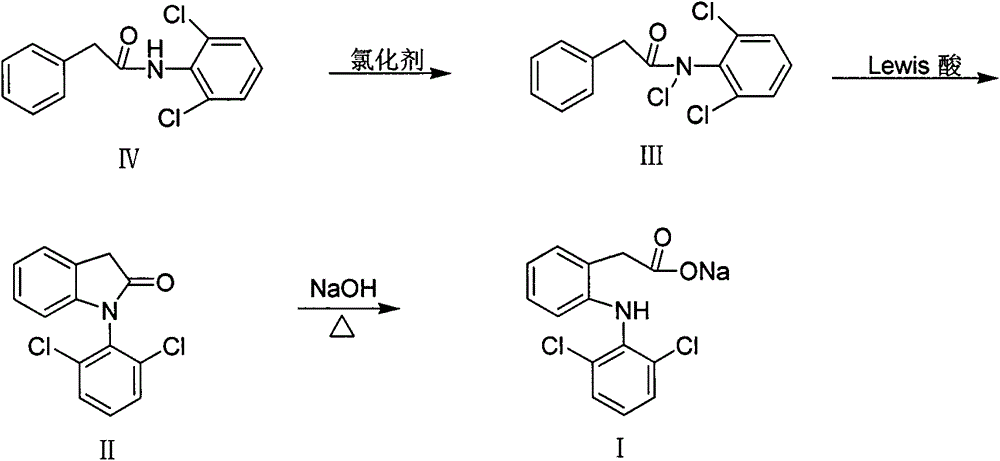
The invention discloses a method for preparing diclofenac sodium. The method is characterized by comprising the following steps of: obtaining N-chloro-N-(2,6-dichlorophenyl)phenylacetamide (III) through reacting a chloride reagent with N-(2,6-dichlorophenyl)phenylacetamide (IV); obtaining 1-(2,6-dichlorophenyl)-2-indolinone (II) through reacting N-chloro-N-(2,6-dichlorophenyl)phenylacetamide (III) with a certain amount of Lewis acid in an organic solvent; and finally obtaining diclofenac sodium (I) through enabling the 1-(2,6-dichlorophenyl)-2-indolinone (II) to be subjected to hydrolysis reaction in a sodium hydroxide solution. The method disclosed by the invention has the characteristics of simplicity in operation, easily-available reagents, low cost, mild conditions and the like, and provides a novel processing route for the synthesis of diclofenac sodium.
Owner:中国人民解放军防化学院
Pharmaceutical dosage form for immediate release of an indolinone derivative
The present invention relates to a pharmaceutical dosage form delivering an immediate release profile containing the active substance 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate.
Owner:BOEHRINGER INGELHEIM INT GMBH
2-indole ketone compound, preparation and uses thereof
InactiveCN101157687AIncreased growth inhibition rateGood antitumor activityOrganic active ingredientsOrganic chemistryKetoneTumor cells
The invention relates to a new 2-indolinone compound and a preparation method and the usage to prepare the compounds. The general formula of the new derivatives of indolinone provided by the invention is the formula above, when in preparation, the 3-position and the 5-position of 2-indolinone are respectively introduced by amide and a carboxamide methyl sulfonyl structure to get the new derivatives. The 2-indolinone compounds of the invention have good activity of inhibition of tumor cell growth.
Owner:EAST CHINA NORMAL UNIV
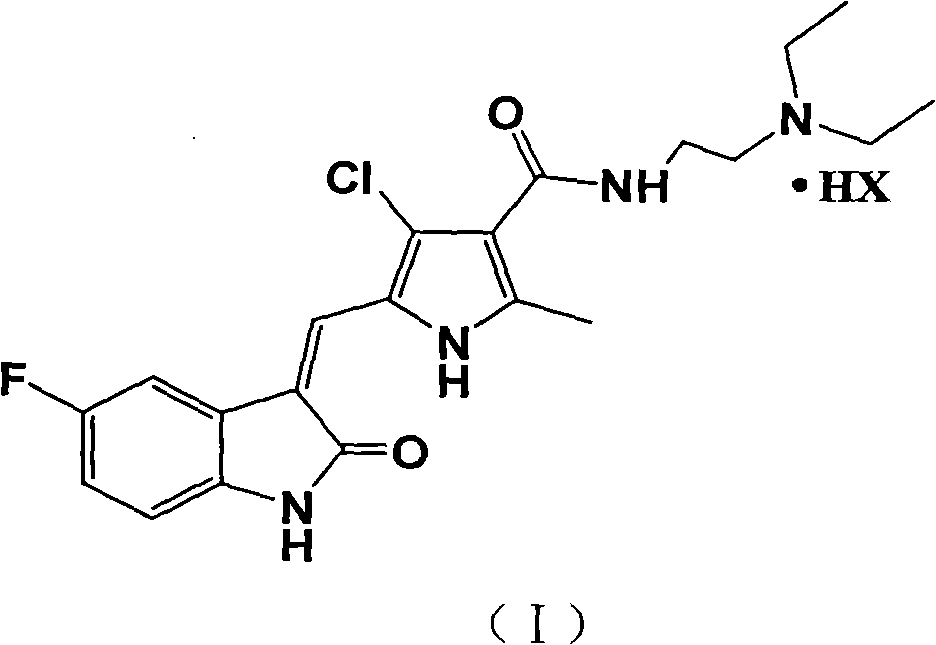
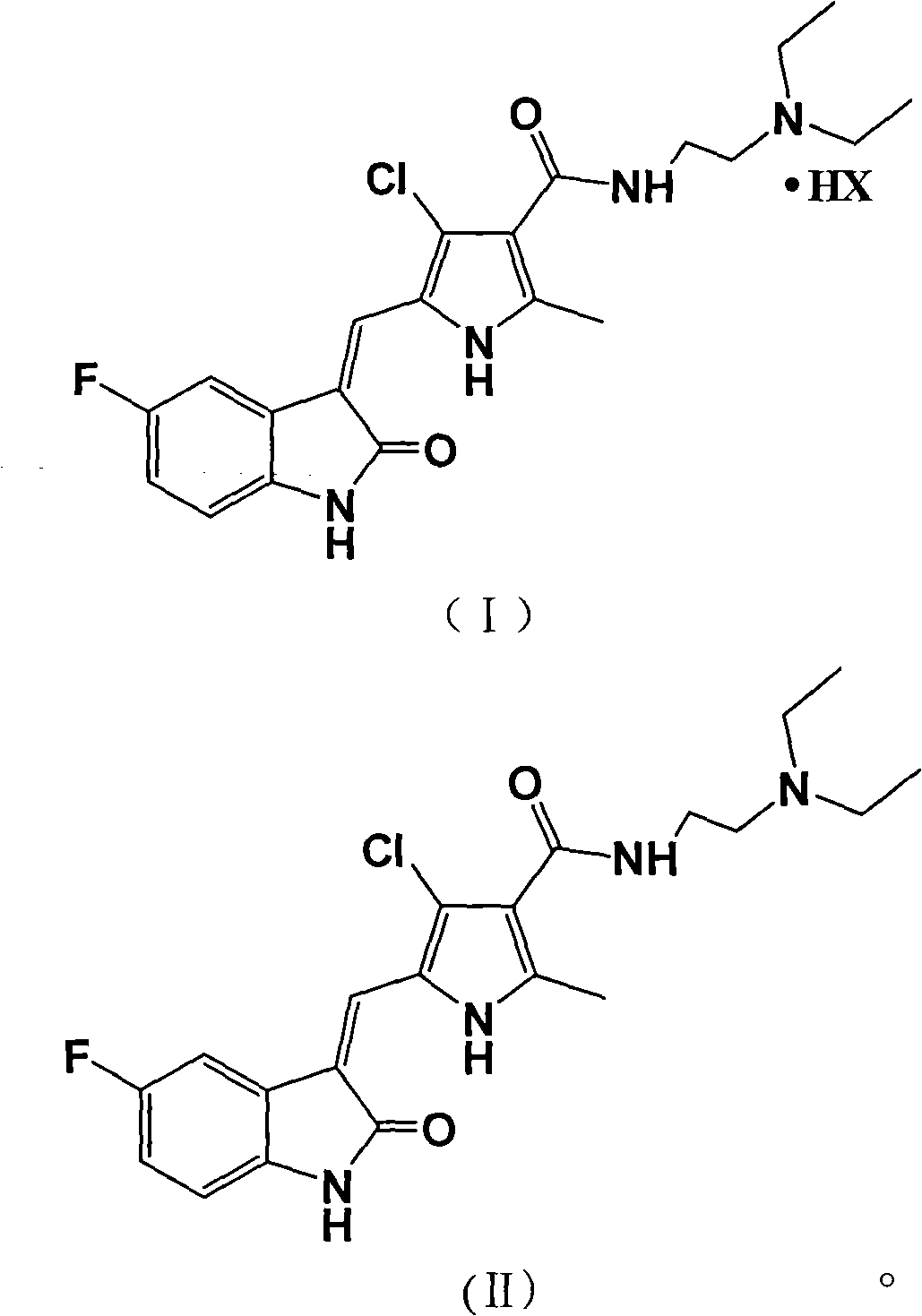
Halogenated pyrrole-substituted 2-indolinone salt and preparation method and application thereof
InactiveCN102030741AImprove solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubilityIn vivo
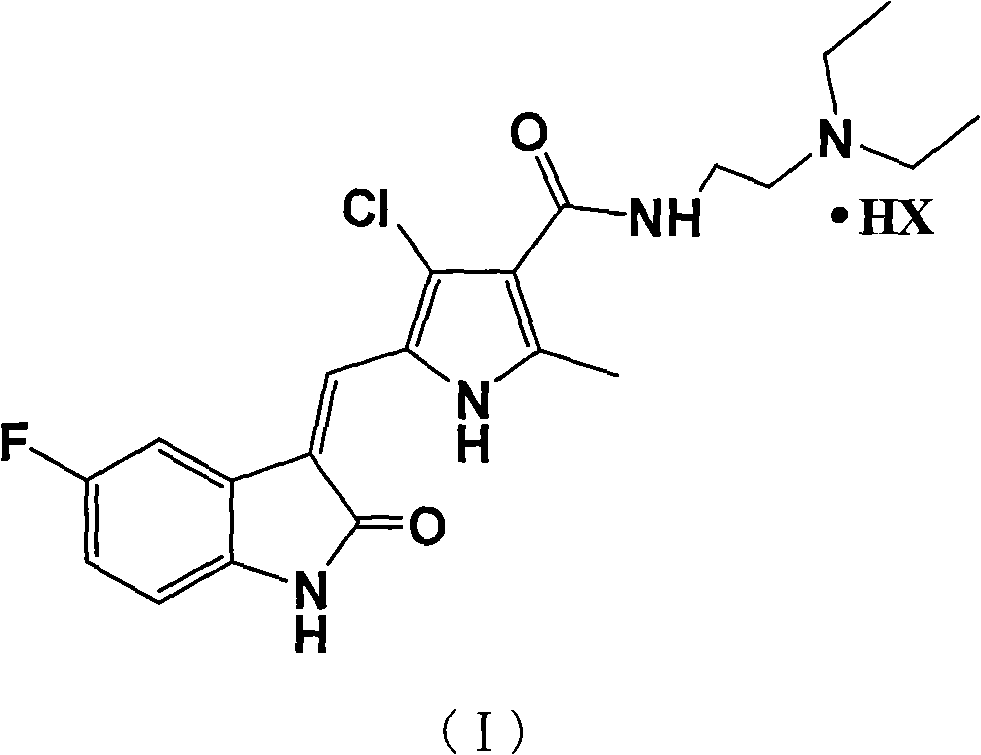
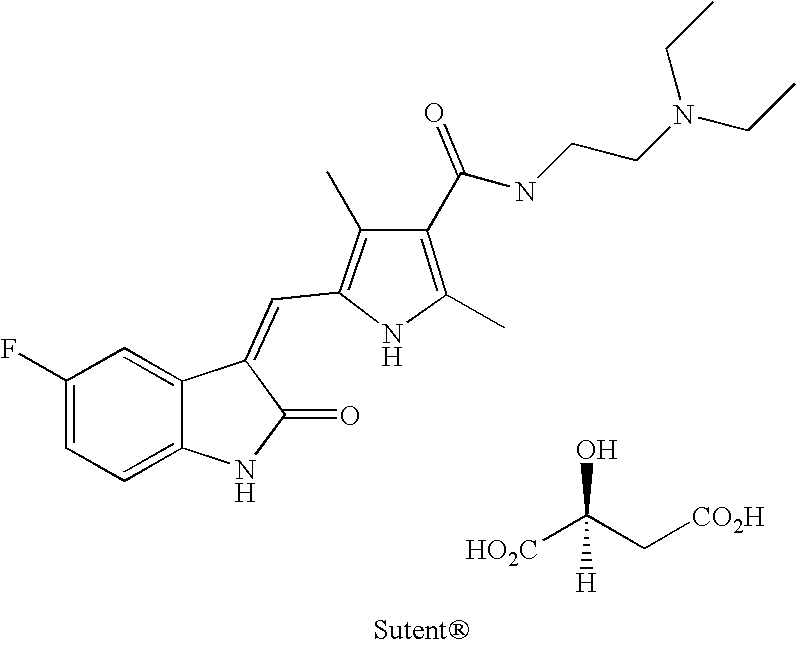
The invention relates to halogenated pyrrole-substituted 2-indolinone salt and a preparation method and application thereof. The salt has a structure which is shown in a formula (I). The preparation method comprises the following step that: the halogenated pyrrole-substituted 2-indolinone salt shown in the formula (I) can be obtained by reacting a compound shown in a formula (II) with acid (HX) in a solvent. The salt has high solubility in water and ethanol and high bioavailability in animal bodies, and is suitable for developing the conventional preparations for treating tumor and suitable to be used as a medicament. The salt prepared by the invention is used for preparing the anti-tumor medicament, has multi-target specificity, and can overcome the disadvantages of poor in-vitro solubility, no obvious in-vitro anti-tumor effect and low in-vivo bioavailability so as to further improve curative effect and toxicity.
Owner:SHANGHAI LANGLAI MEDICINE TECH CENT
Substituted 2-indolinone as PTK inhibitors containing a zinc binding moiety
InactiveUS7928136B2Effective for treating diseaseHigh activityBiocideOrganic chemistryDiseasePTK Inhibitors
The present invention relates to substituted 2-indolinone containing zinc-binding moiety based derivatives that have enhanced or unique properties as inhibitors of protein tyrosine kinase (PTK) receptors and their use in the treatment of PTK related diseases and disorders such as cancer. The said derivatives may further act as HDAC inhibitors.
Owner:CURIS INC
Method for preparing substituted 2-indolinone compound
InactiveCN102432522AMild reaction conditionsHigh yieldOrganic chemistryOrganic solventTrifluoroacetic acid
The invention relates to a method for preparing a substituted 2-indolinone compound, which comprises the following steps of: reducing a substituted isatin compound by triethyl silicane / trifluoroacetic acid; concentrating; and filtering after washing by using an organic solvent so as to obtain the target substituted 2-indolinone compound. In the method, various substituted isatins with wide sources and low price are used as the raw materials; the reaction condition is mild; the substituted 2-indolinone compound is prepared under high yield; and the method has the advantage of convenience and simpleness in posttreatment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
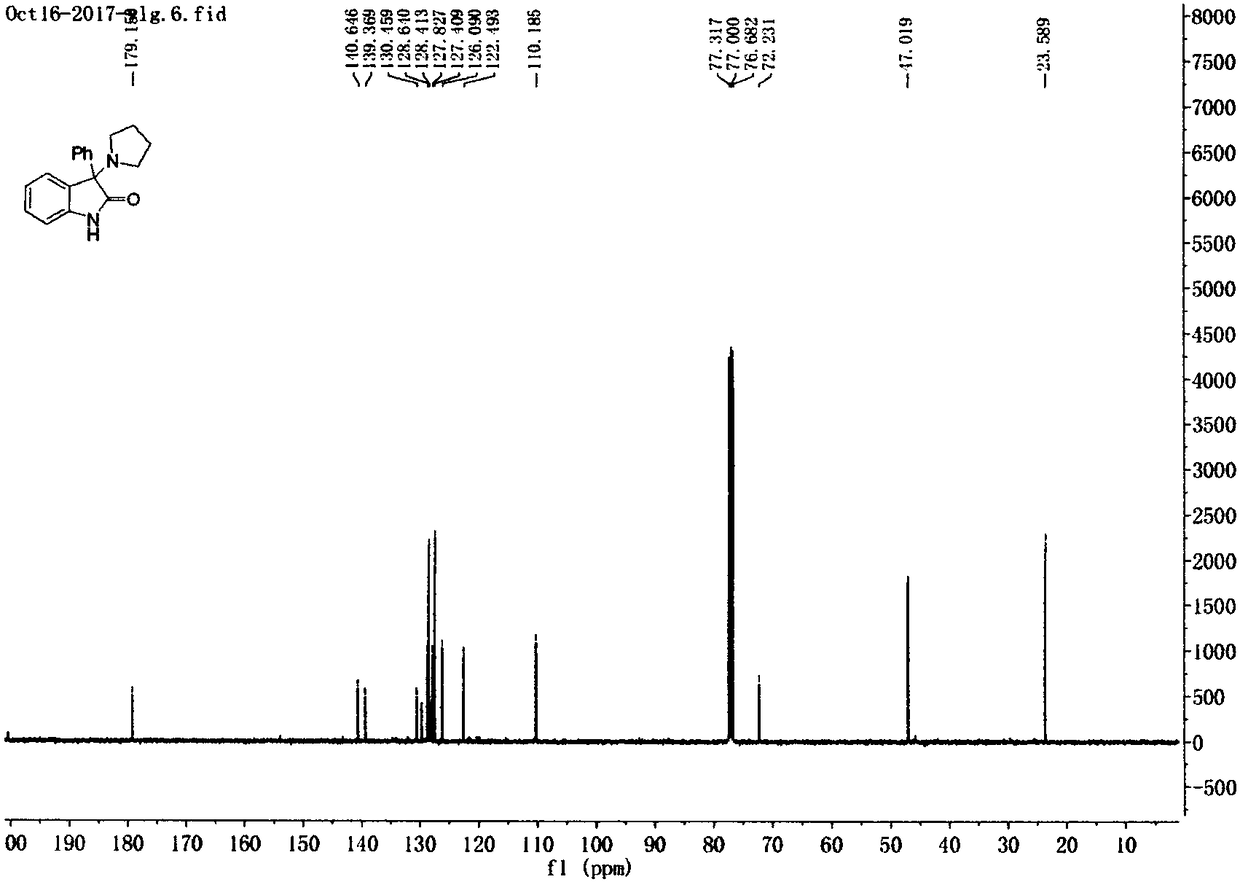
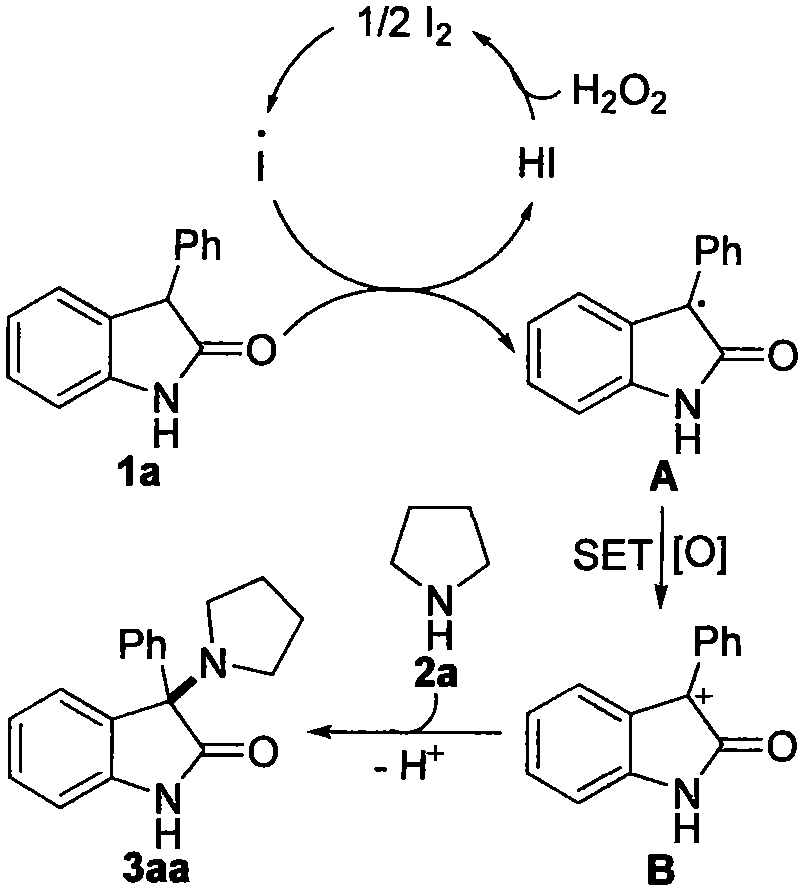
Preparation method of 3-amino-2-indolinone derivative promoted by iodine-hydrogen peroxide at room temperature
The invention provides a preparation method of a 3-amino-2-indolinone derivative. The preparation method is simple in process, economical and green. According to the method, 2-indolinone compound 1a and alkylamine 2a are taken as raw materials and subjected to amination reaction under the conditions of I2-H2O2 system at room temperature, and the 3-amino-2-indolinone derivative I with high yield isprepared simply. The reaction formula of the derivative is as follows in the description.
Owner:NINGBO UNIV
2-indolinone derivatives, preparation and applications thereof
InactiveCN105481751AGood anti-tumorHigh activityOrganic active ingredientsOrganic chemistryChemical structureOrganic acid
The present invention relates to a class of 2-indolinone derivatives, a combination of the class of the compounds, and applications of the 2-indolinone derivatives in anti-tumor and / or anti-cancer and / or anti-angiogenesis drugs. The present invention further relates to a preparation method of the compounds, wherein the chemical structure general formula of the compounds is defined in the specification, and R1, R2, R3 and R4 are individually H, br and OCH3 and / or the combination of R1, R2, R3 and R4 is H, br and OCH3. The present invention further relates to a composition containing at least one compound, or a salt formed from the compound and a physiologically acceptable inorganic or organic acid, or if appropriate, a pharmaceutically acceptable excipient and / or diluent or excipient. The present invention further relates to the compound administration dosage form containing at least one compound having the structure formula or the salt thereof, wherein the dosage form is tablet, capsule, solution for infusion, suppository, patch, powder, suspension and the like.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors](https://images-eureka.patsnap.com/patent_img/727ac101-0a9b-426b-9a6d-81b69cf7b04f/US20100004239A1-20100107-C00001.png)
![Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors](https://images-eureka.patsnap.com/patent_img/727ac101-0a9b-426b-9a6d-81b69cf7b04f/US20100004239A1-20100107-C00002.png)
![Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors Pyrrolo [3,2-C] Pyridine-4-One 2-Indolinone Protein Kinase Inhibitors](https://images-eureka.patsnap.com/patent_img/727ac101-0a9b-426b-9a6d-81b69cf7b04f/US20100004239A1-20100107-C00003.png)
![3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition 3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition](https://images-eureka.patsnap.com/patent_img/d1c97080-4f37-492f-b834-2e254394b83a/US07119093-20061010-D00001.png)
![3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition 3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition](https://images-eureka.patsnap.com/patent_img/d1c97080-4f37-492f-b834-2e254394b83a/US07119093-20061010-D00002.png)
![3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition 3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition](https://images-eureka.patsnap.com/patent_img/d1c97080-4f37-492f-b834-2e254394b83a/US07119093-20061010-C00001.png)
![Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/9eaaea8b-fe90-4ac5-bf72-7e4cb3f24b0a/US08012966-20110906-C00001.png)
![Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/9eaaea8b-fe90-4ac5-bf72-7e4cb3f24b0a/US08012966-20110906-C00002.png)
![Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/9eaaea8b-fe90-4ac5-bf72-7e4cb3f24b0a/US08012966-20110906-C00003.png)
![3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition](https://images-eureka.patsnap.com/patent_img/57cc2a93-7e12-44e6-a0bb-354e6172d834/US20040176392A1-20040909-C00001.png)
![3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition](https://images-eureka.patsnap.com/patent_img/57cc2a93-7e12-44e6-a0bb-354e6172d834/US20040176392A1-20040909-C00002.png)
![3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the use thereof as a pharmaceutical composition](https://images-eureka.patsnap.com/patent_img/57cc2a93-7e12-44e6-a0bb-354e6172d834/US20040176392A1-20040909-D00001.png)
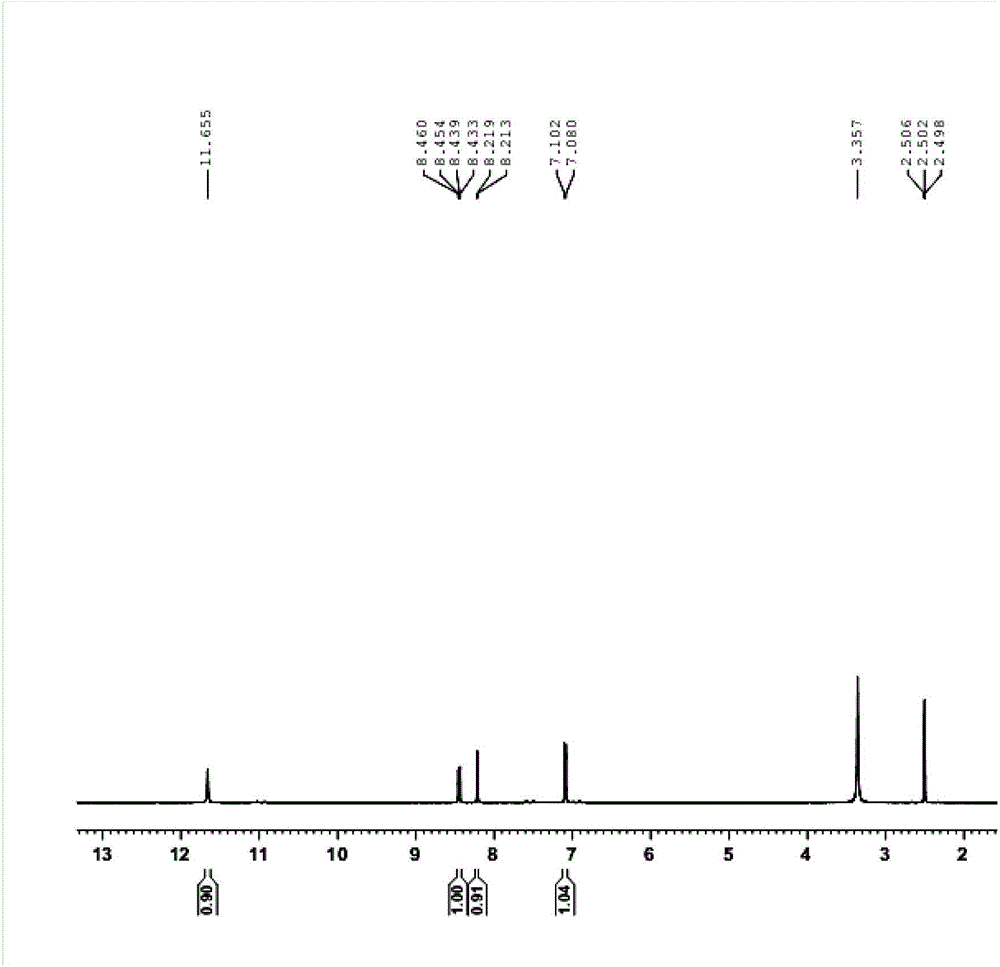
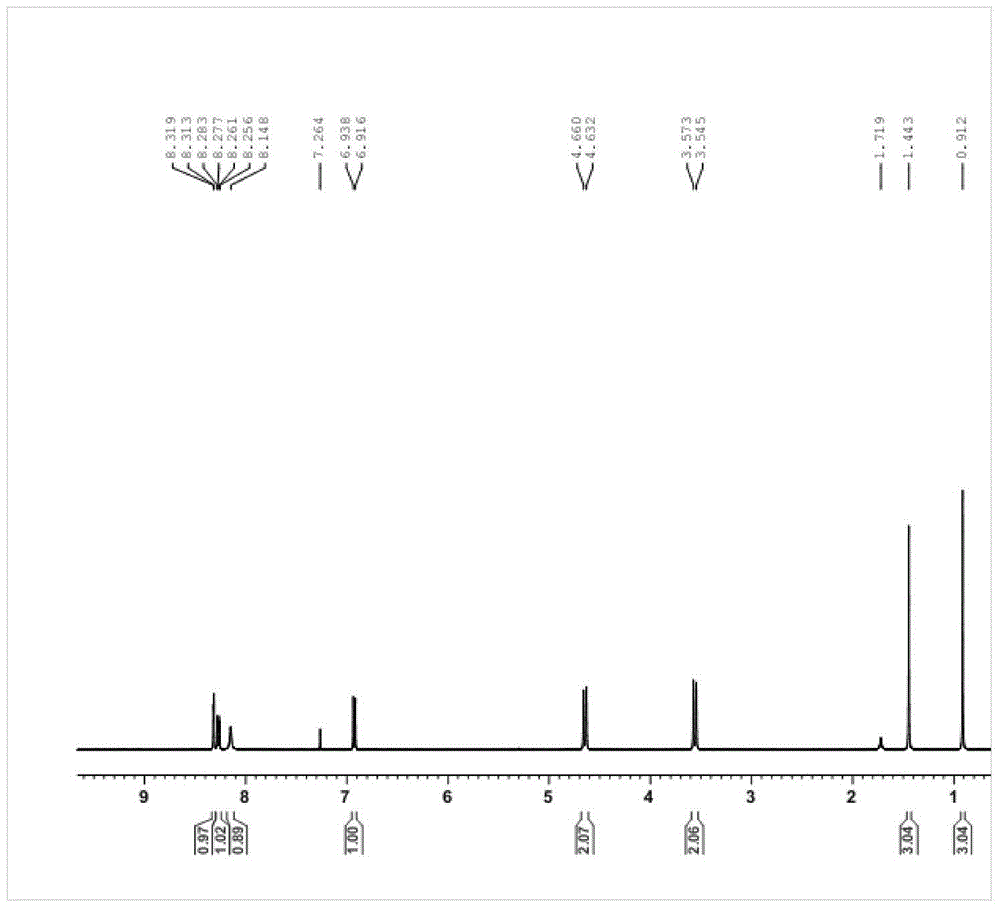
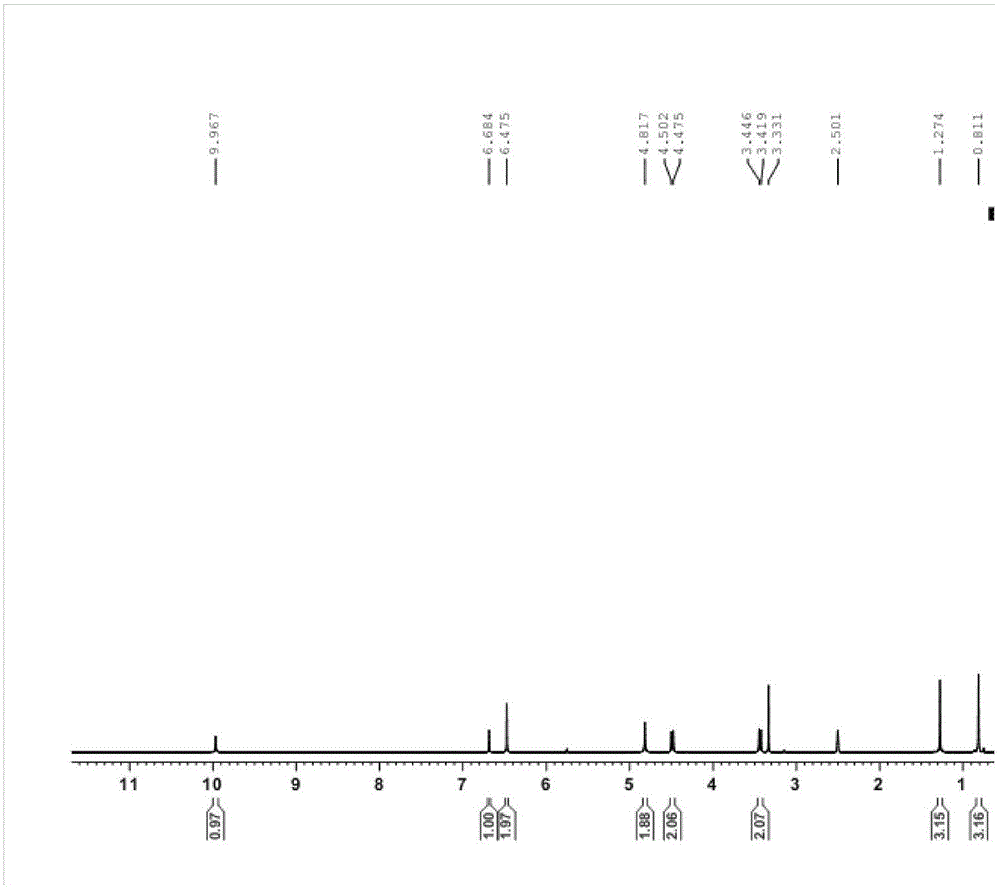
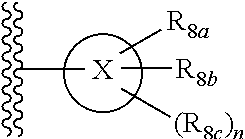
![Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/97a74be5-46a5-4a52-85a4-afb0ba43c753/US20110301353A1-20111208-C00001.png)
![Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/97a74be5-46a5-4a52-85a4-afb0ba43c753/US20110301353A1-20111208-C00002.png)
![Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors Pyrrolo [3,2-c] pyridine-4-one 2-indolinone protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/97a74be5-46a5-4a52-85a4-afb0ba43c753/US20110301353A1-20111208-C00003.png)