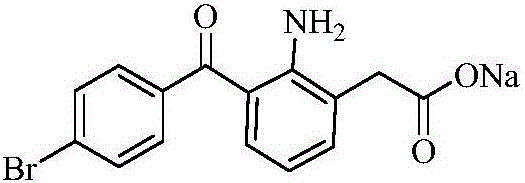
Bromfenac sodium preparation method
A technology of bromfenac sodium and bromfenac, which is applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation and other directions, can solve the problems of high cost, high production cost, complicated process and the like, and achieves a short synthesis route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
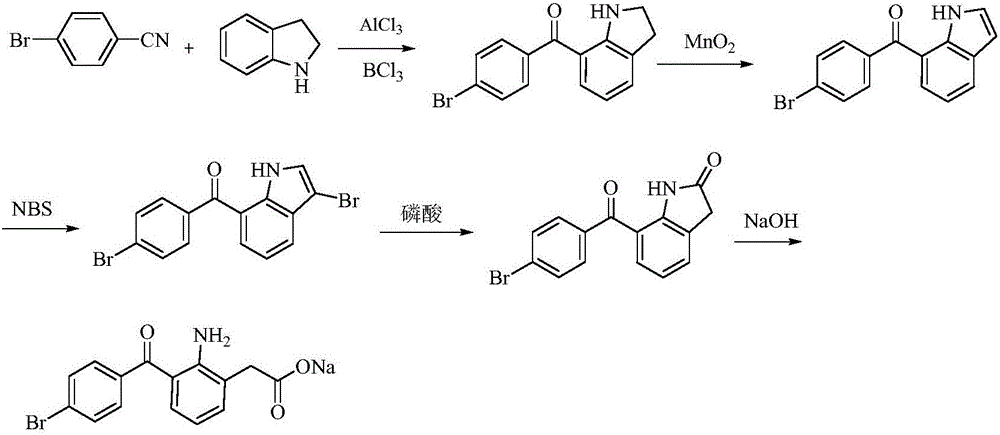
[0023] The synthetic route of bromfenac sodium:
[0024]
[0025] The specific synthetic method of bromfenac sodium is as follows:
[0026] 1) Preparation of 3-bromoindole: Dissolve indole (234.4g, 2.0mol) in 3.5LDMSO, then add NBS (356.0g, 2.0mol), stir at 25°C for 6h, add 6L of water, cool to 10 ℃, stirring and centrifuging, washing the filter cake with 1.5 kg of purified water, and blow drying at 50 ℃ to obtain 380.0 g of the target product 3-bromoindole, with a yield of 96.94%.
[0027] 2) Preparation of 2-indolinone: 3-bromoindole (380.0g, 1.94mol) was added in 9L2-methoxyethanol, then dissolved and refluxed after stirring, phosphoric acid was added in four batches (750ml , add 3h intervals each time, add 3L phosphoric acid in total), react for 12h, cool to room temperature, add 9L purified water, cool to 10°C, centrifuge, wash the filter cake with 1.5kg purified water, and blow dry at 50°C to obtain 2-indole Indolin ketone 245g, the yield is 94.81%.
[0028] 3) Pre...
Embodiment 2
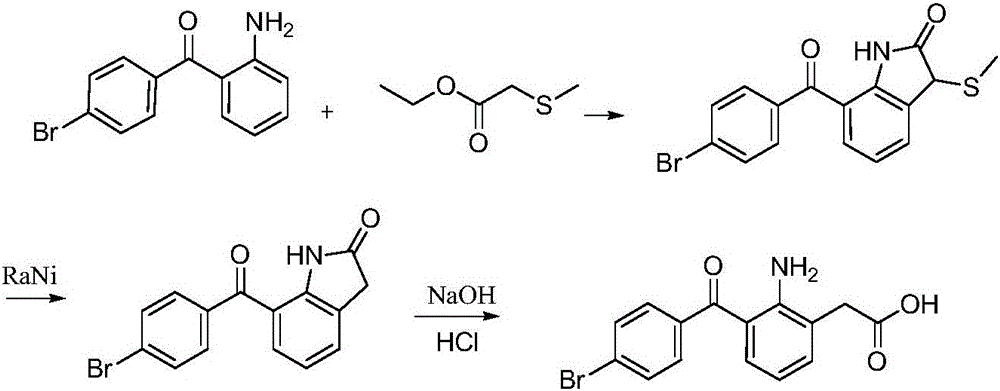
[0032] The synthetic route of bromfenac sodium:
[0033]
[0034] The specific synthetic method of bromfenac sodium is as follows:
[0035] 1) Preparation of 3-bromoindole: Dissolve indole (234.4g, 2.0mol) in 3.5LDMSO, then add NBS (356.0g, 2.0mol), stir at 35°C for 6h, add 6L of water, cool to 15 ℃, stirring and centrifuging, washing the filter cake with 1.5kg of purified water, and blow drying at 50 ℃ to obtain 382.0 g of the target product 3-bromoindole, with a yield of 97.45%.
[0036]2) Preparation of 2-indolinone: 3-bromoindole (382.0g, 1.93mol) was added in 9L2-methoxyethanol, then dissolved and refluxed after stirring, phosphoric acid was added in four batches (750ml , add 3h intervals each time, add 3L phosphoric acid in total), react for 12h, cool to room temperature, add 9L purified water, cool to 15°C, centrifuge, wash the filter cake with 1.5kg purified water, blow dry at 50°C to obtain 2-indole Indolin 247g, the yield is 95.11%.
[0037] 3) Preparation of 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com